Fish Oil Intake during Pregnancy and Lactation in Rats Has Different Long-Term Effects on Glucose-Insulin Relationships in Male Pups Depending on Their Age
Abstract
Introduction
During pregnancy, polyunsaturated fatty acids are essential for fetal development. The fetus is unable to synthesize them and they have to come from the mother. Fish oil supplements (rich in docosahexaenoic acid) are often recommended during the perinatal stage and may contribute to programming future events. The study presented here compared the long-term effects of supplementing the maternal diet of rats with either fish oil or olive oil during pregnancy and lactation on the glucose-insulin axis in the male offspring at different ages.
Methods
Rats were fed diets containing 10% of either fish (FOD) or olive (OOD) oil during pregnancy and lactation. From weaning, male offspring were given a regular diet and were subjected to an oral glucose tolerance test (2 g glucose/kg body weight) at 2, 4, 12 and 18 months of age when plasma glucose and insulin were measured.
Results
At birth there was no difference in the number and weight of the pups in the two groups. During lactation, milk production was lower in dams of FOD than OOD. Neither basal plasma glucose nor insulin differed between the groups; after the glucose load in 2-month-old pups, both groups showed similar increases in glucose concentration, but insulin increased less in FOD pups than in OOD. This difference had disappeared in 4-month-old pups, but in 12- and 18-month old pups the increase in glucose was higher in FOD without differences in insulin.
Conclusions
Biphasic insulin sensitivity in male pups of dams given FOD during pregnancy and lactation depends on their age. Their decreased milk intake and low arachidonic acid availability during the suckling period could influence the response. The findings are consistent with the variable results reported elsewhere on the potential benefits of fish oil supplements during perinatal life.
Keywords
Fish oil supplement, Pregnant rats, Fetal programming, Dietary fatty acids, Insulin sensitivity
Abbreviations
ALA: Alpha-linolenic acid, 18:3 n-3; AA: Arachidonic acid, 20:4 n-6; DHA: Docosahexaenoic acid, 22:6 n-3; EPA: Eicosapentaenoic acid, 20:5 n-3; HOMA: Homeostasis model assessment; LA: Linoleic acid, 18:2 n-6; LCPUFA: Long chain polyunsaturated fatty acids; MUFA: Monounsaturated fatty acids; OA: Oleic acid, 18:1 n-9
Introduction
Linoleic acid (18:2 n-6, LA) and α-linolenic acid (18:3 n-3, ALA) are the essential fatty acids (EFA) from which long-chain polyunsaturated fatty acids (LCPUFA) can be synthesized in adults. The fetus in humans [1] and rats [2], however, has a limited capacity to carry out this synthesis, so both arachidonic acid (20:4 n-6, AA) and docosahexaenoic (22:6 n-3, DHA) (as well as other LCPUFA) become essential. These two acids, which play a major role in fetal development, have therefore to come from maternal sources, meaning that pregnancy is a time of increased risk of LCPUFA deficiency [3-5]. In fact in humans the proportion of both AA and DHA in maternal plasma decreases in late pregnancy, whereas values in cord blood plasma are either higher than or similar to those in the maternal circulation [6]. For this reason, supplementation with fish oil, rich in n-3 LCPUFA, is often recommended during pregnancy. However, some harmful effects of high maternal doses of fish oil or DHA supplements have also been reported in both women [7] and rats [8], and a recent systematic review of data from human studies published on this topic concluded that there is not enough evidence to support the routine use of n 3 LCPUFA or fish oil supplementation during pregnancy [9].
Studies in humans indicate that adverse prenatal and early postnatal nutritional status may contribute to programming future events, including the susceptibility to impaired glucose tolerance [10,11]. However the evidence for programming from fish oil intake during the perinatal stage in humans on later changes in the glucose-insulin axis is limited. Even in a randomized controlled trial of the supplementation with fish oil compared to olive oil during the third trimester of pregnancy, no association with adiposity, plasma insulin and homeostasis model of insulin resistance (HOMA) in 19-year-old children was found [12]. The studies on the long-term effects of fish oil intake during the perinatal stages in rodents are scarce and have yielded quite variable results. In mice, a fish-based diet during pregnancy and lactation resulted in increased insulin sensitivity in offspring at 15 weeks of age [13]. In rats given 7% fish oil throughout pregnancy, no differences were found in plasma glucose and insulin in offspring at 15 weeks of age [14]. In addition, no effect was found either in 105-day old male offspring of maternal rats that were given a diet supplemented with 10% fish oil for 90 days preconception and throughout pregnancy and lactation [15], or in offspring at 12 weeks of age of dams given a diet containing 18% fish oil during the 2 weeks prior to mating and throughout pregnancy and lactation [16]. However, in rats given a diet containing 8% of fish oil during just the first 12 days of pregnancy, we found that at 8 months of age male offspring - but not females - showed increased insulin sensitivity [17]. We also found that the effect was epigenetic and could be explained by the influence of microRNA expression [18].
