Syndecan 1: A Useful Marker for Differential Diagnosis in Pseudocarcinomatous Hyperplasia and Squamous Cell Carcinoma
Introduction
Some proteins have been used as immunohistochemical markers for tumor diagnosis. Such is the case of syndecan-1, one of the four transmembrane heparin sulfate proteoglycans which are mainly present on the cellular surface [1-5].
Syndecan-1 is the principal syndecan on the epithelial cells of adult tissue, involved in numerous processes such as cellular growth, differentiation, adhesion, and migration among others [1].
Normal epithelial cells have a high expression of syndecan-1, and its loss of expression is associated to malignant transformation. Less expression is seen in advanced tumor stages, with more invasion, metastasis and the consequent reduction in patient survival [1,3,4,6,7].
On the other hand, Chromoblastomycosis (Ch) is a subcutaneous mycosis acquired by traumatic inoculation of the fungus which is most commonly Fonsecae pedrosoi, Phialophora verrucosa, Cladosporium carrioni and Rhinocladiella aquaspersa. It has a worldwide distribution with higher prevalence in tropical and subtropical climates isolated from soil or wood debris [8].
Initially, a chancre is formed in the inoculation site and the lesion continues to expand locally, forms verrucous plaques with scar tissue and satellite lesions while no systemic symptoms accompany the disease. There is a chronic inflammatory response with a granulomatous foreign body reaction and tissue remodeling. On long standing lesions, Squamous Cell Carcinoma (SCC) or melanoma may develop, probably due to the presence of chronic inflammation and fibrous scar tissue [9,10].
In the skin biopsy very characteristic Pseudoepitheliomatous Hyperplasia (PH) is observed, with chronic inflammatory infiltrate accompanied by a granulomatous and suppurative reaction [8].
Different treatment options are available, although none is gold standard. Antifungal drugs, heat therapy, cryotherapy, electrosurgery, surgery and radiation and frequently a combination is required [9,10].
Squamous Cell Carcinoma (SCC) is a non-melanoma skin cancer, the second most common form [11-14]. Its incidence increases with age, both in males and females, particularly on those with fair skin and history of prolonged sun exposure. It is more frequent in men older than 55 years of age, and only sometimes it can be seen in teenagers and children with genetic predisposition to this form of cancer [15-19].
Carcinogen exposure such as arsenic and ionizing radiation and Human Papilloma Virus (HPV) (serotypes 16, 18, 31 and 33) can be associated [12,16,19,20].
Another important risk factor for SCC is longstanding inflammation. This can be seen in chronic wounds or scars from different etiologies such as Ch, chronic ulcers of any etiology and burn scars, (Marjolin´s ulcer) [21] that have a high rate of metastasis formation (14-58%) and an aggressive behavior. The exact physiopathology for this is not known [10,22,23].
The gold standard for SCC diagnosis is the histopathological study. With it, one can know the grade of invasion and differentiation degree, enabling the determination of prognostic factors [20,21,24]. SCC histology includes hyperkeratosis and parakeratosis and epidermal hyperplasia with irregular proliferation and atypical squamous cells irregularly placed and may invade the dermis. The most important differential diagnosis is with PH, a reactive process of the epidermis characterized by hyperplasia. This can be secondary to mycotic infections such as chromoblastomycosis, or bacterial infections such as tuberculosis, spirochetes as syphilis, bromoderma or even melanocytic lesions, particularly Spitz nevi and malignant melanoma, as well as trauma sites, chronic inflammation and ulcers [20,24,25].
As both PH and SCC lesions can coexist, it is extremely important to correctly diagnose the patients so the proper treatment can be determined.
Based on this information, we designed a study Syndecan-1 expression in SCC and PH having normal skin samples as controls, with the intention to be able to differentiate accurately via immunohistochemistry benign (PH) from malignant (SCC) lesions.
