Aging Kinetics of Human Hearts and Skin: New Aging Theories and Implications in the Use of Sunscreens
Abstract
Published data on cardiac indexes and superficial nutritive capillary densities from photo-protected and photo-exposed areas as well as collagen contents of skin of forearms from a large number of normal subjects over several decades of lifespan were reanalyzed using a first-order kinetic model. Excellent logarithmic linear fittings were all obtained. Slopes from the photo-protected and photo-exposed studies were virtually parallel. These results appear to directly contradict the widely accepted theory of accelerated aging. It also indicates an apparent lack of effects of mild or moderate sunray exposure on skin aging and appears to contradict the conventional skin aging theory that photoaging or sunray exposure is responsible for about 80 to 90% of total skin aging. In view of many reported health benefits of UV radiation the common practice of using sunscreens can now be seriously questioned, and in fact it may be harmful to one's health. New theories for heart aging and skin aging are postulated. Cardiac index is proposed as an aging marker for studying body aging and enhancement of cardiac index may be explored to enhance health and increase life expectancy. Reversibility of skin aging is re-considered.
Keywords
Aging, Heart, Skin, Anti-aging, Sunlight, Sunscreens
Introduction
As we all wish to live longer, healthier and happier, intense research worldwide has been carried out in the last several decades to explore aging mechanisms and anti-aging methods. Kinetic analysis is a potentially powerful tool in pharmacokinetic studies for understanding the mechanisms or processes involved in the absorption, distribution, metabolism and elimination of a chemical in the body. Past studies on the kinetics of aging of organs and tissues and their significance or implications seem quite limited [1-7] and references therein, although several hundreds of aging theories have been published to date [8-16].
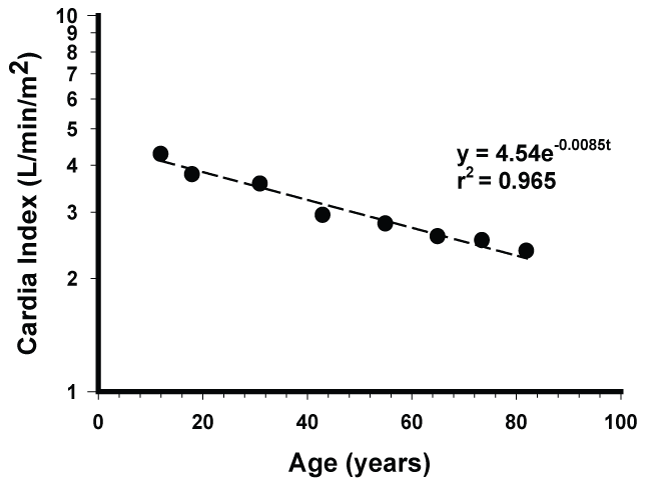
Heart is probably the most important organ of the body. It may be considered as the body motor or body engine that can constantly pump or deliver blood nutrients to all the tissues and organs, including the heart tissue, in order to nourish them and to sustain their functions and vitality. More recently, a potential new role of the heart in body weight control and metabolism has been reported [17]. Based on the cardiac index, i.e., cardiac output per unit of body surface area, the aging kinetics of human hearts were reported to follow a zero order kinetic in subjects ranging from 35 to 85-years-old [2]. The significance of such an observation has not been discussed. Since the cardiac function may peak at age about 8 to 10 [5], inclusion of data from younger ages seems important for more accurate kinetic characterization.
Skin, on the other hand, is the largest organ in the body that protects us from environmental assaults. Furthermore, we all wish to have a younger-looking, healthy facial skin. To date, hundreds or thousands of articles have been published to explore skin aging-related subjects and hundreds of thousands of beauty and anti-aging-related products have been marketed worldwide.
