Multimodal Therapy for Recalcitrant Cutaneous Fusarium Solani Infection in a Patient with Hyper-IgE Syndrome
Abstract
Hyper IgE Syndrome (HIES) is an inherited immunodeficiency syndrome with an increased susceptibility to cutaneous fungal infections. While systemic antifungals are often the therapy of choice, many non-pharmacologic treatment options have been shown to improve clearance of these infections. We report the treatment course of cutaneous Fusarium solani infection in an 8-year-old male with HIES with progression despite a series of systemic antifungals. The patient demonstrated marked improvement upon addition of thermotherapy, blue light aminolevulinic acid, Photodynamic Therapy (PDT), and a regimen of systemic and topical antifungals. A multimodal approach to treatment can be useful for antifungal-resistant infections, potentially decreasing the duration of systemic antifungal therapy and improving patient outcomes.
Keywords
Fusarium solani cutaneous infection, Multimodal therapy, Hyper IgE syndrome
Introduction
Hyper IgE Syndrome (HIES), or Job Syndrome, is a primary immunodeficiency characterized by severe eczema, recurrent skin and pulmonary infections, and elevated IgE levels [1]. HIES is most commonly caused by mutations in the STAT3 gene, which plays a role in Th17 CD4 cell differentiation. In Candida albicans infections in particular, the role of IL-17 is well described. Mannans present on fungal cell walls are recognized by Dectin-2, which induces the Syk-CARD9-NF-κB-dependent signaling pathway, ultimately resulting in IL-1β and IL-23 secretion [2]. IL-23 activates STAT3, which induces and maintains Th17 cell differentiation [3]. Th17 cells produce cytokine IL-17, which is largely responsible for antifungal and antibacterial responses [4]. Additionally, IL-17-A-producing T-cells may have a role in the surveillance and maintenance of the functionality and homeostasis of the immunological barrier by increasing the primary response in exogen pathogens [5]. Chronic mucocutaneous candidiasis is present in approximately 85% of patients with HIES and represents the most common fungal infection in this population [6]. Patients with HIES are susceptible to recurrent infections, specifically staphylococcal abscesses and airway infections [7] in addition to cutaneous fungal infections. In patients with STAT3 mutations such as the one presented in this case, clearance of fungal infections is particularly impaired [4,8]. The Fusarium genus includes to a diverse species of molds that are found mainly as saprophytic organisms in soil with a worldwide distribution [9,10]. The fungus most commonly affects immunocompromised hosts, and causes a wide variety of diseases including foreign-body-associated infections [10], single organ infections [2], and disseminated disease [9]. Fusarium solani is one such species of Fusarium known for causing primarily keratitis, and rarely cutaneous and subcutaneous infections [7,11].
We report a case of a pediatric patient with HIES with a rare cutaneous F. solani infection, whose infection worsened over the course of a several years before showing marked improvement with a multimodal approach to treatment.
Case Presentation
An 8-year-old Hispanic male with history of HIES (S668F positive STAT3 mutation previously confirmed by DNA mutation analysis) was admitted for chronic cutaneous F. solani infection. The infection initially appeared on the right forearm after a dog bite as a 3 × 3 cm erythematous indurated plaque. Biopsy and culture of the lesion revealed F. solani, and he was started on itraconazole 10 mg/mL oral solution twice daily. The patient was subsequently lost to follow-up. He presented two years later with worsening cutaneous infection and was treated over the course of one week with amphotericin B liposomal 3 mg/kg IV daily and isavuconazole 212 mg IV daily. He was discharged on oral isovuconazole 186 mg daily, and was followed up by an interdisciplinary medical team for eight months.
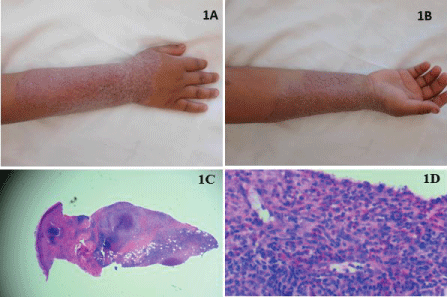
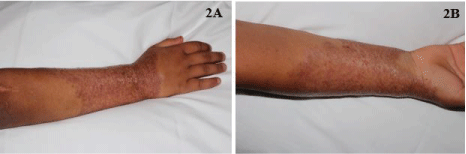
Despite months of aggressive systemic therapy, the patient's infection spread extensively to involve most of his forearm (Figure 1A and Figure 1B) and biopsy again confirmed cutaneous deep fungal infection (Figures 1C and Figure 1D) with cultures growing F. solani. Adjunct treatment with thermotherapy using a heating pad for 30 minutes three times daily was initiated along with aminolevulinic acid, Photodynamic Phototherapy (PDT) twice weekly, and a topical regimen of nystatin ointment once daily, terbinafine cream twice daily, and silver sulfadiazine cream once daily. After one month of adding these adjunct therapies, he showed marked improvement (Figure 2A and Figure 2B).
After initial improvement, the patient was discharged but returned with worsening due to noncompliance with outpatient isovuconazole, PDT, and thermotherapy. At this point, the patient was started on weekly amphotericin B liposomal 5 mg/kg IV infusions along with voriconazole 300 mg twice daily, thermotherapy for 30 minutes three times a day, and PDT twice weekly, resulting in good clinical response, but again noncompliance led to focal relapse after two months of treatment.