In order to determine whether the effect of fish oil intake during the perinatal stage in rats on the glucose-insulin axis in offspring is dependent on the age of the offspring, the work reported here studied oral glucose tolerance tests at different ages in male offspring of dams that had been given a fish-oil supplemented diet and compared the findings with control offspring from dams given the same diet but supplemented with olive oil. This supplementation of the diet with olive oil has been previously used as an appropriate control in dietary fish oil human trials during pregnancy [12,19] and in rats in studies on the effects of fish-oil diet during pregnancy and lactation by ourselves [17,20-22].
Material and Methods
Female Sprague Dawley rats from our own animal quarters were initially given a standard non-purified diet (B&K Universal, Barcelona) and housed under controlled light and temperature conditions (12 h light/dark cycle; 22 ± 1 °C). The experimental protocol was approved by the Animal Research Committee of Universidad San Pablo CEU in Madrid, Spain. Rats were mated when they weighed 184-203 g, and on the day in which spermatozoids were found in vaginal smears (day 0 of pregnancy) they were divided into two groups that were given purified diets that differed only in the nature of the non-vitamin lipid component. The diets contained per kg of diet: 170 g casein, 100 g cellulose, 580 g maize starch, 35 g salt mix, 10 vitamin mix and 100 g of either fish oil (cod liver oil, Cofares, Spain) (FOD) or olive oil (Carbonell, Spain) (OOD). Diets were prepared at the onset of the experiment and were divided into daily portions that were kept at -20°C until use. Dams were housed in collective cages (four per cage) and had free access to the assigned diet and tap water. Two days before delivery, pregnant rats were placed in individual cages. From the 2nd day of postpartum litters were adjusted to 8 pups per dam and the dams were maintained on their experimental diet until day 21 of lactation, when pups were weaned and were maintained in collective cages. From this time on, all pups were fed the standard non purified diet. Milk intake was estimated from pup weight and pup weight gain on days 7-8 and days 15-16 of lactation as previously described [23]. On day 10 of lactation, after being separated from their litters, dams were anesthetized with a cocktail (0.5 ml/200 g body weight) containing 9 mg ketamine (Imalgene 500; Rhone Merieux, Lyon, France) and 0.25 mg chlorpromazine (Largactil; Rhone Poulenc, Madrid, Spain) administered intraperitoneally. They were injected intraperitoneally with a solution of oxytocin (0.25 ml/200 g body weight: 40 mg/L Syntocinon; Novartis Farmacéutica, Barcelona, Spain) and milk was obtained with gentle hand stripping of the teats. An aliquot of milk was immediately placed into chloroform-methanol (2:1, v/v) for lipid extraction and fatty acid profile analysis as described below.
At 2, 4, 12 and 18 months of age each male pup was given an oral glucose tolerance test as follows. All tests were carried out between 09.00 and 11.00 h in rats that had been fasted overnight. After collecting blood (0 time) rats received orally 2 g glucose/kg body weight and blood was collected at 7.5, 15 and 22.5 min in pups that were 2 and 4 months of age and at 7.5, 15, 22.5, 30 and 60 min in the 12- and 18-month old offspring. Blood was always collected from the tail vein into receptacles containing Na2-EDTA and plasma separated by centrifugation at 4 °C and 1,500 g for 10 min. Plasma was kept at -80°C until analysis for glucose and insulin using commercial kits (Boehringer-Mannheim, Manheim, Germany and Mercodia AB, Uppsala, Sweden, respectively).
For the analysis of fatty acids profiles, lipids were extracted in chloroform-methanol (2:1, by volume) and purified [24] from fresh aliquots of milk and from each diet. Lipid extracts were saponified and methylated, and the fatty acid methyl esters were separated and quantified by gas chromatography with a Perkin-Elmer gas chromatograph (Autosystem; Norvalk, Conn.) with a flame ionization detector and a 30 m × 0.25 mm Omegawax capillary column as previously reported [25].
