Amlodipine-Induced Hyperpigmentation
Abstract
Calcium channel blockers are frequently used for treatment of hypertension. Studies have reported hyperpigmentation secondary to calcium channel blockers, although cases are limited and mostly related to diltiazem and nifedipine. To our knowledge, there are only two cases describing hyperpigmentation secondary to amlodipine. We report a patient exhibiting drug-induced hyperpigmentation secondary to chronic amlodipine use and describe what we believe to be the first report of increased pigment on histology, which may be unique to amlodipine and this class of drug. We hope to add to the literature regarding amlodipine-induced hyperpigmentation and to increase awareness of this atypical reaction which can prevent needless biopsy and patient distress in the context of appropriate history and clinical features.
Keywords
Amlodipine, Calcium channel blocker, Hyperpigmentation, Drug-induced hyperpigmentation
Introduction
Calcium channel blockers (CCB) are a group of drugs commonly prescribed for the treatment of hypertension. Due to their potent vasodilatory action in cardiac and vascular smooth muscle, the most common adverse reactions associated with CCBs include edema, flushing, fatigue, palpitations and headache. Amlodipine is considered a first-line option for essential hypertension. Amlodipine rarely causes cutaneous or mucosal side effects, representing only 1-2% of adverse reactions [1,2]. While there have been several cases documenting hyperpigmentation secondary to CCBs such as nifedipine and diltiazem [3-7], only two cases of hyperpigmentation have been published related to amlodipine to our knowledge. We present this case due to the paucity of information linking amlodipine with hyperpigmentation in hopes that physicians recognize this association.
