Statins as an Adjuvant Therapy for Psychotic Disorders: Current Evidence with a Systematic Overview of Double-Blind Placebo Controlled Trials
Abstract
Background
Evidence is accumulating that inflammatory processes play a role in the pathophysiology of schizophrenia. This suggests that anti-inflammatory drugs might have beneficial effects in the treatment of schizophrenia patients. Statins have cholesterol independent effects on inflammation and oxidative stress and could potentially improve positive, negative and/or cognitive symptoms of schizophrenia. We provide an overview of the literature regarding the effects of statins in the treatment of schizophrenia.
Methods
We conducted a systematic search using the PubMed (Medline) and Embase databases. Articles describing the effect of all possible types of statins in patients diagnosed with a schizophrenia spectrum disorder were included.
Results
We found four small Randomized Controlled Trials (RCTs) describing the effects of atorvastatin, lovastatin, pravastatin and simvastatin, in a total of 141 patients. No significant differences were reported when comparing statin treatment to placebo with regard to improvement of positive, negative and cognitive symptoms.
Conclusion
At present, there is no solid evidence for the efficacy of statins in the treatment of schizophrenia spectrum disorders. All four included RCTs evaluated relatively small sample sizes. Furthermore, these studies assessed effects over 6-12 weeks, while the effects of other anti-inflammatory medications have only been observed after 6 months. Large long-term RCTs should be conducted, in order to assess the effects of statins as an adjuvant therapy for schizophrenia more accurately.
Keywords
Statins, Schizophrenia, Psychosis
List of Abbreviations
BACS: Brief Assessment of Cognition in Schizophrenia; BARS: Barnes Akathisia Rating Scale; BBB: Blood-Brain-Barrier penetration; BPRS: Brief Psychiatric Rating Scale; CANTAB: Cambridge Neuropsychological Test Automated Battery; CI: Conference Interval; CGI: Clinical Global Impression; CNS: Central Nerve System; COX-2: Cyclooxygenase-2; CSF: Cerebro Spinal Fluid; CRP: C-Reactive Protein; CVD: Cardiovascular Disease; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders; GAF: Global Assessment of Functioning; MATRICS: Measurement and Treatment Research to Improve Cognition in Schizophrenia; MHC: Major Histocompatibility Complex; MRI: Magnetic Resonance Imaging; NAC: N-Acetylcysteine; NO: Nitrogen Oxide; NOS: Not Otherwise Specified; NSAIDs: Non Steroidal Anti-Inflammatories; PANSS: Positive and Negative Syndrome Scale; QOLS: Quality of Life Scale; RCT: Randomized Controlled Trial; SANS: Scale for the Assessment of Negative Symptoms; SHRS: St. Hans Rating Scale; SNP: Single Nucleotide Poly-Morphism
Introduction
Schizophrenia is a severely debilitating illness, which often results in psychological distress and poor functioning in daily life [1]. While antipsychotic medication is quite effective in reducing positive symptoms, they often cause adverse effects including extra pyramidal symptoms, weight gain, metabolic syndrome and drowsiness [2]. Moreover, negative and cognitive symptoms are insufficiently targeted by antipsychotics [3]. During the past years, the role of inflammatory processes in the pathophysiology of schizophrenia has gained interest, which suggests that anti-inflammatory drugs could serve as candidates for schizophrenia treatment [4].
Although well-regulated immune responses are essential to protect the body from infections, long-lasting inflammatory conditions can be harmful for the host [4]. Different lines of evidence suggest that low-grade inflammation in the central nervous system is involved in the pathogenesis of schizophrenia. These include previous findings of maternal immune alterations and prenatal infections significantly increasing the risk for schizophrenia and schizophrenia-related neurocognitive or neuroanatomical abnormalities in offspring [5]. In addition, autoimmune disorders and the number of infections requiring hospitalization have been identified as risk factors for schizophrenia [6]. Indeed, autoimmune disorders share several features with schizophrenia including onset, course and triggers of disease [7]. Furthermore, the use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and glucocorticosteroids for somatic disorders have been associated with decreased prevalence of schizophrenia in men [8,9]. Research on inflammatory parameters has also shown interesting findings. Numerous studies on cytokine alterations suggest a relationship between immunological processes and schizophrenia [for an extensive review, please refer to [10,11]. However, these studies struggle with small sample sizes and confounding factors such as smoking and metabolic syndrome [12]. A recent post-mortem study reported that signs of inflammation were found in brain tissue in more than 40% of schizophrenia patients [13]. Earlier studies have mentioned degradation products of inflammatory substances in brain tissue of schizophrenic patients [14]. Within the Cerebrospinal Fluid (CSF) of schizophrenia patients, this is as high as 50%. Lastly, genetic studies underline a possible immune component in the pathogenesis of schizophrenia, as a large pooled data-set of Single Nucleotide Polymorphism (SNP)-based genome-wide association studies has shown a link between schizophrenia and various markers located on the Major Histocompatibility Complex (MHC) region on chromosome 6p21.3-22.1 [15].