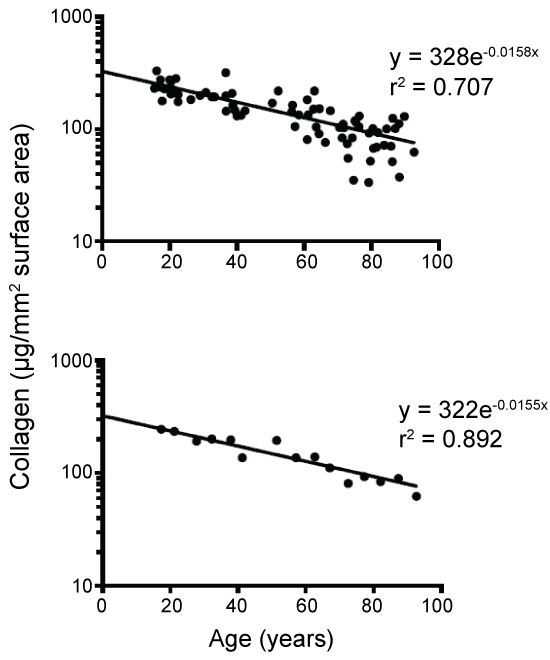
As to skin aging, it has been reported in 1975 by Shuster, et al. [3] that based on data from 154 normal male and female subjects aged 15 to 93, the contents of skin collagen, the main (70 to 80%) composition of the skin [18], from the forearm decreased linearly with age on a Cartesian plot (i.e., zero order process) without elaborating on its potential physiological significance. They also reported [3] that the collagen contents were the same for skin specimens taken from all parts of the body. In other words, there were no differences in collagen contents of samples taken from sun-protected or sun-exposed areas. This interesting finding apparently contradicts the main stream view in the last century that skin aging in adults is overwhelmingly controlled by photoaging, i.e., aging due to exposure to sunlight. It has been commonly cited that the photoaging alone can account for about 80% to 90% of total skin aging [10,19,20].
The main purposes and scopes of this article are three-fold. First, I want to describe results of a re-analysis from reported cardiac index data obtained from 77 subjects aged 12 to 82 [5] using a first order kinetic model and to discuss its implications and to propose an alternative aging theory for the body. Second, first-order aging kinetics will be used to re-analyze nutritive capillary density data reported earlier [4] for photo-protected and photo-exposed skin in 50 female subjects aged 20 to 74. In addition, skin collagen contents from the forearm of 74 Caucasian male subjects aged about 15 to 93 [3] will be similarly re-analyzed. The significance and implications of results from the above studies in skin aging theory and in the use of sunscreens will be discussed. Thirdly, based on the above findings, alternative theories for skin aging symptoms such as age spots, fine lines and wrinkles will be postulated. The current view on the reversibility of skin aging will be re-visited.
Methods
Hearts: Aging kinetics of hearts was analyzed based on the following first-order equation [6]:
Where CI is the cardiac index after age 12, CI0 is the cardiac index at age 12, t is equal to the age in years minus 12 and k is the first-order rate constant. In the present study the cardiac index was assumed to peak at age 12 [5]. The aging half-life t0.5 is calculated by
The cardiac index data were obtained based on reported mean data (Data Supplement) in a standard physiology textbook [5]. Each data point was a mean from about 10 subjects. A standard regression analysis was carried out.
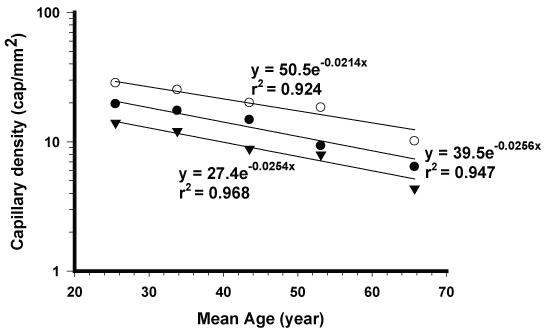
Skin: Mean density data (N = 10 in each 5-year-span age group for a total of 5 age groups (Data Supplement) of the superficial nutritive capillaries from 3 sites were taken from an excellent study by Li, et al. [4]. These sites were:
1. The volar forearm representing a photo-protected area not much affected by external environmental factors,
2. The back of the hand,
3. The forehead
The last 2 sites were regarded as photo-exposed. The obtained data were then analyzed exponentially based on equation 1. As to the collagen contents of the forearm, their individual data were taken from figure 1 in reference [3] and similarly analyzed. Furthermore, their mean values from each of the 5 year periods were also similarly analyzed (Data Supplement).
Results
Results of the present analyses are shown in figure 1, figure 2 and figure 3. In the present study, the cardiac indexes were found to decrease by an apparent first order process with a k of 0.0085/year and a half-life of 82 years over a period of 7 decades. Most interestingly, the correlation coefficient, r, was remarkably high, 0.982, thus providing a strong support that the aging kinetics of normal human hearts in the present study can be adequately described by an apparent first order model. The early report of zero order kinetics [2] can be attributed to their not considering the data below 35 years of age. It is visibly clear from the originally reported graph [5] that the decay of cardiac index with age cannot be described by a zero order process.