Case Presentation
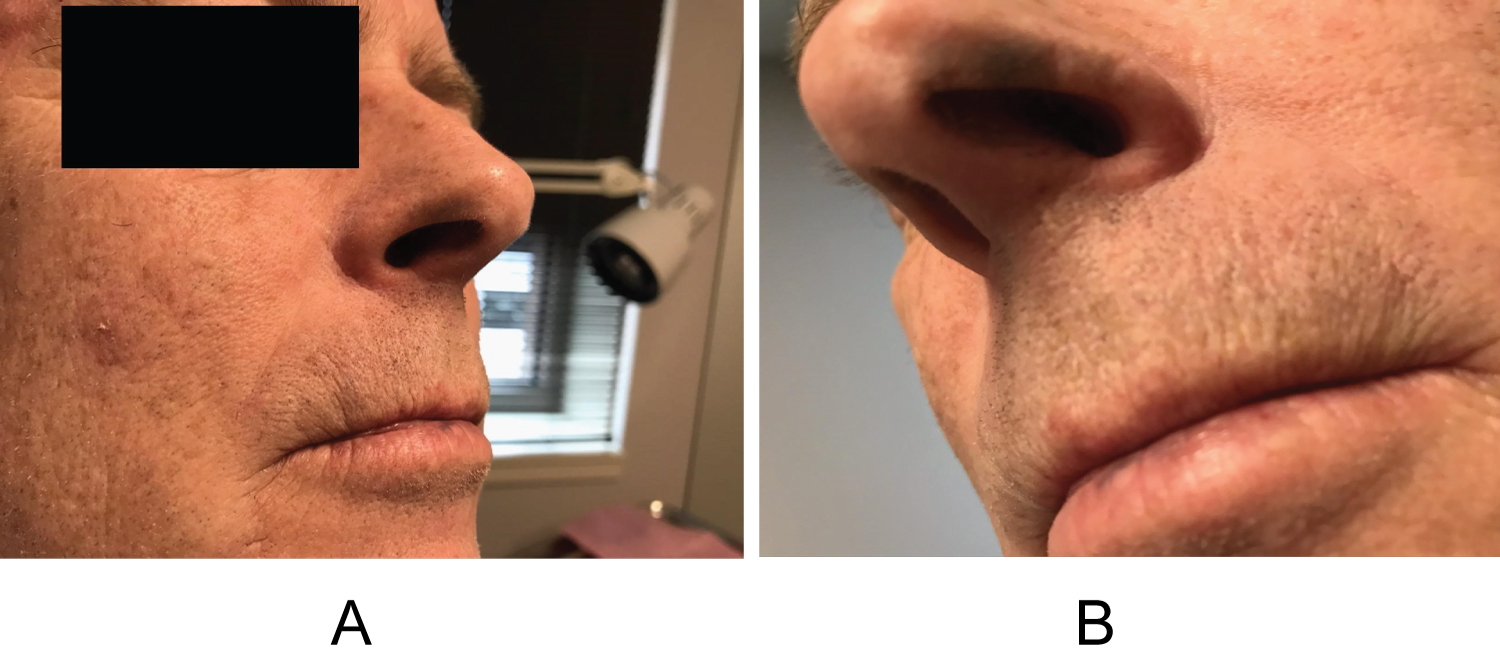
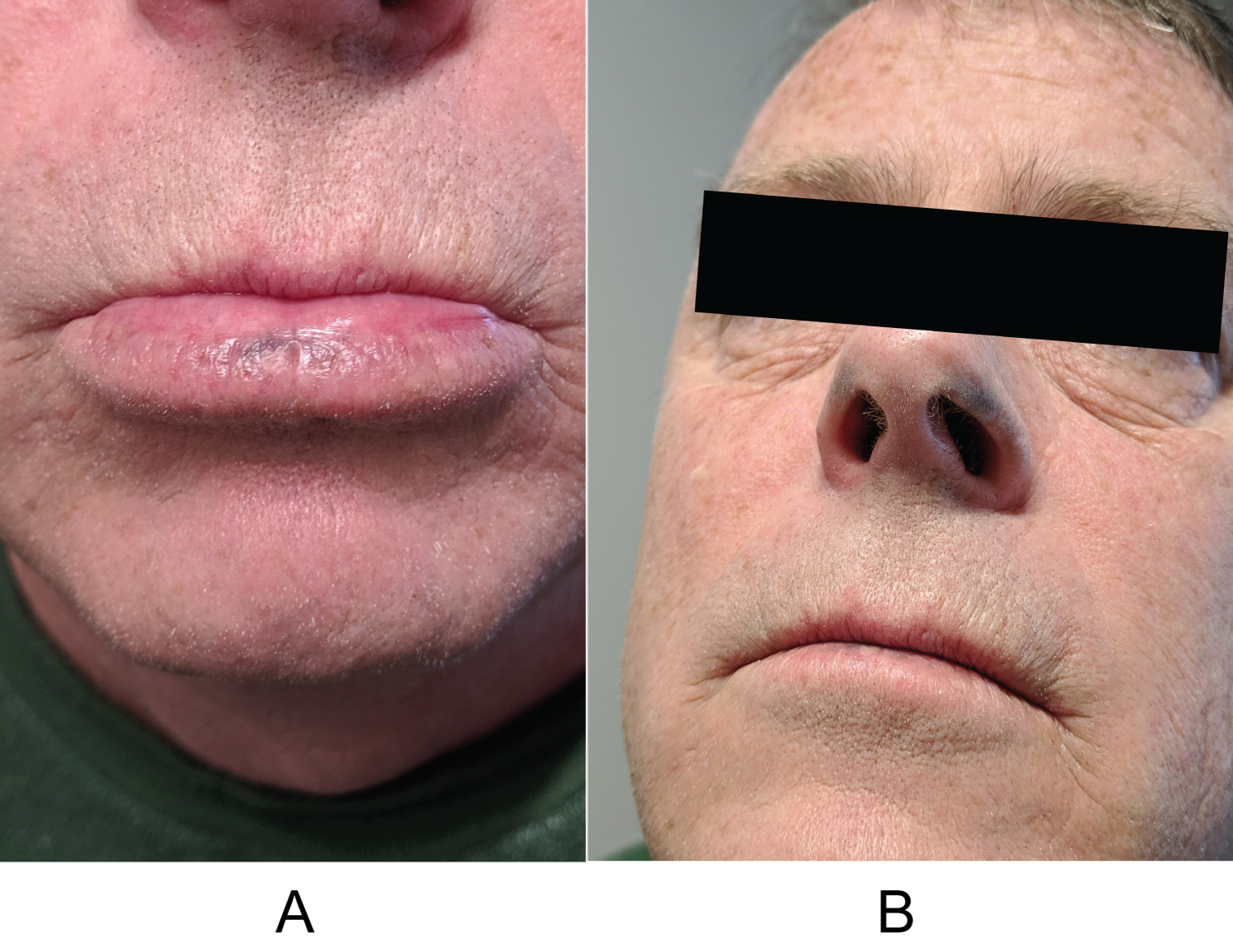
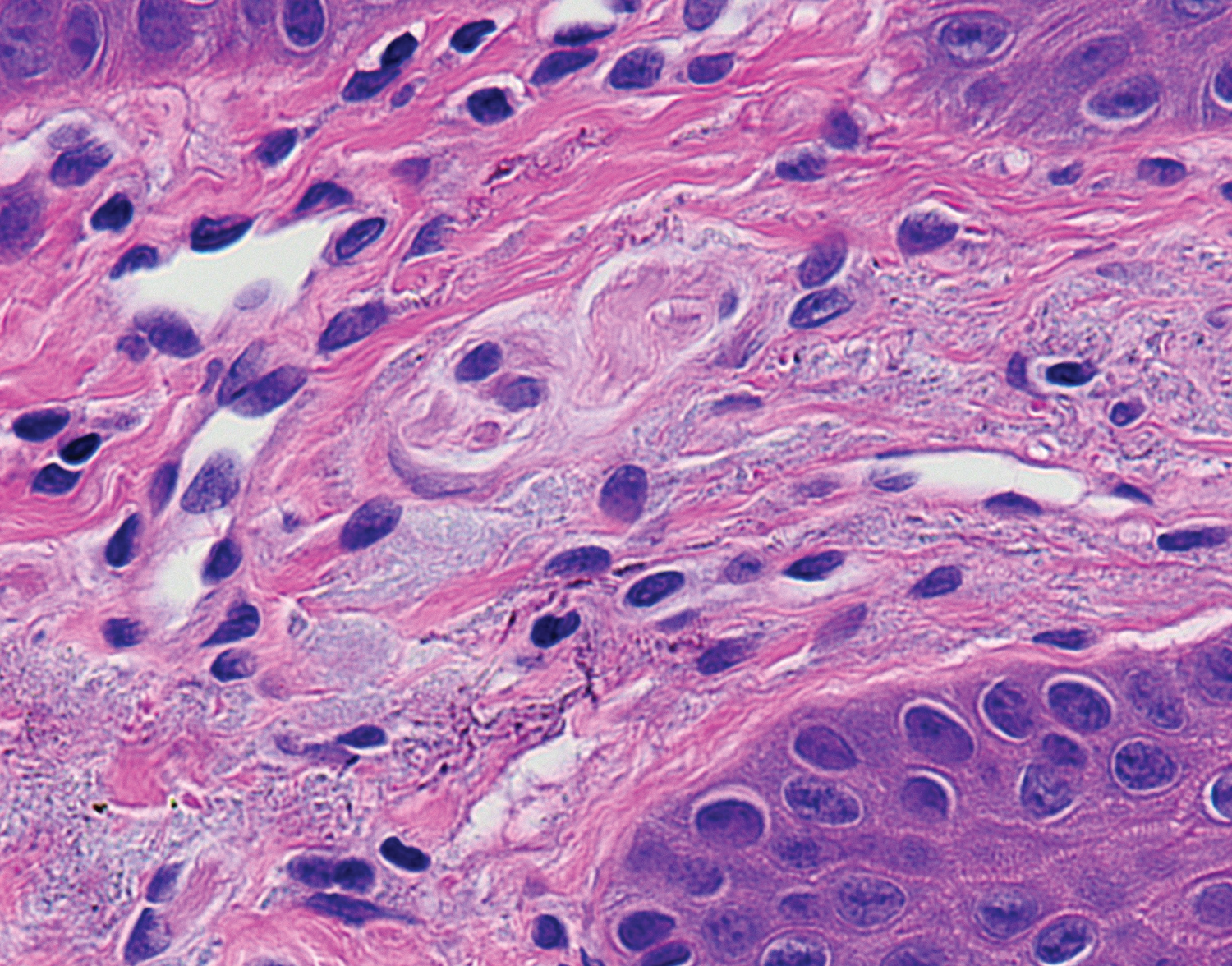
A 53-year-old male presented with concern of hyperpigmentation of his bilateral inferior nasal ala which began over the preceding two months. The patient's only medications were a benzodiazepine as needed for anxiety and amlodipine for hypertension. On exam, there were roughly 3 × 8 mm macular slate-gray hues bilaterally (Figure 1a and Figure 1b); the discoloration being typical of drug-induced hyperpigmentation. The differential diagnosis at that time was a potential drug eruption secondary to amlodipine, ochronosis, melanoma, and other pigmented tumors. Urine homogentisic acid was tested for ochronosis but was negative. A shave biopsy was conducted, but histology did not reveal any explanation for the clinically apparent hyperpigmentation. It was suggested that the biopsy was too shallow to ascertain whether there was increased pigmentation related to drug eruption. The patient returned one year later with progression of hyperpigmentation, now including his lower mucosal lip. The mucosal lip showed macular hyperpigmentation with blue-gray hue (Figure 2a). The inferior nasal ala showed increased hyperpigmentation compared to the year prior (Figure 2b). Shave biopsy of the mucosal lip revealed photodamaged