Effect of Salicylic Acid on Physicochemical Parameters and Accumulation of Compatible Solutes in Three Genotypes of Cowpea under Arsenic Stress
Abstract
Cowpea is a legume widely cultivated across the world but its growth may be limited by contamination with non-essential elements among which are arsenic. The present study was designed to examine the effect of salicylic acid on physicochemical parameters and accumulation of compatible solutes in cowpea under arsenic stress. Three genotypes (Ife brown, ART98-12 and ITOK-568-18) of cowpea seeds were soaked in (0: control, 75 and 150 mg/L) of salicylic acid for 6 hours. They were air-dried and five seeds each were sown in pots of soil containing 0, 250 and 500 mg/L of sodium arsenate. Growth parameters, photosynthetic pigments and compatible solutes were determined. Data were analysed using anova at 5% level of significance. The result shows that in comparing treatment with the control, growth parameters and proline were increased by 4 and 5 folds in Ife brown; 3 and 2 folds in ART 98-12 and 2 and 5 folds in ITOK-568-18,chlorophylls a and b increased by 7.84 ± 0.04 vs. 0.43 ± 0.01 and 5.05 ± 0.03 vs. 0.20 ± 0.01 mg/g/fw in ART98-12; 11.02 ± 0.02 vs. 4.12 ± 0.01 and 7.22 ± 0.01 vs. 1.27 ± 0.02 mg/g/fw in Ife brown; 8.31 ± 0.01 vs. 4.12 ± 0.01 and 5.38 ± 0.01 vs. 1.27 ± 0.02 mg/g/fw in ITOK-568-18, soluble carbohydrate and soluble protein were reduced by 5 and 3 folds, in Ife brown and ITOK-768-18 in 250 mg/L sodium arsenate-treated soil with SA (150 mg/L) but was reduced by 3 folds in 250 mg/L sodium arsenate-treated soil with SA (75 mg/L) for ART98-12. These results show that salicylic acid could alleviate arsenic stress in cowpea and these could be of great value to crop and food producers.
Keywords
SA, Arsenic, Cowpea, Stress, Genotype
Introduction
Cowpea is an annual legume which was invented from Africa. Plant proteins and vitamins obtain from its seed serve as major nutrient for man and feed for animals. Cowpea seeds contain higher amounts of essential nutrients needed for body growth. This serve as a supplement to cereal food in countries that adopt cowpea as source of protein. A seed normally comprises of 25 % protein and has lesser fat content. As a result of great levels of protein found in the leaves and seeds, cowpea is commonly termed "poor man meat". It also serves as great sources of essential minerals [1].
Arsenic is an element that can found in the natural environment in some abundance in the Earth's crust and in small quantities in rock, soil, water and air. It is present in many different minerals. About one third of the arsenic in the atmosphere comes from natural sources such as volcanoes, while the remaining comes from man-made sources. It is ranked as twentieth in abundance among the elements in the Earth's crust [2]. Arsenic contamination may also occurs from the use of agricultural pesticides and in chemicals for timber preservation. There are organic and inorganic arsenic with Organic arsenic species for instance is abundant in seafood, are much less harmful to health, and are readily eliminated by the body, inorganic forms are more toxic. Inorganic forms are arsenite, arsenate, arsine and arsenic metal [3]. There is increased contamination of soil with arsenic both from natural sources and human activities. Therefore amelioration of toxicity of the arsenic is very paramount as reactive oxygen species generated from arsenic-induced toxicity leads to hormonal imbalance in crops. Salicylic acid plays prominent role in promoting plant growth and therefore may serve as potential strategy and show protection against arsenic-mediated injury in plant.