Due to worsening renal function with renal atrophy, an amphotericin-sparing regimen was initiated: oral voriconazole 100 mg twice daily, oral terbinafine 125 mg once daily, natamycin 5% ophthalmic solution topically once daily, silver sulfadiazine topical cream once daily, subcutaneous interferon-gamma three times weekly to stimulate an immune response, PDT every two weeks, and thermotherapy three times daily. The patient has been on the current regimen for four months, and he follows up with the dermatology clinic every two months for continued surveillance and treatment.
Discussion
This patient with a history of HIES presented with a four-year history of cutaneous F. solani infection resistant to systemic antifungals complicated by non-compliance. After years of multiple systemic antifungals, robust improvement was achieved with the addition of non-pharmacologic approaches. Localized heat therapy has been shown effective for superficial and deep skin infections, possibly due to a strengthening in local-host cellular immunity [12]. In a study conducted by De Menezes, et al. PDT was shown to be efficient in reducing the growth of Fusarium species, suggesting that standard antifungal treatment combined with PDT enhances antifungal susceptibility which may be due to increased membrane permeability of molds [6,13]. With minimal improvement of the patient's lesions despite prolonged antifungal treatment, PDT proves itself as an attractive adjunct treatment option.
Lastly, topical nystatin mixed with natamycin ophthalmic ointment was added to the patient's treatment regimen. In considering the Fusarium spp., F. solani most frequently is a cause of fungal keratitis. Indeed, 5% natamycin ophthalmic solution has been shown to effectively treat fungal corneal ulcers caused by Fusarium and Aspergillus [14,15] and was used as an adjunct cutaneous topical therapy due to the refractory nature of this case.
The multimodal approach to the treatment of this patient's F. solani infection was found to be more effective than systemic pharmacologic therapy alone in this case. We hope that this approach to treatment will eventually lead to resolution of this patient's disease and decrease the burden of systemic antifungal treatment, such as the adverse renal effects of prolonged amphotericin treatment.
Conclusion
This is a case of a patient with HIES with a high-risk fungal infection. Even at his young age, the patient has suffered four years of a resistant cutaneous fungal infection with F. solani associated with his immunodeficiency. The multimodal approach with thermotherapy, PDT, and topical antifungals in addition to systemic antifungals was found to be more effective than systemic pharmacologic therapy alone. We hope that this approach to treatment will eventually lead to resolution in this class of patients and decrease the burden of systemic antifungal treatment, such as the adverse renal effects of prolonged amphotericin treatment. Recognizing this risk and utilizing a more multimodal approach to therapy can help decrease side effects of systemic antifungals. Further studies are needed to better investigate the role of new and promising anti-fungal treatments in F. solani infection.
Conflicts of Interest
The authors state no conflict of interest.
References
- Borges WG, Hensley T, Carey JC, et al. (1998) The face of Job. J Pediatr 133: 303-305.
- Saijo S, Ikeda S, Yamabe K, et al. (2010) Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32: 681-691.
- Yang XO, Panopoulos AD, Nurieva R, et al. (2007) STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 282: 9358-9363.
- Minegishi Y, Saito M, Nagasawa M, et al. (2009) Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 206: 1291-1301.
- Grieco T, Faina V, Porzia A, et al. (2017) Atopic dermatitis IL17A- and IFN-γ-producing lymphocytes: investigation in blood, chronic lesions and APT. J Eur Acad Dermatol Venereol.
- De Menezes HD, Bachmann L, Wainwright M, et al. (2016) Photodynamic treatment phenothiazinium photosensitizers kills both ungerminated and germinated microconidia of the pathogenic fungi Fusarium oxysporum, Fusarium moniliforme and Fusarium solani. J Photochem Photobiol 164: 1-12.
- Uchiyama N, Shindo Y, Mikosiba H, et al. (1987) Cutaneous infection caused by Fusarium solani and Fusarium oxysporum. J Dermatol 14: 471-475.
- Vinh DC, Sugui JA, Hsu AP, et al. (2010) Invasive fungal disease in Autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol 125: 1389-1390.
- Nelson PE, Dignani MC, Anaissie EJ (1994) Taxonomy, Biology and Clinical Aspects of Fusarium Species. Clin Microbiol Rev 7: 479-504.
- Nir-Paz R, Strahileitz J, Shapiro M, et al. (2004) Clinical and Epidemiological Aspects of Infections Caused by Fusarium Species: a Collaborative Study from Israel. Journal of Clinical Microbiology 42: 3456-3461.
- Cheikhrouhou F, Makni F, Neji S, et al. (2014) Epidemiological profile of fungal keratitis in Sfax. J Mycol Med 24: 308-312.
- Nakamura Y, Xu X, Saito Y, et al. (2007) Deep cutaneous infection by Fusarium solani in a healthy child: successful treatment with local heat therapy. J Am Acad Dermatol 56: 873-877.
- Lujuan G, Jiang S, Yi S, et al. (2016) Evaluation of the Effects of Photodynamic Therapy Alone and Combined with Standard Antifungal Therapy on Planktonic Cells and Biofilms of Fusarium spp. and Exophiala spp. Front Microbiol 7: 617.
- Prajna NV, Mascarenhas J, Krishnan T, et al. (2010) Comparison of Natamycin and Voriconazole for the treatment of fungal keratitis. Arch Ophthalmol 128: 672-678.
- Sharma S, Das S, Virdi A, et al. (2015) Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomized trail. Br J Opthalmol 99: 1190-1195.
Corresponding Author
Jake E Turrentine, MD, Assistant Professor, Division of Dermatology, Medical College of Georgia, Augusta University, 1004 Chafee Avenue, FH 100, Augusta, GA 30904, USA, Tel: 706-721-6231, Fax: 706-721-6220.
Copyright
© 2017 Belcher MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.