Meta-Analysis of Transcriptomic Responses to Tamoxifen Therapy in Breast Cancer
Abstract
Tamoxifen (Tam) is an effective hormone therapy in order to reduce the risk of cancer recurrence. Investigation of the effect of Tam on transcriptome features by meta-analysis can help us to understand the effect of Tam on biological pathways and expression of key genes. Here, whole-transcriptome (RNA-seq) datasets (19 samples) were derived from European Bioinformatics Institute (EBI). The process of differential gene expression analysis was carried out through CLC Genomics Workbench (12). The total reads of differential expressed genes were analyzed by Meta-seq package in order to identification of common up and down-regulated genes. The outcomes of meta-analysis indicated that several candidate genes contribute to tumor suppression process. As an example, XIAP-associated factor 1 was reported as an up-regulated gene under Tam therapy. It is a tumor suppressor that contributes to the apoptosis and tumor growth inhibition along with TP53 . Estrogen-based growth regulation in breast cancer 1 ( GREB1 ) as another up-regulated gene is an ESR1 (estrogen receptor 1) that could mediate the estrogen action. The results showed that PROM1, KLHL14 and FBN2 were highly down-regulated. Our findings suggested that Tam usage in MCF7 cell line could be associated with apoptosis, proteolysis, and tumor suppression. In addition, Tam may decrease the expression of candidate genes involved in tumor progression, invasion, and metastasis.
Keywords
Tamoxifen, Breast cancer, Meta-analysis, RNA-seq
Introduction
According to the last World Health Organization (WHO) report in 2020, there were 2.3 million women diagnosed with breast cancer and 685,000 deaths globally ( https://www.who.int/ ). Usually, treatment process for breast cancer depends on the subtype of cancer and metastasis stage. Surgery, radiation therapy, chemotherapy and hormonal therapies are the main strategies for breast cancer treatment [1,2]. Tamoxifen (Tam) is a non-steroidal compound and commonly used as an adjuvant hormonal therapy for patients with breast cancer. [3], which inhibits the estrogen activity through binding to the estrogen receptor (ER) competitively [4]. Its beneficial effects in reducing metastasis, tamoxifen can also lower the risk of death from breast cancer [5]. It has been demonstrated that 10 years of Tam in ER-positive disease produces substantial reductions in rates of recurrence and in breast cancer mortality during the first decade [6]. There are several investigations carried out to describe the hormonal therapy effects and drug response mechanism [7-9]. The appropriate drug response is a complex interdependent procedure that is highly dependent upon several factors including the genetic variants background, lifestyle, climate, smoking, and alcohol consumption [10]. Moreover, consumption of Tam can have side effects that may affect the purpose of the treatment [11]. It is shown that Tam induces DNA damages in human endometrial cells and increases the incidence of endometrial tumors [12]. Therefore, investigating the effect of Tam on transcriptome features by meta-analysis can allow us to reduce the side effects and explain the role of key genes under Tam therapy.
Gene expression analysis at transcriptome level is a reliable tool that can show the effects of hormone therapy on genes expression profiles. It has been shown through several studies that there is a close association between transcriptome response and therapeutic drug consumption [13-16]. It was found from one of the main investigations that carried out a comprehensive transcriptomic analysis on Tam resistance that lncRNAs profiling breast cancer cells would provide a new light on the identification of novel endocrine resistance biomarkers [17]. Lanceta, et al. (2020) carried out RNA sequencing (RNA-seq) and pathway analysis in ER+ MCF7, and reported 2183 up-regulated and 1548 down-regulated transcripts that contributed to cell cycle, DNA replication, and DNA repair and autophagy [18]. It was indicated that the effects of Tam on the breast cancer MCF-7 cell line are mediated by the activation of important signaling pathways including Tp53 and Mitogen-Activated Protein Kinase (MAPKs) to induce apoptosis [19]. Meta-analysis of integrated Chip-seq and transcriptome data demonstrated that many transcription factors such as POU5F1B, ZNF662, ZNF442 affected by ER were up-regulated [20]. Interestingly, the meta-analysis investigations showed that that circRNAs could be considered as a good potential clinical biomarkers in breast cancer patients [21,22].
Current study investigates the effect of Tam on the gene expression profile at transcriptome level. Meta-analysis of genes expression studies could be useful to understand the drug response mechanism; also, it would provide a new insight to the increase of the chance of survival, decrease the side effects, and select an appropriate strategy for the therapy period.
Materials and Methods
Data collection
In current study, the whole-transcriptome (RNA-seq) dataset of four investigations were derived from EBI ( https://www.ebi.ac.uk/ ). More details of collected datasets were provided in Table 1.