The role of 24-Epibrassinolide through increasing physiological and biochemical parameters of Cicerarietinum under arsenic stress was reported by [4]. Modulation of arsenic toxicity by salicylic acid via lowering the penetration in Oryza sativa had been reported by [5]. Also, foliar application of salicylic acid had been shown to alleviate the cadmium toxicity by modulating the reactive oxygen species in potato [6]. Plant growth regulators had also been shown to improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint [7]. Moreover, [8] reported modulation of cellular redoxs sand antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. In addition, [9] had reported on minimizing adverse effects of Pb on maize plants by combining treatment with jasmonic, salicylic acids and proline. The effect of kinetin on the physiological and biochemical properties of corn seedlings under arsenic stress had also been reported by [10]. Meanwhile heavy metals component of our locally consumed cowpea in major markets in Ibadan, Oyo State Nigeria had been evaluated and arsenic content were found to be above tolerable standard stipulated by Food and Agricultural Organization [11]. Hence the present study on effect of salicylic acid on physiological parameters and accumulation of compatible solutes in three genotypes of cowpea under arsenic stress.
Materials and Methods
The present work was carried out in a screen at Institute of Agricultural Research and Training (I.A.R&T) Ibadan Oyo State, Nigeria. Pots of 5 kg capacity were filled with soil and were arranged in triplicate in a complete randomised design with 15 treatments for each of the three genotypes of cowpea. Sodium arsenate (250 and 500 mg/L) were prepared. Five hundred millilitres of aqueous sodium arsenate prepared above was applied to each 5 kg of soil in the pot. It was then left for 14 days for proper equilibration. Three genotypes of cowpea seeds were obtained from Institute of Agricultural Research Training [(Ife Brown (Red coat) and ART 98-12 (white coat)] and International Institute of Agricultural Research & Training [ITO7K-568-18 (Red coat)]. Salicylic acid was later prepared by using procedure of [12] and [13].
Three different genotypes of cowpea (Ife brown, ART98-12 and ITO7K-568-18 was soaked in a plastic film with two concentration of salicylic acid (75 and 150 mg/L respectively). Distilled water was used instead of salicylic acid for other part of the seeds which represent the control. All were kept for about 6 hrs without light at room temperature. Thereafter, the solutions were decanted off, and seeds were rinsed thrice with distilled water and air-dried for 1 hour. These air-dried treated and control seeds were sown in the pot of soil containing 0, 250 and 500 mg/L of sodium arsenate (arsenic stress) and allowed to germinate until maturity.
Determination of Compatible Solutes
Soluble protein
Soluble protein was analyzed based on the method of [14]. The result was expressed as mg/g/ dry matter.
Soluble carbohydrate
Soluble carbohydrate was analysed spectrophotometrically based on method of [15]. The result was expressed as mg/g/ fresh weight.
Proline determination
The concentration of proline was estimated according to [16] methods. Proline content was expressed as µMol/g/ fresh weight.
Determination of growth parameters
The physiological effect of arsenic toxicity are suppression in leaf numbers and areas, reduced root elongation, yellowing and rotting of leaf, reduced proliferation and nodulation. SA restores all these physiological function by increasing the rate of photosynthesis, facilitating nutrients uptake, nitrate metabolism, flowering and ethylene production.
Procedure
Fresh weight, dry weight, number of leaf, percentage germination and plant height were determined based on [17] procedures. Percentage germination was determined using the formula
Determination of photosynthetic pigments
Photosynthesis is the process where plants and some microorganisms use light energy to manufacture sugar from carbon dioxide and water. Chlorophyll is a pigment that exist in all plant that performs photosynthesis and it's the most common of the six. Arsenic reduces rates of photosynthesis by interfering with the chlorophyll biosynthesis by inducing insufficient iron or by subduing biosynthesis enzymes involved with the chlorophyll synthesis. Arsenic also induced chloroplast membrane disorganization hindering entire role of photosynthetic process. Therefore Salicylic acid play biomodulatory role by stimulating antioxidant system of plants during arsenic stress.
Procedure
The photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoid) were determine according to the method of [18].The concentrations of chlorophyll a, chlorophyll b, and total carotenoids were determined using Arnon's equation [19].