The skin aging kinetics of superficial capillary densities all followed apparent first order process with high correlations (Figure 2). Most importantly, their slopes are virtually parallel, being 0.0256/year for the sun-protected site, and 0.0254/year and 0.0214/year for the other 2 sun-exposed sites, respectively (Figure 2). Their mean half-life is 29 years. Since the studied ages ranged from 20 to 74 years, about 2 times of the half life, the conclusion of apparent exponential decline for aging seems highly valid. It is to be noted that the superficial capillaries are responsible for delivering nutrients to the top layers of the skin that is critical to the healthy skin appearance.
The changes in forearm collagen in all 74 males also exhibited an exponential decay with a half life of 44 years and an r value of 0.840. As expected, when plotted based on mean 5-year data (Data Supplement), the r value increased to 0.94 with essentially the same half-life.
Discussion
Cardiac output or cardiac index can be practically regarded as the sole measure of capacity of the physiological function of the entire heart. Since it can be quantified, therefore it may serve as a valuable tool to study the aging of hearts. The present data show that like the isotope decay, the decay kinetics of hearts in the studied subjects probably after ages 7 to 8 based on refer [5] can be adequately described by an apparent first order process. This finding seems significant as it appears to contradict the commonly accepted accelerated aging theory [11-16]. If the aging process were to accelerate with age, then its aging rate constant should increase with time and the aging decay will not follow the first order with the same rate constant over a period of 7 decades as found in this study (Figure 1). The reason for the apparent discrepancy between this finding and the acceleration theory is unknown and remains to be explored. It is possible that results from in vitro studies or even from short-term in-vivo studies may not accurately reflect what will happen in vivo over a long period of time (more discussion later). The exponential decline (Figure 1) represents the overall outcome of the extremely complex aging processes occurring at the molecular, tissue and organ levels. Based on equation 1, the cardiac indexes can be calculated to decrease from age 12 by 23, 29, 35, 40 and 49% at ages 30, 40, 50, 60 and 80, respectively. These decreases should be regarded as clinically significant and have implications in aging. For obvious reasons the composition of blood may also vary significantly with age. It is well known that oral absorption and metabolism of foods in the body may change significantly with age. Thus, from this aspect alone the composition of nutrients in blood may also vary significantly with age even if the same food is consumed.
A question may be asked [15]: What is the aging marker? Aging takes place constantly from every part of our body. Since heart can be considered as an engine or motor of our body and its function is measurable, it is proposed that the cardiac output or more preferably cardiac index be used as a marker for studying the aging of whole body. As we age, the amount of nutrients delivered through diffusion across capillary membranes to the heart tissue, brain, lungs, livers, kidneys and others organs or tissues also decreases [5,21]. A person may die when the nutrients delivered are not enough to sustain their vitality especially for heart and lungs.
Our life expectancy may be prolonged by increasing the cardiac index without producing adverse effects. This may be possible if a new heart could be regenerated or the existing heart could be rejuvenated through stem cell engineering (18, 22-24 and references therein). It is envisioned that non-invasive, simple methods for measuring cardiac output could be developed and data base for a large population with diverse backgrounds could be developed. The aging model discussed here may be termed the cardiac output model or nutrition model.
In the present study the first order rate constant for the human heart aging is 0.0085 per year (Figure 1). This is close to the reported [25] turnover rate of about 1% per year for young human cardiomyocytes. The significance of similarity between these two values remains to be explored.
For obvious reasons, skin is probably the most often studied organ in the body in research related to aging. It has been widely accepted in the last century that photoaging is the main cause for skin aging [11-16]. It has been reported to contribute to 80 to 90% of total aging while the intrinsic aging only contributes to 10 to 20% of the total aging [10,12,19,20]. The results of parallel slope between photo-protected and photo-exposed capillary densities (Figure 2) clearly suggest that exposure to sunrays in these studied subjects practically had no effect on the aging of superficial capillaries. The nature of their exponential decay kinetics (Figure 2) also indicates that the aging is not accelerating with age as commonly assumed for skin aging. Interestingly, the collagen contents from skin samples taken from sun-protected or sun-exposed areas from the same individual have been found to be the same in a very large number (N = 154) of male and female adults [3]. Their aging rate was found to decrease exponentially (Figure 3). Based on the above data one may conclude that exposure to mild or moderate sunrays in ordinary people most likely would not have any significant adverse effects on skin aging over a life time. This seems to be inconsistent with the demonstrated in-vitro or in-vivo adverse effects from UV radiation [11-16]. This apparent discrepancy may be largely attributed to the fact that our skin has an enormous ability to self-repair the skin damage. It is of interest to note in this regard that mildly burned skin from exposure to strong sunlight can probably recover or heal by itself within a few weeks after only minor intervention.