Statistics
All analyses were conducted in SPSS (version 14:0; Chicago, Ill., USA). Differences between the two groups were analyzed by Student's t test.
Results
The fatty acid profile of the two diets given to the rats during pregnancy and lactation is shown in table 1. As it would be expected, the main differences between the two diets were the proportions of eicosapentaenoic acid (20:5, n-3, EPA), DHA and total n-3 fatty acids, which were much higher in the FOD than in the OOD. Also as expected, the proportions of oleic acid (18:1, n-9, OA), total monounsaturated fatty acids (MUFA) and total n-6 fatty acids were lower in the FOD than in OOD. Values of the proportion of LA, AA and ALA were low in both diets, although values for LA were higher in OOD than in FOD whereas the opposite was true for both AA and ALA.
The body weight of dams during pregnancy and during lactation did not differ between the two groups (data not shown). As shown in table 2, at delivery the number of pups and birth weight did not differ between the two groups. However from 12 days of age until the end of lactation the body weight of pups from the FOD group was lower than in those from the OOD group. This significant difference between the two groups was seen at 2 and 4 months of age whereas no difference could be found at either 12 or 18 months of age. Milk intake by the suckled pups at day 7-8 of lactation did not differ between the two groups, but at day 15-16 it was lower in the FOD group (Table 3). The fatty acid profiles of milk at mid-lactation (day 10 of lactation) show a higher proportion of saturated fatty acids and EPA, DHA and total n-3 fatty acids but lower of OA, total MUFA, LA and AA in the FOD group than in the OOD group (Table 3).
Oral Glucose Tolerance Tests
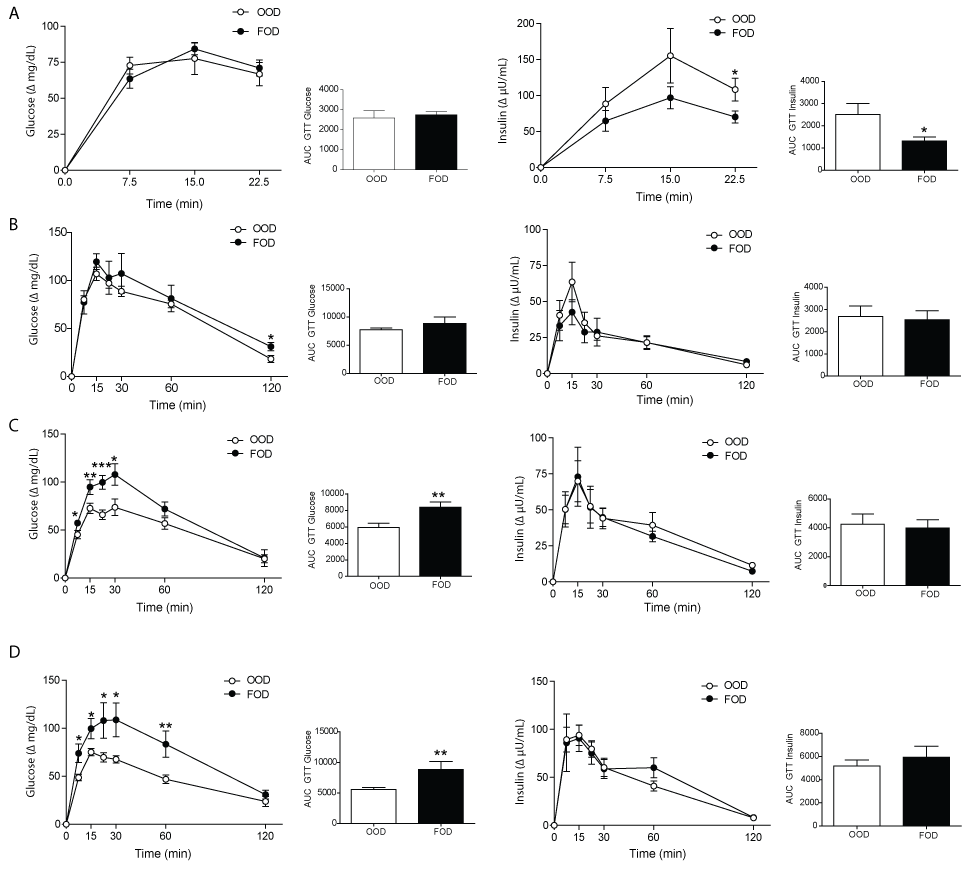
Basal plasma glucose and insulin concentrations (0 time) did not differ between FOD and OOD rats at 2, 4, 12 or 18 months of age (data not shown); however, major differences were found as a response to the oral glucose load. Results are expressed as the change of values at different time points compared to basal values for each rat as well as area under the curve (AUC) for both glucose and insulin. As shown in figure 1A, the 2-month-old pups of the two groups showed a similar increase in plasma glucose, but the FOD group had a smaller increase in insulin concentration than the OOD group, the difference in the AUC for insulin being statistically significant, indicating enhanced insulin sensitivity in the FOD group. When the oral glucose tests were performed at 4 months of age that difference between the two groups disappeared (Figure 1B). However, when these same rats were 12 or 16 months old, the increase in glucose after the glucose load was greater in FOD than in OOD pups whereas no differences in plasma insulin changes were found (Figures 1C and Figure 1D), indicating decreased insulin sensitivity.