Result
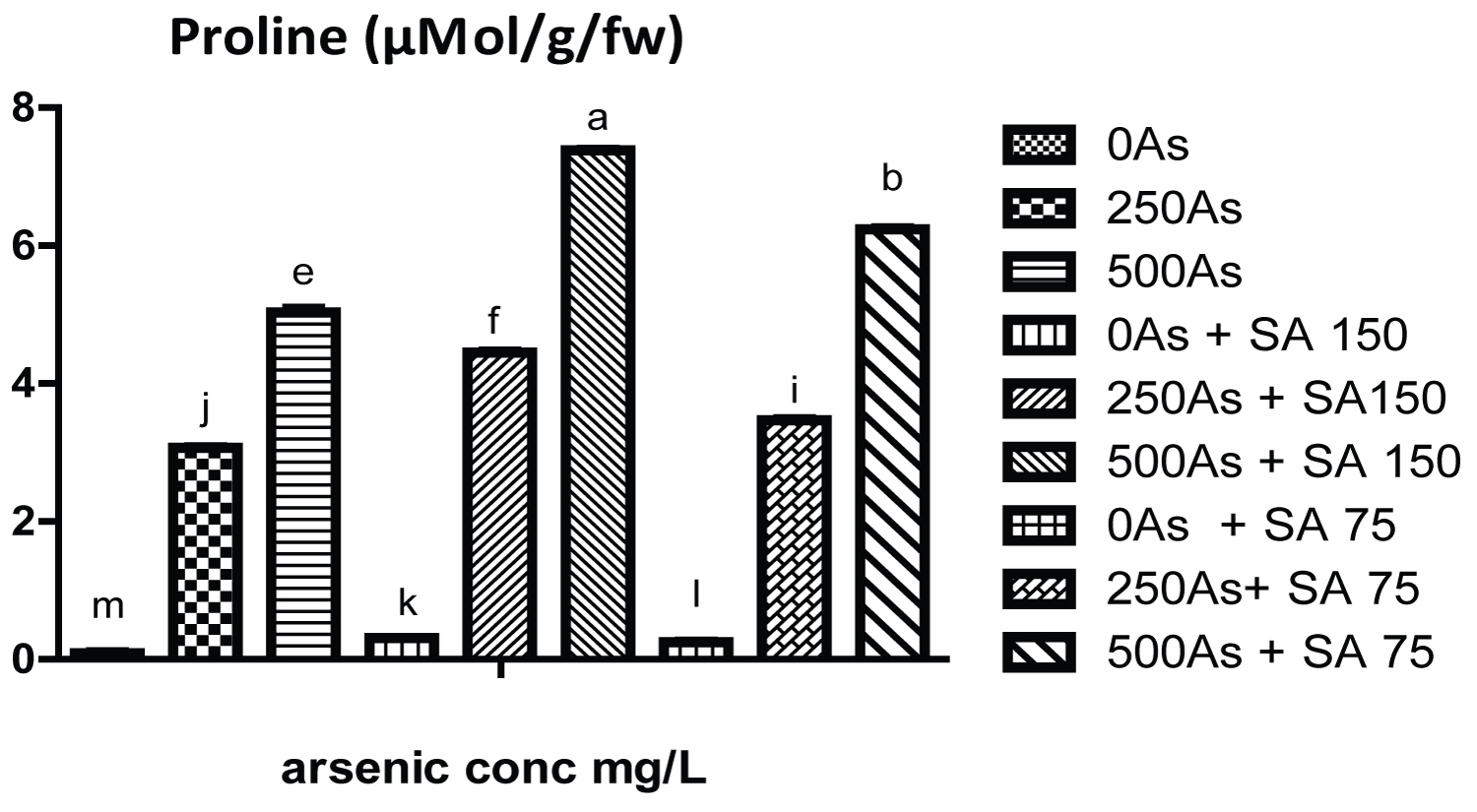
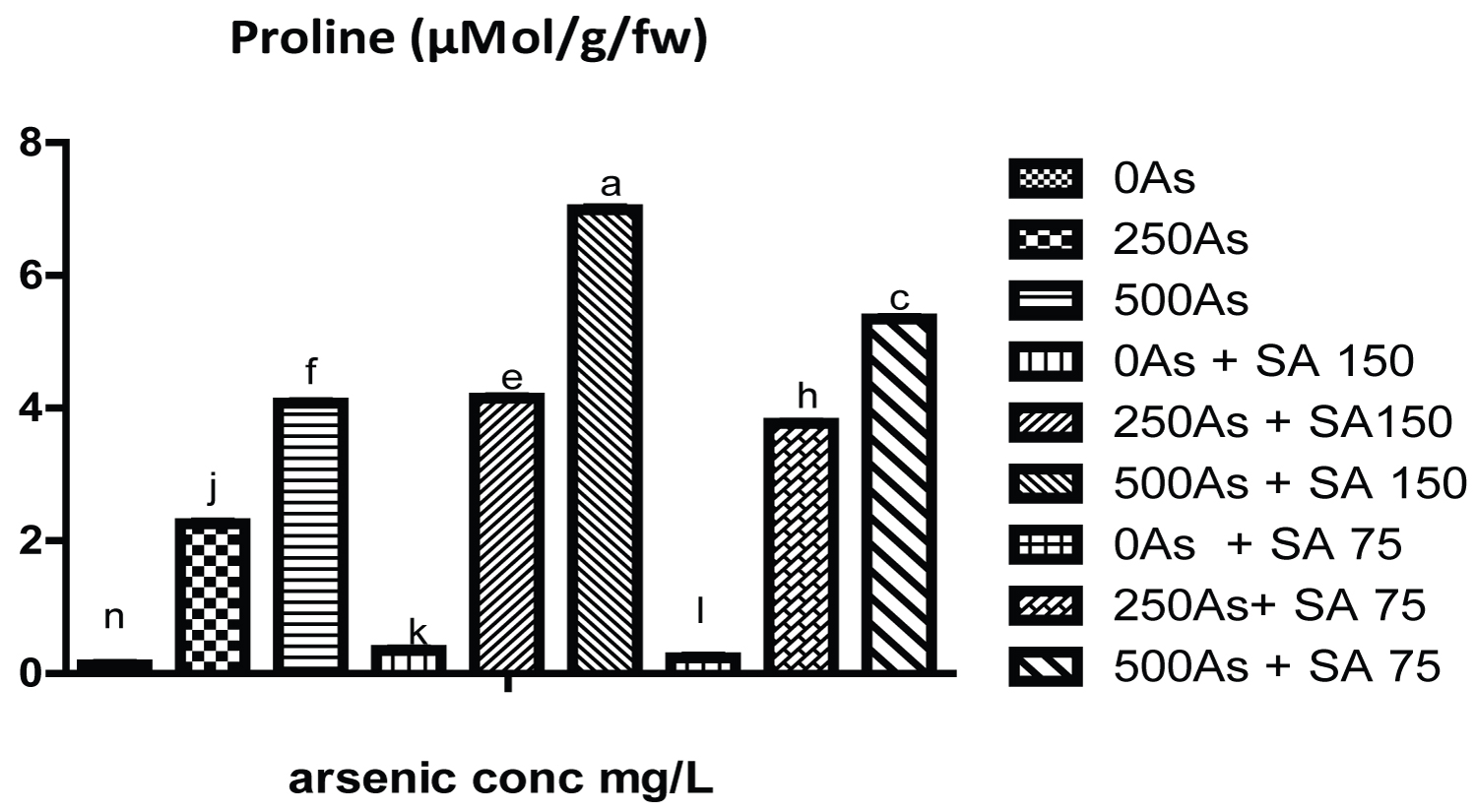
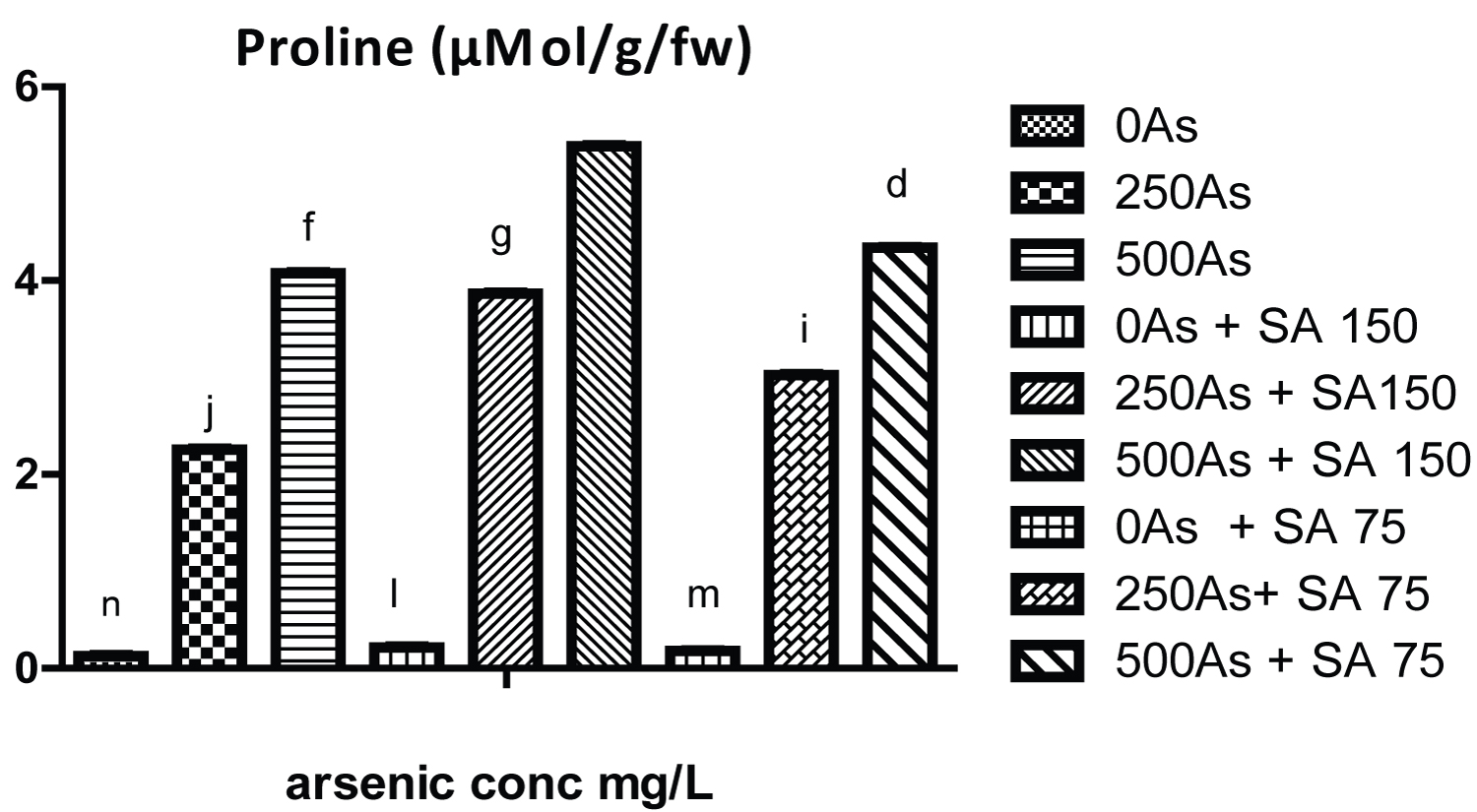
The result of growth parameters for the three genotypes of the cowpea is shown in (Tables 1, Table 2 and Table 3) while that of photosynthetic pigments (chlorophyll a, b and carotenoid) is shown in (Table 4, Table 5 and Table 6). Likewise the result of soluble carbohydrate, soluble protein is shown in (Table 7, Table 8 and Table 9) while that of proline is represent in (Figure 1, Figure 2 and Figure 3).