Use of sunscreens to protect the skin from sun damage has been generally recommended in the last many decades [13]. In recent years numerous studies have reported the health benefits of UV radiation [26,27] and references therein. For example, sunlight can reduce blood pressure, thereby decreasing heart attack and strokes. It can increase the immune function, the bone density, the quality of sleep and healing of certain skin diseases. The above benefits are apparently due to the increase of body production of nitric oxide and vitamin D as well as the increased secretion of serotonin from exposure of skin to sunlight [26,27]. Since the use of sunscreens can block the sunlight from reaching the skin, therefore, its use may actually be harmful to our health by depriving us from the benefits of sunlight. Furthermore, the long-term adverse effects of many sunscreens including their metabolites are really unknown. Based on the above considerations it is recommended that for general public, sunscreens should not be used unless they are going to be excessively exposed to strong sunlight. This is important in view of the fact that the chance of having a skin cancer is very low and the benefits of sunlight far outweigh its risks [26,27]. In this regard, a sunscreen product with a sun protection factor (SPF) of more than 33 may be regarded as unnecessary. This is because in addition to higher cost for the product, there may be harm due to virtually complete blockade of sunlight and potential adverse effects of the sunscreen and its metabolite. It is suggested that a broad- spectrum sunscreen with a SPF of 10 to 20 should be generally adequate when exposed excessively to strong sunrays. Obviously, extensive clinical studies are needed to confirm or refute the above suggestion.
It is reasoned that skin aging is predominantly controlled by the amount of blood or nutrients delivered to the skin. It is of interest to observe that when normal young kids or even young adults are exposed to strong sunrays for many hours daily for weeks or months, they may not develop any sagging skin, rough skin, wrinkles or age spots on their faces. This may be rationalized by their having had very healthy, tight, firm skin cells due to the nourishment from high blood flow. Their skin can more effectively resist assaults by UV rays and have a more robust self-repair mechanism. This seems consistent with the report that the body immune power peaks at puberty [28]. Based on skin collagen contents, age for age, women are about 15 years older than men throughout their adult life [5]. Since mean cardiac index in women is about 10% lower than that in man for the same age [5], therefore, the faster skin aging in women compared to men may be possibly related to their lower cardiac index. It is well known that in adults it usually takes about one year to grow a new toe nail while it only takes about 6 months to grow a finger nail. This difference may be mainly attributed to much lower blood flow to the toe than to the finger due to their location difference from the heart.
The formation of age spots and wrinkles has been commonly attributed to photoaging. A very preliminary study was conducted to determine the percent of normal adults in various age groups that had developed two or more age spots in their upper thighs and the back of the body that were protected from sunrays. The findings are as follows: 40% in ages 50 to 60 (N = 10); about 60% in ages 60 to 70 (N = 12); 80% in ages 70 and above (N = 10). At age 68 in 2006, I, the author, had 42 age spots on the upper arms, 36 age spots on the thighs and 18 age spots on the back even although I have been a non-smoker, and have minimally stayed outdoors. The above observations indicate that intrinsic aging alone can cause age spots. Another view not in favor of the photoaging theory is that the whole face is almost uniformly exposed to sunrays and it appears impossible for the sunrays to constantly attack certain specific areas to form age spots or wrinkles. It is postulated that an age spot is formed primarily due to a natural or injured defect in skin microcirculation that will result in an insufficient supply of nutrients to that area. As to fine lines and wrinkles, it is hypothesized that they are formed in order to decrease the effective surface area of the skin so that it can minimize the water evaporation from the skin surface. In other words, the formation of fine lines and wrinkles may primarily reflect the body's natural self-defense mechanism. Obviously, direct exposure to strong sunlight without any protection can worsen the age spots or wrinkles due to higher water loss from the skin and the damage caused by UV-generated free radicals [8-16].