Discussion
The present study shows that maternal fish oil supplementation during pregnancy and lactation did not affect glucose tolerance but diminished the increase of plasma insulin after oral glucose load in 2-month-old male pups, indicating an enhanced insulin sensitivity. However, the effect disappeared when these pups were studied at 4 months old and was reversed to show decreased insulin sensitivity when studied at both 12 and 18 months old. Although the precise correlation between the ages of laboratory rats and of humans is still a subject of debate and in adulthood one rat month is approximately equivalent to three human years [26, 27], these findings clearly fit with the variable and contradictory results found in randomized-controlled trials performed in humans to determine if n-3 LCPUFA supplementation affects pregnancy short- and long-term outcomes, as recently reviewed [9]. In fact, the intake of n-3 LCPUFA from fish has been associated with a reduction in the major risk factors of the metabolic syndrome, including adiposity, inflammation, dyslipidemia, hypertension, insulin resistance and type 2 diabetes [28]. In rats fish oil has also been shown to improve insulin sensitivity in obesity [29] and to prevent insulin resistance in high-fat feeding [30]. However, prospective studies in humans don't support the supposed benefits of fish and seafood or EPA and DHA on the development of diabetes mellitus [31]. From randomized controlled human trials it can be concluded that supplementation with fish oil during the second or third trimester of pregnancy is not associated with a reduced risk of gestational diabetes mellitus and associated pathologies [32].
The evidence for programming from fish oil intake during the perinatal stage in human trials on later changes in the glucose-insulin axis is limited. In a randomized controlled trial of daily supplementation with fish oil capsules no association with differences in plasma insulin, glucose or the homeostasis model assessment of insulin resistance (HOMA) in 19-year-old offspring was found [12]. In experimental animals it has been shown that nutritional disturbances may program the fetus for later development of altered functioning of the adult insulin axis, the response being specific for the time-window of exposure in a sex-dependent manner [33]. The data on long-term effects of fish oil intake during pregnancy and lactation on the offspring glucose-insulin axis from rats have also been shown to be variable, going from no differences [15] to enhanced insulin sensitivity [17], the process being sex-dependent and caused by an epigenetic mechanism [18].
The findings we report, of different changes in insulin sensitivity depending on the age of offspring of rats fed fish oil during pregnancy and lactation could be caused or influenced by different factors: a specific action of the higher availability of n-3 LCPUFA during the perinatal stage; a decreased milk yield during lactation causing under-nutrition of pups during suckling; or the decreased availability of AA, which was lower in milk of FOD rats than of those on OOD. Concerning the specific action of the higher availability of n-3 LCPUFA during the perinatal stage, it is known that LCPUFA can have effects on fetal programming (for a review see [34]) and it is known that fish oil consumption in rats even during just the first 12 days of pregnancy have epigenetic effects [18] that result in an enhanced insulin sensitivity in one-year old pups [17]. However, high-fat diets supplemented with fish oil given to rats 2 weeks before mating and until the end of the weaning period had no effect on glucose tolerance at the age of 12 weeks in male offspring [16]. On the other hand, the under-nourished condition during suckling in pups of FOD could be the result of a reduced ability of their dams to synthesize AA as suggested by its low proportion in milk. This probably results from the increased concentrations of DHA and EPA, which are known to inhibit the delta-6 desaturase activity, causing a reduction in AA synthesis and a subsequent decrease of the milk yield [8]. On its own, such a condition of under-nutrition during suckling could result in a permanently decreased insulin sensitivity as we have previously reported also in male rats [35], but the present study shows that insulin sensitivity is actually increased in young (2-months-old) pups of FOD dams, the effect disappearing when they reach 4 months of age and reversing by the time the pups were old (12 and 18 months old).