Quality control and trimming
Various parameters were applied in CLC Genomic Workbench (12) in order to determine the quality for each sample including length distribution, GC content, ambiguous base content, Phred score, nucleotide contribution, enrich 5 mers, and duplicate sequences [23]. Due to the fact that the adaptor sequences were cleaned in the achieved datasets, the adaptor trimming was not achieved.
RNA-seq analysis, meta-analysis and gene ontology enrichment analysis
The reference genome (hg38) and all of the annotations were downloaded from Ensembl database ( www.ensembl.org ). Also, the process of differential expressed genes (DEGs) analysis was carried out through CLC Genomics Workbench (12) on the basis of following parameters including mismatch cost = 2, insertion cost = 3, deletion cost = 3, length fraction = 0.7, similarity fraction = 0.8, expression value = total reads, calculate RPKM for genes without transcripts = Yes [24]. The total reads of DEGs were analyzed by Meta-seq package in order to identification of common up and down-regulated genes among experiments [25]. The outputs of meta-analysis were used in gene ontology enrichment analysis (P ≤ 0.01) by gene ontology consortium ( http://geneontology.org ). Heat map and volcano plot were implemented to visualize the Meta-analysis results.
Results
Meta-analysis outcomes
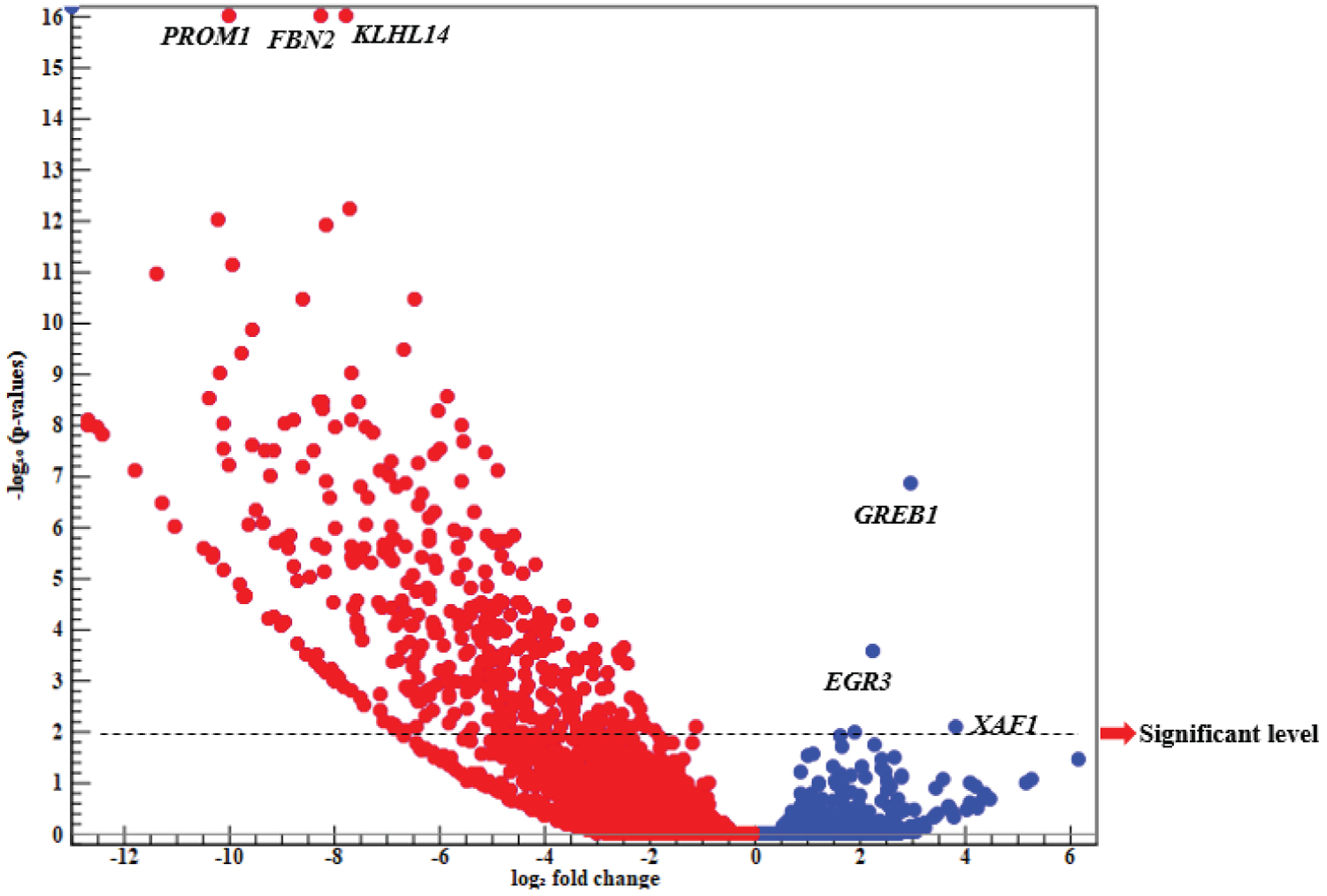
Volcano plot: Results of the meta-analysis showed that there were 21515 common up and down-regulated genes, while findings of Volcano plot indicated that most of DEGs were classified as the down-regulated genes. At significant levels (P < 0.01, -Log 10 (P-value) > 2), there were 910 and 3 candidate genes reported as the significant down- and up-regulated ones (Figure 1).