Discussion
Growth parameters is one of the measurement of physiological attributes of plants. The result in (Tables 1, Table 2 and Table 3) shows that fresh weight, dry weight, number of leaf, plant height and % germination of plant exposed to 250 and 500 mg/L arsenate significantly reduce when compare to control plant. This is due to arsenic inhibition of some important mineral elements necessary for plant growth hence diseased symptoms like chlorosis, blackening of the plant, reduction in the biomass and necrosis at later stage. However, SA (75 and 150 mg/L) significantly increase their level in all three genotypes respectively to different extents. It was reported by [20] that studies conducted on Triticumaestivum L. shows that arsenic application at higher levels pose greater risks. Symptoms were leaf shading, reduced plant growth and brown necrotic spots on the leaves. It was also reported by [3] that 1.5 mg/L kinetin application cause greater rise in the root numbers by 28% with respect to those at arsenic treatment alone in maize. Moreover, it was reported by [5] that SA pre- treated plants also experienced less toxicity during exposure to AsV. Arsenate induced reduction in biomass was also significant.
Also (Table 4, Table 5 and Table 6) shows that 250 and 500 mg/L sodium arsenate significantly reduce chlorophyll a, b and carotenoids level when compared control plant in all the three genotypes. This might be as a result of the arsenic intervention with the synthesis of chlorophyll either by suppression of biosynthetic enzymes involved with chlorophyll synthesis or through induction of mineral element deficiency however, SA at the concentration of 150 mg/L and 75 mg/L significantly increased the level of Chlorophyll a, b and carotenoids respectively. It was reported by [17] that [21] find out that a concentrations of arsenic which was 50 and 100 mg/kg of arsenic subdued the photosynthesis by 42.1 and 32.1 %, respectively.
Moreover, the result in (Figure 1, Figure 2 and Figure 3) and (Table 7, Table 8 and Table 9) shows that the proline content, soluble carbohydrate and soluble protein of plants exposed to 250 and 500 mg/L of arsenate significantly increased with respect to control plant. However, SA and GA 150 mg/L significantly increase the proline content in all the genotypes. Initial increase in proline content might be due to the plant response to the stress which result from reduction in water potential of the plant under arsenic stress, therefore proline, an amino acid function to maintain least water intensity needed by the plant thereby keeping them hydrated under arsenic stress. It is also regarded as proteinogenic five-carbon α-amino acid compound [22].
On the contrary, SA (75 and 150 mg/L) significantly reduce the level of soluble protein and carbohydrate in all the three genotypes respectively which is an indication of moderation of alleviation of the stress as the increase in their level signify response to the stress. In addition with the proline, soluble carbohydrate and protein serve to protect plant from injury by reactive oxygen species which was generated from arsenic toxicity Therefore, the results obtained in this work indicate that salicylic acid could alleviate arsenic stress in cowpea by increasing its tolerance to promote growth and production.
Conclusion
From the present study, arsenic shows high level of toxicity to plants and has been demonstrated in the contents of the physiological and biochemical parameters examined which is an indication of their response to stress. Meanwhile, the effects are characterised by chloros is as a result of inhibition of photosynthesis which shows in their level of photosynthetic pigments, reduction in plants biomass as well as necrosis at continuous and higher exposure. Also, human exposure is highly detrimental to health which could result in terminal illness such as cancers. However, bio regulator such as salicylic acid has been implicated in regulating, stabilizing some biochemical process in plant due to hormonal imbalance caused by these arsenic toxicity. Hormone determined growth and some functions in plants. Three genotypes of cowpea seed pre-treated with salicylic acid show varying modulator effects against arsenic stress through increase in the level of photosynthetic pigments, growth parameters via increase in compatible solutes, and other molecules that serve to protect plants against stress. Therefore, pre-treatment of cowpea with salicylic acid could serve ameliorative role in militating against arsenic stress.
Acknowledgement
The author is grateful to the Institute of Agricultural Research & Training and International Institute of Tropical Agriculture both at Ibadan, Oyo State Nigeria for providing cowpea seed used for the work.
Funding Agents
The present work was independently funded by the authors.
Conflict of Interest
All authors declared that there is no conflict of interest in publishing this research.
References
- Davis DW, Oelke EA, Oplinger ES, et al. (1991) "Cowpea". Alternative field crops manual. University of Wisconsin Extension, Cooperative Extension 20: 11-19.
- Ghinwa NM, Bohumil V (2009) Toxicity and sources of lead, cadmium, mercury, copper arsenic and radionuclides in the environment. Toxicology Science Journal 24:70-75.