The availability of numerous anti-aging commercial products and approaches has been extensively compiled and the reversal of facial skin aging has been commonly regarded as impossible [13]. In this regard, it is of interest to note that there is a proprietary product marketed as Dr. Win's Eternal Spring Serum from Winlind Skincare LLC. Burr Ridge, IL 60527, USA (website at http://www.eternalspringserum.com) developed by me about 12 years ago based on the above nutritional theory. It has been observed to be able to reverse skin aging among many users. One of the most dramatic examples is shown in a 75-year female who has used the product for about 12 years and who has rarely used sunscreens (Figure 4). Her skin is age-spot and wrinkle free and was found to be much firmer and tighter than many other females 25 to 35 years younger. The aging-reversal phenomenon has been noticed by many of her friends who have not seen her for 10 to 20 years and found her facial skin appearing much younger than before. Interestingly, she has developed more than 50 age spots on her lower hind legs. This seems to indicate that a properly formulated topical product may minimize, prevent or even reverse skin aging due to both intrinsic and extrinsic factors. Her numerous age spots on the legs were apparently caused predominantly by the intrinsic aging. In view of some apparent differences between this work and conventional viewpoints, it is hoped that this commentary may stimulate further studies and discussion.
References
- Shock N (1957) Age changes in some physiologic processes. Geriatrics 12: 40-48.
- Strehler B, Mildvan A (1960) General theory of mortality and aging. Science 132: 14-21.
- Shuster S, Black M, McVitie E (1975) The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol 93: 639-643.
- Li L, Mac-Mary S, Sainthillier JM, et al. (2006) Age-related changes of the cutaneous microcirculation in vivo. Gerontology 52: 142-153.
- Guyton A (1971) Textbook of medical physiology. (4th edn), Saunders, USA, 31-375.
- Sehl M, Yates F (2001) Kinetics of human aging: 1. Rates of senescence between age 30 and 70 in healthy people. J Gerontology Bio Sci 56: B198-B208.
- Nikolakis G, Makrantonaki, Zouboulis C (2013) Skin mirrors human aging. Horm Mol Biol Clin Invest 16: 13-28.
- Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerotolol 11: 298-300.
- Kirkwood T (2005) Understanding the science of aging. Cell 120: 437-447.
- Giorgio M, Trenei M, Migliaccio E, et al. (2007) Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Biol 8: 722-728.
- Farkas J, Pessa J, Hubbard B, et al. (2013) The science and theory behind facial aging. Plast Reconstr Surg Glob Open 1: e8-e15.
- Kammeyer A, Luiten RM (2015) Oxidation events and skin aging. Ageing Res Rev 21: 16-29.
- Jung M, Kelly K, McCullough J (2006) Skin aging: pathogenesis, prevention and treatment, In: Rattan S, Kassem M, Prevention and Treatment of Age-Related Diseases. USA, 175-192.
- Butov A, Volkov N, Aniismov V, et al. (2002) A model of accelerated aging induced by 5-bromodeoxyuridine. Biogerontology 3: 175-182.
- Jin K (2010) Modern biological theories of aging. Aging Dis 1: 72-74.
- Chung J, Seo JY, Choi HR, et al. (2001) Modulation of skin collagen metabolism in aged and photoaged humans. J Invest Dermatol 117: 1218-1224.
- Grueter CE, Rooij E, Johnson BA, et al. (2012) A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell 149: 671-683.
- Oikarinen A (1994) Aging of the skin connective tissue: how to measure the biochemical and mechanical properties of aging dermis. Photodermatol Photoimmunol Photomed 10: 47-52.
- Rigel D, Weiss R, Lim H, et al. (2004) Clinical and histological changes of photoaging. In Photoaging 33-54.
- Sohal R, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273: 59-63.
- Chiou W (1989) The phenomenon and rationale of marked dependence of drug concentration on blood sampling site (Part I). Clin Pharmacokinet 17: 175-199.
- Bergmann O, Bhardwaj R, Bernard S, et al. (2009) Evidence for cardiomyocyte renewal in humans. Science 324: 98-102.
- D'Uva G, Aharonony A, Lauriola M, et al. (2015) ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nature Cell Biol 17: 627-638.
- Lazaro D, Kostarelos K (2016) Engineering cell fate for tissue regeneration by in vivo transdifferentiation. Stem Cell Rev 12: 129-139.
- Schultz M, Sinciair DA (2016) When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development 143: 3-14.
- Weller P (2016) Sunlight has cardiovascular benefit independent of vitamin D. Blow Purif 41: 131-134.
- Wright F, Weller R (2015) Risks and benefits of UV radiation in older people: More of a friend than a foe? Maturitas 81: 425-431.
- Hirokawa K, Usuyama M, Kurashima C (1992) Aging and immunity. Acta Pathol Jpn 42: 537-548.
Corresponding Author
Win L Chiou, Chiou Consulting Inc and Winlind Skincare LLC, Burr Ridge, Illinois 60527, USA.
Copyright
© 2017 Chiou WL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.