Results of Volcano plot showed that three candidate genes including GREB1, EGR3, and XAF1 were clustered as the up-regulated genes. It was also found that the estrogen-based growth regulation in breast cancer 1 ( GREB1 ) was an early estrogen-responsive gene, and there was a close association between GREB1 expression and estrogen levels in breast cancer patients. In fact, GREB1 was an ESR1 (estrogen receptor 1) that could mediate the estrogen action. It was reported that the optimal level of GREB1 expression was required for breast cancer cells proliferation [26]. However, GREB1 knockdown could prevent the breast cancer cell lines proliferation; therefore, it was found that targeting GREB1 could provide a possible treatment strategy through inhibiting the tumor-promoting pathways [27]. Early growth response ( EGR ) is a family of transcription factors that contributes to various biological pathways [28]. It was reported that EGR3 could be induced by estrogen in breast cancer MCF-7 cells and consequently, become involved in the estrogen-signaling pathway [29]. Moreover, EGR3 levels were significantly higher within tissue samples derived from patients with recurrent breast cancer compared to those with primary tumors [30]. XIAP-associated factor 1 ( XAF1 ) is a tumor suppressor observed in the multiple human neoplasm’s [31]. It was shown that XAF1 loss expression would be resulted from tumor staging and its dysfunction was associated with tumor progression. Moreover, its appropriate expression could play a critical role in the apoptosis inductions and tumor growth inhibition in the gastric cancer [32]. Pinto, et al. (2020) reported that XAF1 may be considered as a TP53 function modifier through increasing the transcriptional activity of hypo orphic TP53 variants [33]. TP53 is one of the most significant tumor suppressor genes, which is commonly mutated in various cancers such as breast cancer [34].
It is noteworthy that PROM1, FBN2, and KLHL14 were highly down-regulated (Figure 1). Prominin 1 ( PROM1or CD133 ) is known as a biomarker of cancer stem cells; however, its biological role is not illustrated perfectly [35]. Findings showed that there was an association between PROM1 levels and malignancy properties stages including initiation, progression, and metastasis. Moreover, it was reported that PROM1 would contribute to the cell motility and invasion, and may affect the malignancy of breast tumors. Also, PROM1 genes were highly expressed in TNBC cell lines [36,37]. Fibrillin-2 ( FBN2 ) is an extracellular calcium-binding micro fibril that contributes to several biological pathways including the bone mineralization, osteoblast maturation, and calcium binding (UniProtKB: P35556). FBN2 is considered as a biomarker of cancers early diagnosis. For example, Promotor hypermethylation of FBN2 is associated with colorectal cancer as an early event. In fact, Methylation may lead to FBN2 down-regulation in primary tumors [38], while Kelch-like protein 14 ( KLHL14 ) belongs to Kelch family genes and interacts with torsin-1A (UniProtKB: Q9P2G3). It was shown that KLHL14 was significantly over-expressed in breast cancer compared to normal breast tissues, and had a positive relationship with tumor aggressiveness [39]. Moreover, findings indicated the vital role of KLHL14 in the development of various cancers including ovarian cancer [40].