- Gills, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in tolerance to abiotic stress in crop plants. Plant Physiol Biochem 48: 909-930.
- Anjana SK, Kaur I, Bhatnaga AK (2016) Impact of 24-Epibrassinolide on tolerance, accumulation, growth, photosynthesis and biochemical parameters in the stressed arsenic Cicerarietinum L. Agricultural Science Research Journal 6: 201-212.
- Amit PS, Garima D, Seema M, et al. (2015) Modulation of arsenic toxicity by Salicylic acid via reduction of the root for the translocation of the outbreak in rice. Front Genet 6: 34.
- Qian Lia, Gang W, Yurong W, et al. (2019) Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato, Ecotoxicology and Environmental Safety 172: 317-325.
- Abbu Z, FirozM, Qazi F (2020) Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Menthaarvensis L.). Physiology and Molecular Biology of Plants 26: 25-39.
- Mahmoud RS, Mahmoud FS, Bushra AA, et al. (2020) Minimizing adverse effects of pb on maize plants by combined treatment with jasmonic, Salicylic acids and proline. Agronomy 10: 699.
- Mohammad F, Shafaque S, Vishnu Rajput D, et al. (2021) Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 10: 2254.
- Haijuan W, Biyu D, Xiaogang S, et al. (2015) Effect of kinetin on the physiological and biochemical properties of corn seedlings under arsenic stress. Adv Mater Sci Hindawi 7: 45-49.
- Ailenokhuoria BV, Omolekan TO (2019) Heavy metal, proximate and antioxidant composition of five cultivars of (Vignaunguiculata L.) in Ibadan, Oyo State, Nigeria. Journal of Food Science and Nutrition Resources 2: 327-332.
- Coolbear P (1977) Seed treatments for improved performance-survey and attempted Prognosis. Seed Science. Technology 5: 353-425.
- Heydecker W (1977) Stress and seed germination: An agronomic view. In: The physiology and biochemistry of seed dormancy and germination. Biomedical Press 1997: 237-282.
- Lowry OH, Rosebrough NJ, Fail AL, et al. (1951) Protein measurements with flinephenol reagent. J Biol Chem 193: 265-275.
- Dubey RS, Singh AK (1999) Salinity induces accumulation of soluble sugars and alters the activity of sugar metabolizing enzyme in rice plants. Biologia Plantaium 42: 233-239.
- Saumya Srivastava, Yogesh Kumar Sharma (2013) Impact of arsenic toxicity on black gram and its amelioration using phosphate. Toxicology 2013: 1-8.
- Sing SK, Ghosh AK (2010) Effect of arsenic on photosynthesis, growth and its accumulation in the tissues of allium cepa (Onion) International Journal of Environmental Engineering and Management 1: 39-50.
- Richardson A, Shane PD, Graeme PB (2002) An evaluation of non-invasive methods to estimate foliar chlorophyll content. New phytologist 153: 185-194.
- Arnon DI, Stout PR (1949) The essentially of certain elements in minute quantity for plants with special reference to copper. Plant Physiology 14: 371-375.
- Vibhuti C, Subhas C, Sahu K (2016) Arsenic-induction of metabolic disorders and their mechanisms of mitigation in crop plants: A review. Biologia 71: 367-377.
- Miteva E, Merakchiyska M (2009) Chlorophyll and photosynthetic pigments mechanism of bean plants responses to excess arsenic in soil Bulgaria. Journal Agricultural Science 8: 151-156.
- Chen ML, Lin CC, Kao HC (2000) Copper Toxicity of copper in rice seedlings: Changes in antioxidant enzyme activities, level of hydrogen peroxide and peroxidase activity of the cell wall in the roots. Botanical Bulletin of Academia Sinica 41: 99-103.
Corresponding Author
Bukola Victoria Ailenokhuoria, Agricultural Value Addition Programme, Institute of Agricultural Research and Training Obafemi Awolowo University P.M.B 5029 Moor Plantation, Ibadan Nigeria, Tel: 234-803-464-8471
Copyright
© 2022 Ailenokhuoria BV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.