Material and Methods
We included all registered cases of Ch and PH from the Dermatopathology area of the Dermatology Service of the General Hospital Dr. Manuel Gea González from 1997 to 2011. Specimens of invasive SCC and normal skin served as controls for immunohistochemistry (Table 1). The diagnosis of Chromoblastomycosis was established by two certified dermatopathologist, as well as a positive KOH (potassium hydroxide) mount and/or a positive fungal culture. The diagnosis of SCC and of normal skin was equally performed by the same two certified dermatopathologists (Table 1).
The expression of Syndecan 1 was evaluated in every biopsy included, and was considered positive when the immunohistochemistry was positive outside the cellular membrane (Table 2). Syndecan-1 was measured by immunohistochemistry with a visual scale 0-3+.
The paraffin embedded specimens were sectioned in 2 µm thick samples, mounted in a slide covered with poly-lysine and left during the night to dry at room temperature. After removing the paraffin and rehydrating the sections, these were treated with 0.1 M sodium citrate (pH 6.2) and Tween 20 to unravel the epitopes. The endogenous peroxidases were blocked with 0.9% hydrogen peroxide after being incubated in 1% bovine albumin serum in saline phosphate tampon during 5 minutes to eliminate the non-specific unions. Monoclonal antibodies against syndecan-1 (CD 138) (clone M115, dilution 1:100, Dako, Carpinteria, CA) were used. The tissue samples were incubated with primary antibodies during 45 minutes. After this, the samples were incubated with an anti mice/rabbit biotinylated antibody and with a peroxidase/streptavidin complex during 30 minutes each one (SLAB+ labeled streptavidin-biotin, Dako). To visualize the reaction a substrate 3,3'-deaminobencidine-H20 (Dako) was used (2,5).
Afterwards, the tissue sections were counter stained with Mayer hematoxylin solution. For negative controls the primary antibody was substituted for a saline phosphate tampon.
All immunohistochemistry samples were blindly evaluated by two experts. Syndecan-1 expression was evaluated using a light microscope with a 10X objective, which corresponds to a 5.3 mm2 area. With a digital camera (Olympus C-7070) microphotographs were taken of each of the stained tissue sections. The index of agreement kappa inter an intra observer was determined with a concordance equal or greater than 80% before initiating the reading of the immunohistochemistry in the samples and for the statistical analysis Kruskal-Wallis test, percentages and proportions were applied.
Results
Between the years 1997 to 2011, we found 11 patients with confirmed diagnosis of Chromoblastomycosis in 21 biopsies stained with hematoxylin and eosin and sclerotic bodies, fungal culture and/or positive KOH mount.
The history of the fungal infection ranged from 4 to 50 years (average 19.8 yr). Ten of the cases were male and only one female, with an age range between 42-72 years (average 63.6).
We also included 22 biopsies of invasive SCC, form 21 patients. The age range was 50-84 years (average 74.59). The history of the disease ranged from one month to 70 years (average 5.45 years). Twelve of these cases were classified as well differentiated invasive SCC, 7 as moderately differentiated and 2 cases of ill differentiated SCC.
For the normal skin samples, we included 20 biopsies from 13 patients, 7 female and 6 male.
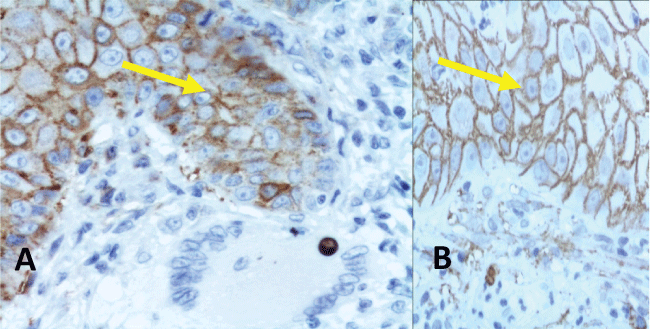
Of the 21 biopsies with Chromoblastomycosis and Pseudoepitheliomatous Hyperplasia (Ch/PH) 6 were 1+, 10 were 2+ and 5 were 3+ for the immunohistochemistry of the membrane (Syndecan-1). None of the studied biopsies was negative (Figure 1).