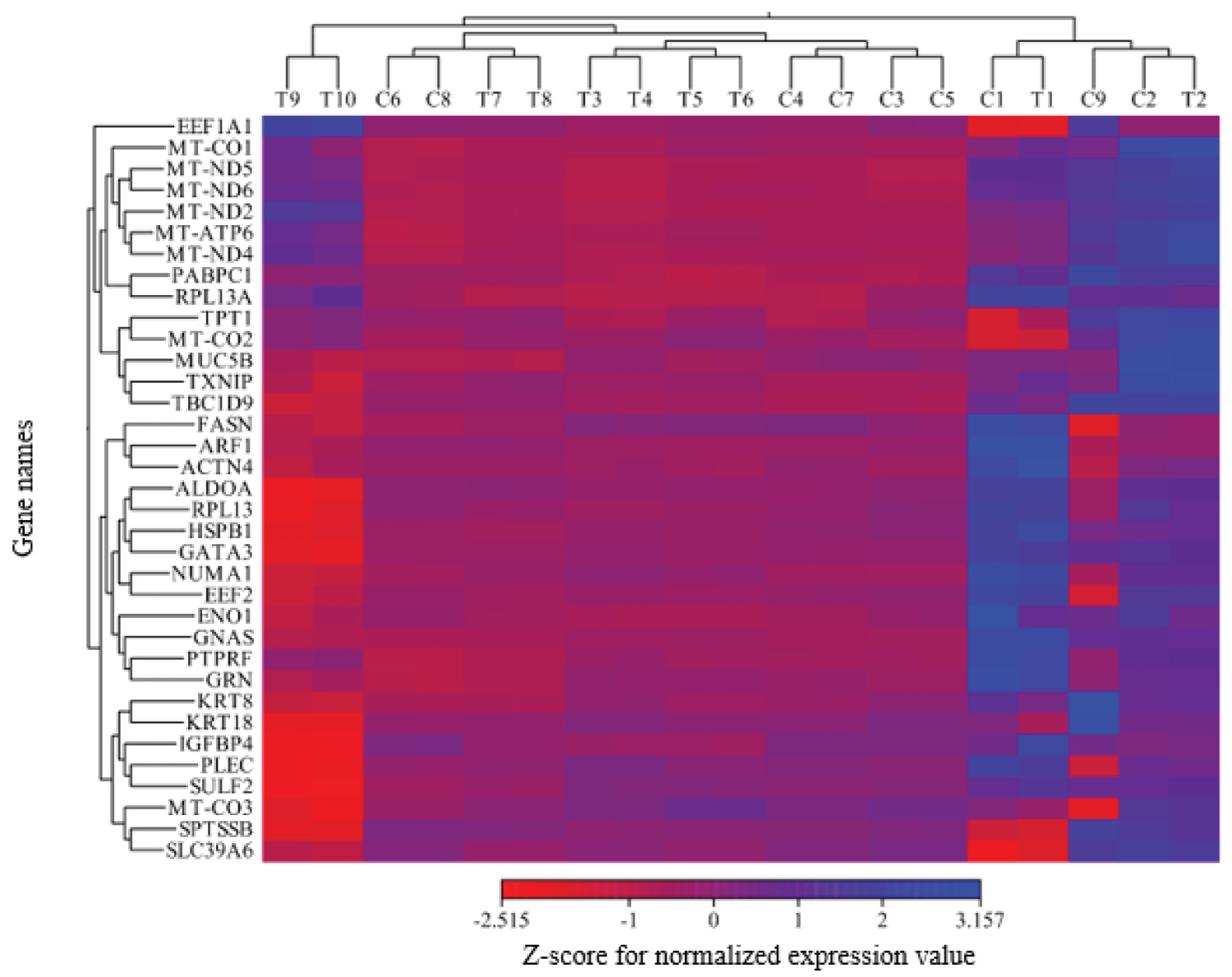
Heat map analysis: The visualization of meta-analysis results derived from control and treated samples were shown in a heat map (Figure 2).
It was also shown that mitochondrial respiratory genes were expressed at lower levels within treated samples compared to control ones. In current study, it was reported that MT-CO1, MT-CO3, MT-ND2, MT-ND4, MT-ND5, MT-ND6, and MT-ATP6 were the mitochondrion respiratory genes. MT-ND genes provide NADH dehydrogenise. This protein is a part of a large enzyme complex encoded by the mitochondrial genome. Moreover, the dysfunction of MT-ND proteins would lead to the electron transport chain disruption and ATP production. MT-CO genes encode Cytochrome C Oxidase subunits within mitochondria. It is found that they were the last enzyme in the mitochondrial electron transport chain for ATP synthesis [23]. Findings derived from heat map analysis suggested that several candidate genes including ALDOA, RPL13, HSPB1, GATA3, KRT18, IGFBP4 , and SULF2 were associated with the lowest gene expression level in treated samples. Aldolase (ALDOA) is known as an oncogene, which is a glycolytic enzyme that promotes the metastatic progression of cancers [41,42]. It was shown that there was an association between ALDOA knock down and proliferation reduction of breast cancer cells [43]. RPL13 encodes a ribosomal protein, which is a component of 60S subunit. Ribosomal proteins (RP) expression patterns were implemented as a diagnostic strategy in human cancers. Reports of several cancers indicated the deregulation of RP expression (e.g. RPL13 ) [44]; therefore, it could be said that RPL13 would be expressed at significantly higher levels in benign breast lesions compared to that of breast carcinomas (Gene Cards: GC16P089674). HSPB1 is a member of heat shock proteins, which are considered as a large family of proteins with breast cancer behavior [45]. It was reported that the down-regulation of HSPB1 protein may induce the expression of phosphatase and tensin homologue (PTEN) as a tumor suppressor gene. In other words, PTEN stabilization depends upon HSPB1 low-level expression [46]. GATA binding protein 3 ( GATA3 ) is a highly conserved transcription factor that belongs to GATA family and leads to the expression of a large number of important genes [47]. Furthermore, it contributes to the human growth and differentiation cells including the mammary tissue. Lower levels of GATA3 expression in breast tumors are associated with larger tumors. Therefore, GATA3 is considered as an important gene in breast cancer development; however, its exact role as an oncogene or tumor suppressor is unclear [48,49]. Keratin 18 ( KRT18 ) is a member of the intermediate filament family of cytoskeleton protein that is involved in the tissue integrity, and its over-expression has been reported in many cancers [50]. It was also re-ported that KT18 was over-expressed in breast cancer and played a vital role in the breast tumorigenesis and tumor dedifferentiation [51]. Insulin-like growth factor binding proteins ( IGFBPs ) would regulate many cellular processes such as cell proliferation. IGFBPs act as binding proteins for insulin-like growth factor (IGF); furthermore, it is evidenced that they play a critical role in the cancer progression, especially in breast cancer [52]. However, there are various reports regarding their activities as oncogene or tumor suppressors. IGFBP5 may be considered as an oncogene due to its contribution to metastasis, proliferation, and limited responses to endocrine treatment; also, it acts as a tumor suppressor because of its apoptotic role, anti-metastatic function, and anti-migratory effects [53]. Sulphates family, which includes sulfatase1 ( SULF1 ) and sulphates 2 ( SULF2 ), plays an important role in the multiple biological pathways through regulating the sulfation status [54]. It was confirmed that SULF2 would promote the breast cancer progression and regulate the tumor-related genes expression in breast cancer [55].