mucosal lip with acanthosis, confluent solar elastosis, and subtle fine brown-grey granular pigmentation of microfibrils in the papillary dermis. The biopsy was devoid of substantial cellular inflammation (Figure 3). We instructed the patient to follow up with his primary care provider to discuss switching to an alternate anti-hypertensive medication. At 6-week follow up, the patient had discontinued amlodipine for one month and noticed subtle improvements in his discoloration. We educated the patient that the lesions may take up to a year to resolve and possibly not have complete resolution.
Discussion
Amlodipine is a dihydropyridine-derivative that is a first-line treatment of essential hypertension. Cutaneous reactions are rare, and only two cases have been found according to literature review related specifically to hyperpigmentation.
Erbagci describes a case of amlodipine-induced oral and cutaneous hyperpigmentation in a Turkish male patient. The morphological appearance was dark-brown pigmentation of the face, neck, forearms and posterior hands with slate-gray pigmentation of the lips, blue-gray patches on the lateral tongue and brown pigmented macules on the hard palate [2]. As with our case, there was pigmentation in areas unassociated with sun exposure.
Unlike in the case study presented by Erbagci in which the palms, soles and nails were spared, Sladden, et al. describe a case of amlodipine associated longitudinal melanonychia [8]. The patient presented with dark longitudinal streaks within the nail plate affecting several fingernails and toenails.
