Same Patient, Same Site: Using Different Topical Medicine for Psoriasis
Dear editor,
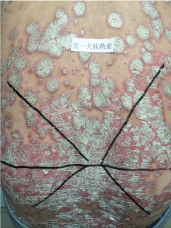
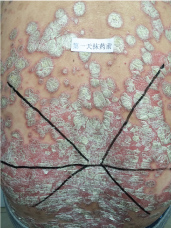
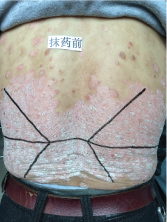
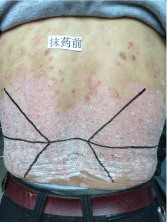
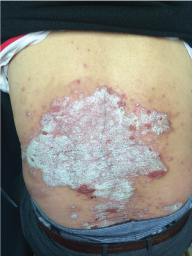
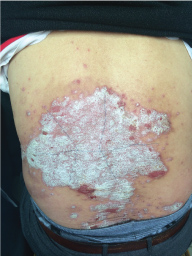
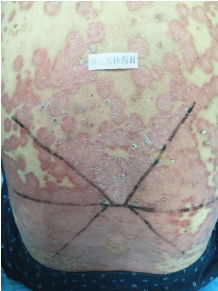
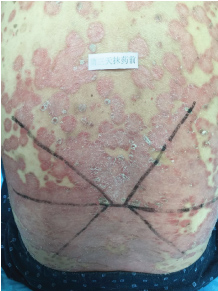
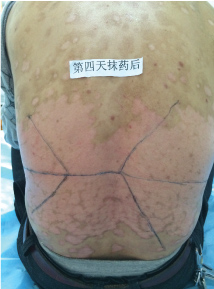
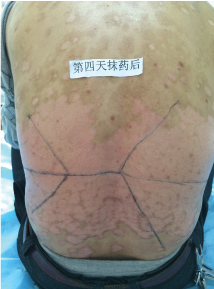
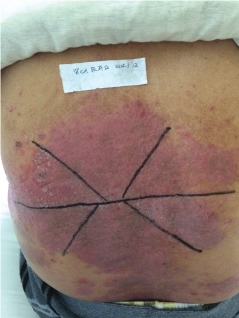
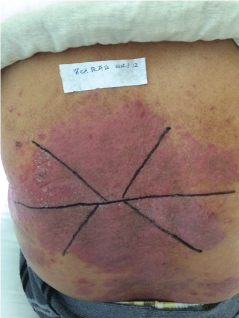
Topical use is the main advantage for dermatological therapy due to the outer-layer features of skin. Consequently, numerous preparations are available for the dermatosis. However, the efficacy evaluation of different preparations accurately is always very difficult. Psoriasis is a common skin condition characterized by persistent erythematous scaly plaque. It is estimated about 3% population suffered from this notorious calcitrant disease. The traditional and accepted method (until now it is also popular in practice). To observe treatment effect of cream is to apply one kind of drug to a group of patients with a specific disease who received different systemic approaches that might influence the effect of topical appliance [1]. Here we show 3 very severely affected psoriatic patients, with several years of history, whose lumber regions were marked into six different areas (Figure 1, Figure 2, and Figure 3) being applied with six different kinds of cream (Figure 4), respectively in the following seven days. The topical cream was spread, and clinical pictures were taken by a designated nurse each day. The patients did not know which cream be used to which area, so they are blinded. The creams include 0.1% tacrolimus ointment (Protopic, Astellas), 0.1% tazarotene cream (China Chongqing Huapont Pharm. Co. LTD), calcipotriol ointment (Bright Future), 0.1% mometasone furoate cream (Eloson, Bayer) and two hospital self-made creams (one is piyanjin cream containing clobetasol, the other vehicle cream named danchun cream without any medicine component). On the third day before topical appliance (Figure 5), the fourth day (Figure 6) and the seventh day (Figure 7), different efficacy could be seen clearly. To our great surprise, most of the creams have similar effects, even the vehicle cream without any medicine component. This method avoids the influences of different systemic medicine, patients' personal response, anatomical sites and disease duration on the efficacy evaluation of topical preparations. We coined the SPSS rule which stands for the acronym of same patient, same site or symmetrical site for different creams. We propose it be implemented in the future evaluation of topical preparations.
References
Corresponding Author
Yi-Guo Feng, Department of Dermatology, The Second Affiliated Hospital, Xi'an Jiaotong University, 157 Xiwu Road, Xi'an, China.
Copyright
© 2018 Yi-Guo F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.