This is the first time that an age-dependent biphasic response in pups to the treatment with fish oil in pregnant and lactating rats has been reported. It may be the reason for the variable response found by others studying the consequences in offspring of LCPUFA supplementation during pregnancy or lactation (or both) [34]. We have even found enhanced insulin sensitivity in 1-year-old male pups (although not in females) of dams receiving the dietary fish oil supplement during only the first half of pregnancy [17] Taken together, the evidence indicates that different doses of LCPUFA and the sex of the offspring, the timing of window of treatment during the perinatal stage and now the age of the offspring can all affect the response to the fish oil supplement.
Extrapolation of the present findings to the human situation should be made with caution for 2 main reasons: the doses of fish oil used in experimental animals, including those applied here, are much higher than those applied in humans; and secondly, comparison of rat and human ages depends on several factors that could impede the appropriate correlation between both species [26, 27]. However, findings reported herein begin to explain the variable and sometimes contradictory results reported in both humans and experimental animals on maternal fish oil intake and insulin sensitivity in the offspring. Additional studies are necessary to establish a consistent pattern of responsiveness.
Acknowledgments
The authors thank Milagros Morante for excellent technical help and pp-science-editing.com for editing and linguistic revision of the manuscript. Sources of financial support: Fundación Ramón Areces (CIVP16A1835) of Spain and European Community (specific RTD programme "Quality of Life and Management of Living Resources", QLKI-2001-00138. Perilip).
References
- Chambaz J, Ravel D, Manier MC, et al. (1985) Essential fatty acids interconversion in the human fetal liver. Biol Neonate 47: 136-140.
- Green P, Yavin E (1993) Elongation, desaturation, and esterification of essential fatty acids by fetal rat brain in vivo. J Lipid Res 34: 2099-2107.
- Makrides M, Gibson RA (2000) Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am J Clin Nutr 71: 307S-311S.
- Al MD, van Houwelingen AC, Kester AD, et al. (1995) Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br J Nutr 74: 55-68.
- Otto SJ, Houwelingen AC, Antal M, et al. (1997) Maternal and neonatal essential fatty acid status in phospholipids: an international comparative study. Eur J Clin Nutr 51: 232-242.
- Herrera E, Ortega H, Alvino G, et al. (2004) Relationship between plasma fatty acid profile and antioxidant vitamins during normal pregnancy. European journal of clinical nutrition 58: 1231-1238.
- Thorsdottir I, Birgisdottir BE, Halldorsdottir S, et al. (2004) Association of fish and fish liver oil intake in pregnancy with infant size at birth among women of normal weight before pregnancy in a fishing community. Am J Epidemiol 160: 460-465.
- Amusquivar E, Ruperez FJ, Barbas C, et al. (2000) Low arachidonic acid rather than alpha-tocopherol is responsible for the delayed postnatal development in offspring of rats fed fish oil instead of olive oil during pregnancy and lactation. J Nutr 130: 2855-2865.
- Saccone G, Saccone I, Berghella V (2016) Omega-3 long-chain polyunsaturated fatty acids and fish oil supplementation during pregnancy: which evidence? J Matern Fetal Neonatal Med 29: 2389-2397.
- Lucas A (1991) Programming by early nutrition in man. Ciba Found Symp 156: 38-50.
- Duque-Guimaraes DE, Ozanne SE (2013) Nutritional programming of insulin resistance: causes and consequences. Trends Endocrinol Metab 24: 525-535.
- Rytter D, Bech BH, Christensen JH, et al. (2011) Intake of fish oil during pregnancy and adiposity in 19-y-old offspring: follow-up on a randomized controlled trial. Am J Clin Nutr 94: 701-708.
- Hussain A, Nookaew I, Khoomrung S, et al. (2013) A maternal diet of fatty fish reduces body fat of offspring compared with a maternal diet of beef and a post-weaning diet of fish improves insulin sensitivity and lipid profile in adult C57BL/6 male mice. Acta Physiol (Oxf) 209: 220-234.