Gene ontology enrichment analysis: Results derived from GO enrichment analysis of DEGs showed that most of DEGs were enriched in the regulations of apoptosis and cell death pathways (Table 2). Cell cycle damage is considered as the main cause of cancer incidence; therefore, the balance between proliferation and cell death is disrupted in cancers. It was shown that apoptosis inactivation would play a vital role in the process of cancer development [56]. Therefore, it could be said that significant GO term of apoptosis pathways could contribute to cancerous cell death under Tamoxifen therapy. Proteolysis is a hydrolysis reaction that occurs when peptide bonds and proteins are broken down into smaller polypeptides or amino acids. There is an association between the metastasis of malignancy tumor and overexpression of proteolytic enzyme. More importantly, proteolysis inactivation in cancerous tissue plays a critical role in the inhibition of tumor invasion, angiogenesis, and migration [57]. Interestingly, our findings suggested that negative proteolysis regulation and consequent regulations of proteolytic pathways could be regarded as considerable GO terms that control the cancer under Tamoxifen treatment (Table 2).
Discussion
Generally, breast cancer tumors are hormone receptor-positive with highly estrogen- and progesterone-dependent growth rates. Tamoxifen is a type of hormonal therapy implemented with the purpose of treating the estrogen receptor-positive breast cancer; also, it can decrease the risk of invasive cancer development. However, it is able to affect the gene expression profile at transcriptome level [58]. Results achieved from RNA-seq analysis in MCF7 cell line under Tamoxifen therapy revealed that there were several candidate genes and biological pathways that could result in the tumor suppression and consequently, effective treatments. XIAP-associated factor 1 ( XAF1 ) can be up-regulated under Tamoxifen therapy; also, it is a tumor suppressor that plays a critical role in the apoptosis induction and tumor growth inhibition in gastric cancer [32]. Interestingly, it was reported that the combination of XAF1 with TP53 would act as a modifier. TP53 is a key tumor suppressor gene, which is generally mutated in various cancers such as breast cancer [33,34]. It was found in current study that PROM1 and KLHL14 were down-regulated under hormone therapy; also, an association was observed between PROM1 levels and malignancy features including initiation, progression, and metastasis. Results showed that PROM1 led to the cell motility and invasion and may impact on the breast tumors malignancy [36,37]. It was observed that there was a high level of KLHL14 expression in breast cancer compared to the normal breast tissues, which was associated with considerable tumor aggressiveness. KLHL14 also played a vital role in the development of various cancers such as ovarian cancer [40], and results derived from GO enrichment analysis of DGEs indicated that most of DGEs could considerably result in the cell death, apoptosis, and negative regulation of proteolysis process. Generally, apoptosis has a critical role in the inhibition of cancerous cell growth. Proteolysis activation in cancerous tissues would result in the tumor invasion [56,57]. All of the above-mentioned findings were in accordance with those of Rouhimoghadam, et al. (2018) who showed that tamoxifen treatment on the breast cancer MCF-7 cell line could lead to the apoptosis induction through activating the apoptotic signaling pathways including Tp53 and MAPKs [59]. Comparable results reported that Tamoxifen induced the apoptosis through inhibiting the cancerous inhibitor of protein phosphatase 2A (CIP2A) and phospho-Akt (p-Akt) in ER-negative breast cancer cell lines [60]. Frasor, et al. (2006) also reported that Tamoxifen could regulate the gene expression in breast cancer cells and indicated several Tamoxifen-regulated candidate genes including SOCS1 and IEX-1 , which was in accordance with our findings. Cytokine Signaling 1 (SOCS1) suppressor is considered as a tumor suppressor that plays a negative regulatory role for cytokine action through JAK/STAT pathway and suppresses the growth of hepatocellular carcinomas. IEX-1 , which is an immediate early response gene that can widely be expressed in various tissues, is over expressed in breast cancer cells and has an inhibitory effect on breast cancer cell proliferation [61]. Generally, it could be concluded that Tamoxifen implementation in the treatment procedures could be beneficial at the transcriptome level; however, it may induce the oncogene expression in rodent uterine [62].