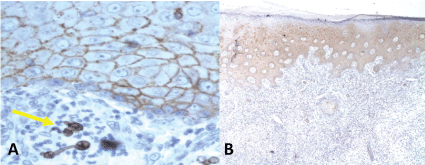
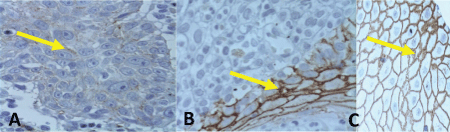
Of the 22 samples of SCC, 12 were negative to Syndecan-1, 7 expressed 1+, 3 expressed 2+ and none of them had 3+. The loss of Syndecan-1 expression in the cellular membrane correlated to the loss of differentiation of the SCC analyzed. So, overall, 3 invasive SCC were positive while 19 were negative (Table 3) (Figure 2 and Figure 3).
The normal skin biopsies, used as control for the expression of Syndecan-1 in the cellular membrane, all reported 3+ in the immunohistochemistry (Table 3).
So, Syndecan-1 expressed more in Ch/PH cases when compared with SCC, with a statistically significant difference (p = 0.001).
The median expression of Syndecan-1 in normal skin was 3, and 1.95 in Ch/PH with a confidence interval of 95% (1.63-2.27) and 0.52 in SCC CI 95% (0.21-0.83).
The positivity percentage (2+ and 3+) of Syndecan-1 by immunohistochemistry in Ch/PH was 71.4% compared to 13.6% in SCC (Table 3).
Discussion
Chromoblastomycosis is an endemic subcutaneous mycosis in Mexico, which affects mainly men that live in rural areas [8].
This chronic mycosis develops into verrucous plaques and scar areas and like many chronic inflammatory processes, it is related to an important Pseudoepitheliomatous Hyperplasia (PH) of which SCC is the main differential diagnosis [25] because one of the most severe complications of chronic inflammatory processes is the development of SCC. Although the mechanism for malignant transformation is not completely understood, it is probable that the scarring process acts as tumor promotor and conditions mutations in the Fas gene, avoiding apoptosis in the mutated cells [10,22,23].
The differential diagnosis between PH and SCC is fundamental for the treatment and prognosis of the patients. Making this differentiation can sometimes be complicated based only on morphological characteristics of routine histopathology and some markers of cellular proliferation [25]. Syndecan-1 is typically located in well differentiated epithelia [1].
The loss of its expression is associated to malignant transformation and directly related to advanced stages of the disease, more invasion and metastasis presence, with the consequent reduction in survival rates [2-4] and can be used as a useful marker to distinguish between these two dermatosis, as the malignant transformation to SCC is one of the complications of longstanding Ch with PH.
Conclusion
Loss of syndecan-1 can be used as marker to distinguish PH from PH with malignant transformation. In further studies we must study PH associated to other inflammatory processes different to Chromoblastomycosis, and compare those results with this work.
References
- Kopper L, Sebestyén A, Galla M, et al. (1997) Syndecan1-A New piece in B-cell Puzzle. Pathol Oncol Res 3: 183-191.
- Hashimoto Y, Skacel M, Adams JC (2008) Association of loss of epithelial syndecan-1 with stage and local metastasis of colorectal adenocarcinomas: An immunohistochemical study of clinically annotated tumors. BMC Cancer 8: 185.
- Chu YQ, Ye ZY, Tao HQ, et al. (2008) Relationship between cell adhesion molecules expression and the biological behavior of gastric carcinoma. World J Gastroenterol 14: 1990-1996.