Several types of medication have been proposed to investigate the beneficial effects of anti-inflammatory medication. A meta-analysis including 26 double-blind RCTs described beneficial effects for aspirin (n = 270), estrogens (n = 262) and N-Acetylcysteine (NAC) (n = 140) [16]. These promising results have increased interest for the potential augmentation of antipsychotic therapy with anti-inflammatory agents for patients with a schizophrenia spectrum disorder. However, many of the investigated anti-inflammatory agents also exert side effects, especially within long-term use. Therefore, the search for effective anti-inflammatory agents that can be used on a long-term basis has continued [17].
Recently, the anti-inflammatory potential of statins has been highlighted [18]. Until now, statins are almost exclusively used in the treatment of cardiovascular diseases and have established themselves as safe and efficient cholesterol inhibitors. Apart from their lipid lowering effects, studies have shown that statins have cholesterol independent effects on inflammation and oxidative stress [19]. Statins have been widely prescribed during the past thirty years, thereby generating a vast amount of data on their efficacy and safety [19]. While statin therapy may cause symptomatic adverse events (e.g. muscle pain or weakness), a major review recently stated that adverse effects seem to have been disproportionally highlighted in previous reports, as these are only observed in up to 50-100 patients per 10,000 treated for five years [19].
In the CNS, cholesterol metabolism seems to be independently regulated from other parts of the body, which means that Blood-Brain-Barrier (BBB) penetration could be an important factor for statin efficacy [20]. Several types of statins, including lovastatin, pravastatin, atorvastatin and simvastatin, have been tested for their neuroprotective potential by Sierra, et al. [21]. It was described that statins with a high order of lipophilicity have a higher potential to cross the BBB, implying that lipophilic statins could have a more significant impact on the CNS. Not only potential BBB penetration, but also hypocholesterolaemia activity in neurons and biosafety of the statins were analyzed. The researchers described that among the nine statins studied, simvastatin presented the best characteristics for preventing neurodegenerative conditions, mainly for its high capacity to penetrate the BBB, its cholesterol lowering effect in neurons and neuroprotective potential in vitro.
The aim of this review is to provide insight into the influence of statins on the positive, negative and cognitive symptom severity in patients with a schizophrenia spectrum disorder.
Methods
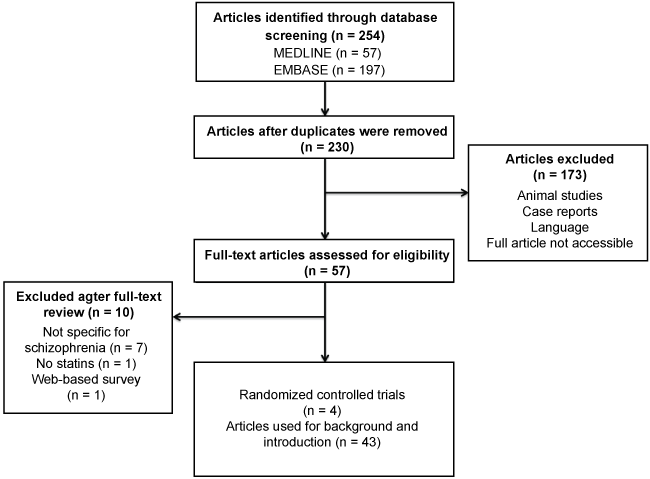
A systematic search was performed in the PubMed (Medline) and Embase databases, using combinations and synonyms of the following search terms: "Statin", "Cholesterol inhibitor", Hydroxymethylglutaryl-Coenzyme A inhibitor" in addition to one of the following diagnoses or syndromes: "Schizophrenia","negative syndrome", "positive syndrome", "dementia praecox" or "Psychosis". More details on the search are presented in Figure 1. After the duplicates were removed, two investigators independently screened all titles and abstracts. Articles describing both the direct effect of statins on psychotic symptoms and general effects of statins in psychotic patients were initially selected. Articles were excluded if they encompassed (i) animal studies, (ii) case reports or (iii) articles that were not originally available or translated in English or Dutch. Publication date was not an exclusion criterion, as the total number of results was limited, and older articles were still considered relevant. The full search strategy is supplemented as Appendix A. The search string was built by one investigator and reviewed by an experienced librarian. Search cut-off date was January 1st 2017.