Conclusion
Results of current study that was carried out at transcriptome level showed that Tamoxifen consumption in MCF7 cell line could be associated with candidate genes and biological pathways that contribute to the apoptosis, proteolysis, and tumor suppression. Moreover, Tamoxifen could decrease the expression of candidate genes involved in tumor progression, invasion, and metastasis.
Ethics Approval and Consent to Participate
This investigation is in accordance with relevant guidelines and regulations of Shiraz University of Medical Science (permit number: IR.SUMS.REC.1399.1276).
Author Contributions
HKK and STH conceived the study. HKK contributed to data analysis and prepared the primary draft of manuscript. STH and KBL revised the manuscript. All authors read and approve the manuscript.
Funding
Current study was financially supported by Shiraz University of Medical Science (grant number: 23234-106-01-99).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets which were analyzed during the current study are available in the European Bioinformatics Institution (EBI) repository (Table 1).
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Preprint Repository
The most parts of the manuscript have been published previously in Research Square website (https://doi.org/10.21203/rs.3.rs-783422/v1).
References
- Waks AG, Winer EP (2019) Breast cancer treatment: A review. JAMA 321: 288-300.
- Akram M, Iqbal M, Daniyal M, et al. (2017) Awareness and current knowledge of breast cancer. Biol Res 50: 33.
- Abo-Touk NA, Sakr HA, El-Lattef AA (2010) Switching to letrozole versus continued tamoxifen therapy in treatment of postmenopausal women with early breast cancer. J Egypt Natl Canc Inst 22: 79-85.
- Geisler J, Sasano H, Chen S, et al. (2011) Steroid sulfatase inhibitors: Promising new tools for breast cancer therapy? J Steroid Biochem Mol Biol 125: 39-45.
- Huber-Keener K J, Liu X, Wang Z, et al. (2012) Differential gene expression in tamoxifen-resistant breast cancer cells revealed by a new analytical model of RNA-Seq data. PLoS One 7: e41333.
- Davies C, Pan H, Godwin J, et al. (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. The Lancet 381: 805-816.
- AlFakeeh A, Brezden-Masley C (2018) Overcoming endocrine resistance in hormone receptor-positive breast cancer. Curr Oncol 25: S18-S27.
- Szostakowska M, Trebinska-Stryjewska A, Grzybowska EA, et al. (2019) Resistance to endocrine therapy in breast cancer: Molecular mechanisms and future goals. Breast Cancer Res Treat 173: 489-497.
- Moiseenko F, Volkov N, Bogdanov A, et al. (2017) Resistance mechanisms to drug therapy in breast cancer and other solid tumors: An opinion. F1000Res 6: 288.
- Bachtiar M, Lee CG (2013) Genetics of population differences in drug response. Current Genetic Medicine Reports 1: 162-170.
- Nezamivand-Chegini M, Kharrati-Koopaee H, Lankarani KB, et al. (2023) Characterization of small genetic variants in breast cancer cell line under tamoxifen therapy. Galen Medical Journal 12: e2598.
- White IN, Smith LL (1996) Mechanisms of tamoxifen-induced genotoxicity and carcinogenicity. In: Hormonal Carcino-genesis II. 228-239.
- Fekete JT, Gyorffy B (2019) ROCplot.org: Validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3,104 breast cancer patients. Int J Cancer 145: 3140-3151.
- Sjöström M, Chang SL, Fishbane N, et al. (2020) Comprehensive transcriptomic profiling identifies breast cancer patients who may be spared adjuvant systemic therapy. Clin Cancer Res 26: 171-182.
- Kumari K, Keshari S, Sengupta D, et al. (2017) Transcriptome analysis of genes associated with breast cancer cell motility in response to Artemisinin treatment. BMC Cancer 17: 858.
- Selli C, Dixon JM, Sims AH (2016) Accurate prediction of response to endocrine therapy in breast cancer patients: current and future biomarkers. Breast Cancer Research 18: 1-10.