- Joshi S, Rao S, Golwilkar A, et al. (2003) Fish oil supplementation of rats during pregnancy reduces adult disease risks in their offspring. J Nutr 133: 3170-3174.
- Ibrahim A, Ghafoorunissa, Basak S, et al. (2009) Impact of maternal dietary fatty acid composition on glucose and lipid metabolism in male rat offspring aged 105 d. Br J Nutr 102: 233-241.
- Siemelink M, Verhoef A, Dormans JA, et al. (2002) Dietary fatty acid composition during pregnancy and lactation in the rat programs growth and glucose metabolism in the offspring. Diabetologia 45: 1397-1403.
- Sardinha FL, Fernandes FS, Tavares do Carmo MG, et al. (2013) Sex-dependent nutritional programming: fish oil intake during early pregnancy in rats reduces age-dependent insulin resistance in male, but not female, offspring. Am J Physiol Regul Integr Comp Physiol 304: R313-320.
- Casas-Agustench P, Fernandes FS, Tavares do Carmo MG, et al. (2015) Consumption of distinct dietary lipids during early pregnancy differentially modulates the expression of microRNAs in mothers and offspring. PLoS One 10: e0117858.
- Salvig JD, Olsen SF, Secher NJ (1996) Effects of fish oil supplementation in late pregnancy on blood pressure: a randomised controlled trial. Br J Obstet Gynaecol 103: 529-533.
- Fernandes FS, Sardinha FL, Badia-Villanueva M, et al. (2012) Dietary lipids during early pregnancy differently influence adipose tissue metabolism and fatty acid composition in pregnant rats with repercussions on pup's development. Prostaglandins Leukot Essent Fatty Acids 86: 167-174.
- Fernandes FS, Tavares do Carmo M, Herrera E (2012) Influence of maternal diet during early pregnancy on the fatty acid profile in the fetus at late pregnancy in rats. Lipids 47: 505-517.
- Jimenez MJ, Bocos C, Panadero M, et al. (2015) Fish oil diet in pregnancy and lactation reduces pup weight and modifies newborn hepatic metabolic adaptations in rats. Eur J Nutr.
- Sampson DA, Jansen GR (1984) Measurement of milk yield in the lactating rat from pup weight and weight gain. J Pediatr Gastroenterol Nutr 3: 613-617.
- Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497-509.
- Amusquivar E, Schiffner S, Herrera E (2011) Evaluation of two methods for plasma fatty acid analysis by GC. Eur J Lipid Sci Technol 113: 711-716.
- Quinn R (2005) Comparing rat's to human's age: how old is my rat in people years? Nutrition 21: 775-777.
- Sengupta P (2013) The Laboratory Rat: Relating Its Age With Human's. Int J Prev Med 4: 624-630.
- Poudyal H, Panchal SK, Diwan V, et al. (2011) Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog Lipid Res 50: 372-387.
- Yamazaki RK, Brito GA, Coelho I, et al. (2011) Low fish oil intake improves insulin sensitivity, lipid profile and muscle metabolism on insulin resistant MSG-obese rats. Lipids Health Dis 10: 66.
- Storlien LH, Kraegen EW, Chisholm DJ, et al. (1987) Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237: 885-888.
- Wu JH, Micha R, Imamura F, et al. (2012) Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 107: S214-227.
- Chen B, Ji X, Zhang L, et al. (2015) Fish Oil Supplementation does not Reduce Risks of Gestational Diabetes Mellitus, Pregnancy-Induced Hypertension, or Pre-Eclampsia: A Meta-Analysis of Randomized Controlled Trials. Med Sci Monit 21: 2322-2330.
- Zambrano E, Bautista CJ, Deas M, et al. (2006) A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol 571: 221-230.
- Mennitti LV, Oliveira JL, Morais CA, et al. (2015) Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J Nutr Biochem 26: 99-111.
- Lopez-Soldado I, Munilla MA, Herrera E (2006) Long-term consequences of under-nutrition during suckling on glucose tolerance and lipoprotein profile in female and male rats. Br J Nutr 96: 1030-1037.
Corresponding Author
Dr. Emilio Herrera, Department of Chemistry and Biochemistry, Facultad de Farmacia, Universidad San Pablo CEU, Ctra. Boadilla del Monte km 5.3, 28668 Boadilla del Monte (Madrid), Spain.
Copyright
© 2016 López-Soldado I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.