Both investigators checked fifty-seven articles for full-text eligibility. Seven articles were excluded in which patients with more general psychiatric disorders were described, as specific information on schizophrenia or psychosis was not found. Another article did not specifically describe effects of statins. The final article that was excluded contained a web-based survey in which the methodology was not described explicitly. Judgment differences were resolved through a discussion between the two authors and consensus was achieved. Reference lists of included articles were used to further complete the information used for this review. The remaining forty-seven articles were divided within Randomized Controlled Trials (RCTs) and articles that were used for background information.
Results
Conducted clinical trials
Four RCTs were retrieved that met our inclusion criteria, assessing the effects of four different statins on symptoms of schizophrenia in patients with a schizophrenia spectrum disorder. More detailed information on these four studies is presented in Table 1.
Atorvastatin
Atorvastatin was described as a drug that can potentially influence endothelial Nitric Oxide (NO) synthesis and suppress inflammation [22]. This study mainly focused on the effects of atorvastatin on negative symptoms. Participants had a minimum score of 50 on the Positive and Negative Syndrome Scale (PANSS)-[23] and at least 10 points on the Scale for the Assessment of Negative Symptoms (SANS)-[24]. The participants were randomly assigned to receive 20 mg atorvastatin plus risperidone (6 mg/day) or risperidone (6 mg/day) plus placebo. Both groups showed a significant reduction of negative symptoms over the course of 6 weeks, which could largely be attributed to risperidone. It was described that the SANS score in the treatment group did decrease more in the treatment group compared to the placebo group, but this result was not statistically significant (p = 0.068). The authors did calculate that the result would have been significant after 8 to 12 weeks, provided that the difference in SANS score between the two groups would increase at equal speed. This line of reasoning is questionable however, as another study with pravastatin only showed an increased difference in symptoms between statin and placebo groups during the early treatment phase, while this difference could not be maintained for a longer period of time. The limited treatment period, small study population, and absence of monitoring of positive symptoms are major limitations of this study.
Lovastatin
Ghanizadeh, et al. [25] performed a clinical trial to assess the effect of lovastatin on the overall symptoms of schizophrenia, given its anti-inflammatory effects. Patients with a schizophrenia diagnosis were randomized in a 1:1 ratio to lovastatin (20 mg/day) plus risperidone (2 to 8 mg/day) or placebo plus risperidone. Results were obtained after 60 days, using the PANSS as main outcome measure. The PANSS scores did significantly decrease in both groups over time but there was no significant difference between the two groups (p = 0.3). The decrease in PANSS scores might be caused by risperidone treatment. The hypothesis that statin treatment would lead to symptomatic improvement was not supported by these results. The authors provided several explanations. Firstly, they stated that the immune hypothesis and the role of IL-6 are still not fully understood, while it is also unclear which cytokines are suppressed by lovastatin. Secondly, all included patients were inpatients, who are generally severely ill. Additional limitations were a small sample size and short study duration. Similarly to pravastatin, lovastatin's ability to cross the BBB is poor [21].
Pravastatin
Vincenzi, et al. [26] chose to investigate the role of pravastatin on inflammatory markers (including CRP), lipid and glucose metabolism, PANSS scores and cognition in subjects with schizophrenia and schizo affective disorder [26]. Outcome measures were assessed at baseline, 6 weeks, and 12 weeks. No significant reduction in CRP levels, PANSS scores or cognitive functioning was observed in the treatment group over 12 weeks of treatment compared with placebo. The authors did find a significant decrease in the PANSS positive symptoms score at 6 weeks as compared to baseline scores for the pravastatin group, but this difference failed to remain significant after 12 weeks. The authors proposed that the pravastatin dose used in their study was too low to maintain effects after 6 weeks. In addition, the authors propose that increasing the sample size (n = 49) could yield more accurate results, as it allows for multiple testing and subgroup analysis.