- Gao L, Shen K, Yin N, et al. (2020) Comprehensive transcriptomic analysis reveals dysregulated competing endogenous RNA network in endocrine resistant breast cancer cells. Front Oncol 10: 600487.
- Lanceta L, O'Neill C, Lypova N, et al. (2020) Transcriptomic profiling identifies differentially expressed genes in palbociclib-resistant ER+ MCF7 breast cancer cells. Genes 11: 467.
- Rouhimoghadam M, Safarian S, Carroll JS, et al. (2018) Tamoxifen-induced apoptosis of MCF-7 cells via GPR30/PI3K/MAPKs interactions: Verification by ODE modeling and RNA sequencing. Front in Physiol 9: 907.
- Piryaei Z, Salehi Z, Ebrahimie E, et al. (2023) Meta-analysis of integrated chip-seq and transcriptome data revealed genomic regions affected by estrogen receptor alpha in breast cancer. BMC Med Genomics 16: 219.
- Zhu Z, Jiang H, Xie J, et al. (2022) Current evidence on circRNAs as potential theranostic markers for detecting chemo resistance in breast cancer: A systematic review and meta analysis. Sci Rep 12: 22016.
- Chu M, Fang Y, Jin Y (2021) CircRNAs as promising biomarker in diagnosis of breast cancer: An updated meta-analysis. J Clin Lab Anal 35: e23934.
- Kharrati-Koopaee H, Ebrahimie E, Dadpasand M, et al. (2019) Genomic analysis reveals variant association with high altitude adaptation in native chickens. Sci Rep 9: 9224.
- Kasirajan L, Hoang NV, Furtado A, et al. (2018) Transcriptome analysis highlights key differentially expressed genes involved in cellulose and lignin biosynthesis of sugarcane genotypes varying in fiber content. Sci Rep 8: 11612.
- Tsuyuzaki K, Nikaido I (2013) metaSeq: Meta-analysis of RNA-seq count data. Tokyo University of Science, Tokyo.
- Cheng M, Michalski S, Kommagani R (2018) Role for growth regulation by estrogen in breast cancer 1 (GREB1) in hormone-dependent cancers. Int J Mol Sci 19: 2543.
- Hodgkinson K, Forrest LA, Vuong N, et al. (2018) GREB1 is an estrogen receptor-regulated tumour promoter that is frequently expressed in ovarian cancer. Oncogene 37: 5873-5886.
- Pio R, Jia Z, Baron VT, et al. (2013) Early growth response 3 (Egr3) is highly over-expressed in non-relapsing prostate cancer but not in relapsing prostate cancer. PLoS One 8: e54096.
- Inoue A, Omoto Y, Yamaguchi Y, et al. (2004) Transcription factor EGR3 is involved in the estrogen-signaling pathway in breast cancer cells. J Mol Endocrinol 32: 649-661.
- Knudsen AM, Eilertsen I, Kielland S, et al. (2020) Expression and prognostic value of the transcription factors EGR1 and EGR3 in gliomas. Sci Rep 10: 1-13.
- Shin CH, Lee MG, Han J, et al. (2017) Identification of XAF1–MT2A mutual antagonism as a molecular switch in cell-fate decisions under stressful conditions. Proc Natl Acad Sci USA 114: 5683-5688.
- Tu SP, Liston P, Cui JT, et al. (2009) Restoration of XAF1 expression induces apoptosis and inhibits tumor growth in gastric cancer. Int J Cancer 125: 688-697.
- Pinto EM, Zambetti GP (2020) What 20 years of research has taught us about the TP53 p. R337H mutation. Cancer 126: 4678-4686.
- Børresen-Dale AL (2003) TP53 and breast cancer. Human Mutation 21: 292-300.
- Saha SK, Islam SR, Kwak KS, et al. (2020) PROM1 and PROM2 expression differentially modulates clinical prognosis of cancer: A multiomics analysis. Cancer Gene Ther 27: 147-167.
- Brugnoli F, Grassilli S, Al-Qassab Y, et al. (2019) CD133 in breast cancer cells: more than a stem cell marker. J Oncol 2019: 7512632.
- Guo J, Gong G, Zhang B (2017) Screening and identification of potential biomarkers in triple-negative breast cancer by integrated analysis. Oncol Rep 38: 2219-2228.
- Yi JM, Dhir M, Guzzetta AA, et al. (2012) DNA methylation biomarker candidates for early detection of colon cancer. Tumor Biol 33: 363-372.
- Wang X, Sun R, Hong X, et al. (2022) KLHL14: A novel prognostic biomarker and therapeutic target for ovarian cancer. J Oncol 2022: 9799346.