- Bologna R, Mosqueda A, Lopez E, et al. (2007) Syndecan-1 (CD138) and Ki-67 expression in different subtypes of ameloblastomas. Oral Oncol 44: 805-811.
- Mukunyadzi P, Liu K, Hanna EY, et al. (2003) Induced Expresion of Syndecan-1 in the Stroma of Head and Neck Squamous Cell Carcinoma. Mod Pathol 16: 796-801.
- Szatmári T, Ötvös R, Hjerpe A, et al. (2015) Syndecan-1 in cáncer: implications for cell signaling, differentiation and prognostication. Dis Markers 2015: 796052.
- Bosch R, Philips N, Suárez-Pérez JA, et al. (2015) Mechanisms of photoaging and cutaneous photocarcinogenesis and photoprotective strategies with phytochemicals. Antioxidants (Basel) 4: 248-268.
- Correia RT, Valente NY, Criado PR, et al. (2010) Chromoblastomycosis: study of 27 cases and review of medical literature. An Bras Dermatol 85: 448-454.
- Elgart GW (1996) Chromoblastomycosis. Dermatologic Clinics 14.
- Torres E, Beristain J, Lievanos Z, et al. (2010) Chromoblastomycosis associated with letal squamous cell carcinoma. An Bras Dermatol 85: 267-270.
- Sachs DL, Marghoob AA, Halpern A (2001) Skin cancer in the elderly. Clin Geriatr Med 17: 715-738.
- Arenas R (2007) Carcinoma espinocelular. Dermatología ATLAS, diagnóstico y tratamiento. (2nd edn), México: McGraw-Hill Interamericana, 141-143.
- Thomas B (2007) Management of basal and squamous cell carcinoma. Fitzpatrick's Dermatology in General Medicine. (6th edn), Fitzpatrick RAJKW, McGraw-Hill.
- Thomas P Habif (2004) Premalignant and Malignant Nonmelanoma Skin Tumors. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. (4th edn), Philadelphia, Pennsylvania: Mosby.
- Armstrong BK, Kricker A (1996) Epidemiology of sun exposure and skin cancer. Cancer Surv 26: 133-153.
- Aubry F, MacGibbon B (1985) Risk factors of squamous cell carcinoma of the skin. A case-control study in the Montreal region. Cancer 55: 907-911.
- Hartley BE, Searle AE, Breach NM, et al. (1996) Aggressive cutaneous squamous cell carcinoma of the head and neck in patients with chronic lymphocytic leukaemia. J Laryngol Otol 110: 694-695.
- Johnson TM, Rowe DE, Nelson BR, et al. (1992) Squamous cell carcinoma of the skin (excluding lip and oral mucosa). J Am Acad Dermatol 26: 467-484.
- Rowe DE, Carroll RJ, Day CL (1992) Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 26: 976-990.
- Rudolph R, Zelac DE (2004) Squamous cell carcinoma of the skin. Plast Reconstr Surg 114: 82e-94e.
- Bernstein SC, Lim KK, Brodland DG, et al. (1996) The many faces of squamous cell carcinoma. Dermatol Surg 22: 243-254.
- García I, Pérez-Gil A, Cmacho FM (2006) Marjolin's ulcer: burn scar carcinoma. Actas Dermosifilogr 97: 529-532.
- Esterre P, Pecarrère JL, Raharisolo C, et al. (1999) Squamous cell carcinoma arising from chromoblastomycosis. Report of two cases. Ann Pathol 19: 516-520.
- Madan V, Lear JT, Szeimies RM (2010) Non-melanoma skin cancer. Lancet 375: 673-685.
- Civatte J (1985) Pseudo-carcinomatous hyperplasia. J Cutan Pathol 12: 214-223.
Corresponding Author
Gabriela Moreno Coutiño, MD, Mycology Section, Hospital General "Dr. Manuel Gea González", Tlalpan, México City, México, Tel: 52-55-40003058.
Copyright
© 2017 Juárez MCDA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.