The pathogenesis of drug-induced pigmentation varies depending on the causative medication. Several mechanisms have been proposed related to the general pathogenesis of drug-induced pigmentation including increased melanin accumulation due to inflammation caused by the drug, increased melanin production induced directly by the drug, and accumulation of the drug or its metabolites with free-radical formation [2,5,9-11].
Scherschun, et al. performed an analysis of four patients with hyperpigmentation taking diltiazem and found that on histologic exam, there were no deposits of the drug or its metabolites in the patients' melanosomes, which is in contrast to findings of hyperpigmentation induced by other drugs or chemicals [4,5]. Additionally, while the UVA range spectrum has been reported as the major contributor in photosensitive reactions, they found that there was no absorption of diltiazem in the UVA, UVB, or visible light spectrum and it has been hypothesized that an unidentified metabolite rather than the drug itself could be a photosensitizing agent [4,11]. Saladi, et al. analyzed a group of four patients with diltiazem-induced hyperpigmentation and showed diltiazem's absorption range to be 220 to 300 nm within the UVB spectrum [3]. These results are supported by Desai, et al. who described a case of photodistributed hyperpigmentation due to diltiazem with a phototest reaction to UVB [6]. Because further clinical evidence is required to determine the relevance of these findings, broad-spectrum sun protection covering both UVA and UVB should be recommended to these patients.