- Chen Z, Wu M, Liu J (2020) Kelch-like protein 14 promotes proliferation and migration of ovarian cancer cells. Int J Clin Exp Pathol 13: 2950-2961.
- Chang YC, Chiou J, Yang YF, et al. (2019) Therapeutic targeting of aldolase an interaction inhibits lung cancer metastasis and prolongs survival. Cancer Res 79: 4754-4766.
- Ji S, Zhang B, Liu J, et al. (2016) ALDOA functions as an oncogene in the highly metastatic pancreatic cancer. Cancer Lett 374: 127-135.
- Zhang F, Lin J D, Zuo XY, et al. (2017) Elevated transcriptional levels of aldolase A (ALDOA) associates with cell cycle-related genes in patients with NSCLC and several solid tumors. BioData Min 10: 6.
- Dolezal JM, Dash AP, Prochownik EV (2018) Diagnostic and prognostic implications of ribosomal protein transcript expression patterns in human cancers. BMC Cancer 18: 275.
- Zoppino FC, Guerrero-Gimenez ME, Castro GN, et al. (2018) Comprehensive transcriptomic analysis of heat shock proteins in the molecular subtypes of human breast cancer. BMC Cancer 18: 700.
- Cayado-Gutiérrez N, Moncalero VL, Rosales EM, et al. (2013) Downregulation of Hsp27 (HSPB1) in MCF-7 human breast cancer cells induces upregulation of PTEN. Cell Stress Chaperones 18: 243-249.
- Voduc D, Cheang M, Nielsen T (2008) GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer Epidemiol Biomarkers Prev 17: 365-373.
- Afzaljavan F, Sadr AS, Savas S, et al. (2021) GATA3 somatic mutations are associated with clinicopathological features and expression profile in TCGA breast cancer patients. Sci Rep 11: 1679.
- Takaku M, Grimm SA, Wade PA (2015) GATA3 in breast cancer: Tumor suppressor or oncogene? Gene Expr 16: 163-168.
- Zhang J, Hu S, Li Y (2019) KRT18 is correlated with the malignant status and acts as an oncogene in colorectal cancer. Biosci Rep 39: 141-149.
- Ha SA, Lee YS, Kim HK, et al. (2012) The prognostic potential of keratin 18 in breast cancer associated with tumor dedifferentiation, and the loss of estrogen and progesterone receptors. Cancer Biomark 10: 219-231.
- Hermani A, Shukla A, Medunjanin S, et al. (2013) Insulin-like growth factor binding protein-4 and-5 modulate ligand-dependent estrogen receptor-α activation in breast cancer cells in an IGF-independent manner. Cell Signal 25: 1395-1402.
- Akkiprik M, Peker I, Özmen T, et al. (2015) Identification of differentially expressed IGFBP5-related genes in breast cancer tumor tissues using cDNA microarray experiments. Genes 6: 1201-1214.
- Jiang T, Chen Z H, Chen Z, et al. (2020) SULF2 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells through the ERK/AKT signaling pathway. Braz J Med Biol Res 53: e8901.
- Zhu C, He L, Zhou X, et al. (2016) Sulfatase 2 promotes breast cancer progression through regulating some tumor-related factors. Oncology Rep 35: 1318-1328.
- Brown JM, Attardi LD (2005) The role of apoptosis in cancer development and treatment response. Nat Rev Cancer 5: 231-237.
- Wyganowska Swiatkowska M, Tarnowski M, Murtagh D, et al. (2019) Proteolysis is the most fundamental property of malignancy and its inhibition may be used therapeutically. Int J Mol Med 43: 15-25.
- Taylor KJ, Sims AH, Liang L, et al. (2010) Dynamic changes in gene expression in vivo predict prognosis of tamoxifen-treated patients with breast cancer. Breast Cancer Res 12: R39.
- Rouhimoghadam M, Safarian S, Carroll JS, et al. (2018) Tamoxifen-induced apoptosis of MCF-7 cells via GPR30/PI3K/MAPKs interactions: Verification by ODE modeling and RNA sequencing. Front Physiol 9: 907.
- Liu CY, Hung MH, Wang DS, et al. (2014) Tamoxifen induces apoptosis through cancerous inhibitor of protein phosphatase 2A-dependent phospho-Akt inactivation in estrogen receptor-negative human breast cancer cells. Breast Cancer Res 6: 1-15.
- Frasor J, Chang EC, Komm B, et al. (2006) Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res 66: 7334-7340.
- Nephew KP, Polek TC, Khan SA (1996) Tamoxifen-induced proto-oncogene expression persists in uterine endometrial epithelium. Endocrinology 137: 219-224.
Corresponding Author
Seyed Taghi Heydari, Health Policy Research Center, Institute of Heath, Shiraz University of Medical Sciences, Shiraz, Iran.
Copyright
© 2024 Koopaee HK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.