Although structurally unrelated, cross-reactivity has been found between diltiazem, a nondihydropyridine CCB and nifedipine, a dihydropyridine CCB. Seggev describes a case in which a patient developed a photosensitive rash to nifedipine and so was switched to diltiazem, only to develop a similar reaction [10]. Another case of facial telangiectasia induced by nifedipine suggests cross-reactivity with the closely related drug amlodipine, as the telangiectasia recurred when the patient switched drugs 3 years later [7]. This cross-reactivity between diltiazem, nifedipine, and amlodipine is poorly understood and has not been well established [4,7,10,12,13], but we hypothesize that there may also be a cross-reactivity regarding hyperpigmentation, although further investigation is required.
For medical management, discontinuation of the offending drug is recommended. Although switching to a different CCB has been found to be safe [6,13], due to the potential cross-reactivity of CCBs, an antihypertensive agent from a different drug class is preferred [4]. Studies have reported that hyperpigmentation improves gradually with some cases reporting resolution at 1 year follow up [3,5] after discontinuation of the drug along with strict sun avoidance, although pigmentation may remain permanent in some patients and may be unrelated to photosensitivity, as in our case. Other treatments may include skin bleaching agents such as hydroquinone or laser therapy [2,9].
Conclusion
We present a case linking hyperpigmentation to the calcium channel blocker amlodipine. Treatment of hypertension with amlodipine is common and it should be considered as a potential source of hyperpigmentation within the appropriate clinical context. This side effect, while well known for other drug classes, is not normally seen in patients taking amlodipine, so we hope to increase knowledge about this potential reaction. We propose the addition of calcium channel blockers to the list of drugs with potential for causing hyperpigmentation, including tetracyclines, antimalarials, nonsteroidal anti-inflammatories, amiodarone, antipsychotics, cytotoxic drugs, phenytoin, and containing nose drops.
Disclosures
The authors declare no conflicts of interest.
References
- Lexicomp I (2020) Amlodipine: Drug information. In: Post TW, UpToDate, Waltham, MA.
- Erbagci Z (2004) Amlodipine associated hyperpigmentation. Saudi Med J 25: 103-105.
- Saladi RN, Cohen SR, Phelps RG, et al. (2006) Diltiazem induces severe photodistributed hyperpigmentation: Case series, histoimmunopathology, management, and review of the literature. Arch Dermatol 142: 206-210.
- Scherschun L, Lee MW, Lim HW (2001) Diltiazem-associated photodistributed hyperpigmentation: A review of 4 cases. Arch Dermatol 137: 179-182.
- Boyer M, Katta R, Markus R (2003) Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J 9: 10.
- Desai N, Alexis AF, DeLeo VA (2010) Facial hyperpigmentation caused by diltiazem hydrochloride. Cutis 86: 82-84.
- Collins P, Ferguson J (1993) Photodistributed nifedipine-induced facial telangiectasia. Br J Dermatol 129: 630-633.
- Sladden MJ, Mortimer NJ, Osborne JE (2005) Longitudinal melanonychia and pseudo-Hutchinson sign associated with amlodipine. Br J Dermatol 153: 219-220.
- Dereure O (2001) Drug-induced skin pigmentation. Epidemiology, diagnosis and treatment. Am J Clin Dermatol 2: 253-262.
- Seggev JS, Lagstein Z (1996) Photosensitivity skin reactions to calcium channel blockers. J Allergy Clin Immunol 97: 852-855.
- Hashimoto M (1979) Photosensitivity due to diltiazem hydrochloride. Acta Derm Kyoto 74: 181-186.
- Stern R, Khalsa JH (1989) Cutaneous adverse reactions associated with calcium channel blockers. Arch Intern Med 149: 829-832.
- Gonzalo Garijo MA, Perez Calderon R, de Argila Fernandez-Duran D, et al. (2005) Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr) 33: 238-240.
Corresponding Author
Matthew DaCunha, MD, University of Kansas City Missouri School of Medicine, 2411 Holmes St, Kansas City, MO 64108, USA, Tel: (816)-235-1808
Copyright
© 2021 Harper HE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.