Simvastatin
Chaudhry, et al. [27] investigated the effects on PANSS scores of simvastatin or ondansetron, a serotonin (5-HT3) receptor antagonist with limited effects on cytokine production, in addition to treatment as usual, for a period of 12 weeks. PANSS scores were the main outcome of this rater-blind, placebo-controlled study. The PANSS outcomes do not differ between simvastatin compared to treatment as usual after 12 weeks (95% Confidence Interval (CI): PANSS positive: - 6.12 to + 0.73; PANSS negative: - 3.12 to + 3.11; PANSS total: - 17.05 to + 3.81). Chaudhry, et al. [27] proposed that simvastatin's anti-inflammatory properties might work better in patients experiencing early psychosis, compared to patients with established schizophrenia.
Side effects
The four RCTs included within this review did not describe increased side effects caused by statin treatment compared to placebo. The reported side-effects mentioned in both groups where headache, head cold, slowness of movement, increased appetite, weight gain, decreased energy, muscle rigidity, weakness, memory problems, dizziness, restlessness, constipation and drowsiness during the day [22,25-27]. Vincenzi, et al. [26] did describe muscle soreness reported by 5 subjects within the pravastatin group (n = 24) compared to 1 subject within the placebo group (n = 25); it was not tested for significance. None of the included RCTs described serious adverse events.
Pending trials
Currently, there are three ongoing trials in which statins are used to treat schizophrenia spectrum disorder patients, which have been found by searching the ClinicalTrials.gov database.
Simvastatin and ondansetron
After conducting the initial pilot study, Chaudhry, et al. [27] are now conducting a second, larger RCT to assess the effects of simvastatin and/or ondansetron to treatment as usual (ClinicalTrials.gov identifier: NCT01602029). A large number of patients (n = 216) will be randomized into four different groups, which is slightly different compared to their pilot study in which the patients were randomised into three groups (ondansetron, simvastatin or placebo). The four study treatment arms are as follows: ondansetron (8 mg) and simvastatin (40 mg) (n = 54), ondansetron and placebo (8 mg) (n = 54), simvastatin and placebo (40 mg) (n = 54) versus two placebos (n = 54) will be used in a 2 × 2 design. The first four weeks, the patients will use 20 mg once daily; then the dose will be titrated to 40 mg once daily. Patients will be treated with study medication for 26 weeks in total. The following outcomes will be evaluated after treatment duration of 26 weeks: improving positive symptoms, social functioning, cognitive functioning and other additive effects of adding study medication on treatment as usual. Symptom severity will be assessed by using the PANSS, Clinical Global Impression (CGI), Global Assessment of Functioning (GAF), Quality of Life Scale (QOLS-[28]) social functioning scale and additionally cognitive assessments will be assessed using the seven domains of the Cambridge Neuropsychological Test Automated Battery (CANTAB-[29]). This study has been completed and a total of 303 patients have been enrolled. Results are not yet published.
Simvastatin
Currently, a large RCT is being conducted by our group (ClincialTrials.gov identifier: NCT01999309) on the effects of 40 mg/day simvastatin. This study will include a larger sample size compared to previous clinical trials (n = 250), as well as a longer treatment duration (12 months). The study focuses on recent-onset patients with a schizophrenia spectrum disorder DSM-IV diagnosis. The onset of the first psychotic episode can be no longer than three years ago. PANSS scores and cognitive functioning as measured by the Brief Assessment of Cognition in Schizophrenia (BACS-[30]) are the primary outcomes. Secondary outcomes include the evaluation brain tissue loss by Magnetic Resonance Imaging (MRI). Additionally, the presence and severity of metabolic syndrome and degree of movement disorders will be evaluated by using the Barnes Akathisia Rating Scale (BARS-[31]) and the St. Hanss Rating Scale (SHRS-[32]). A total of 100 patients are currently enrolled, the final inclusion is estimated around March 2018.
Fluvastatin
Buchanan, et al. assesses the effects of combined anti-inflammatory therapy, including: fluvastatin (40 mg/day), salsate (4mg/day) and omega-3-fatty acids eicosapentaenoic and docosahexaenoic (2 mg/day), as compared to placebo therapy (ClinicalTrials.gov identifier: NCT01514682). Fluvastatin is thought to have similar anti-inflammatory properties as earlier described statins (e.g. atorvastatin, simvastatin). However, a combination therapy is chosen to maximize the possibility of pronounced therapeutic effects. Primary outcomes are positive symptoms using the Brief Psychiatric Rating Scale (BPRS-[33]) positive item total score, in addition to cognitive performance for which the Measurement and Treatment Research to Improve Cognition in Schizophrenia Concensus Cognitive Battery (MATRICS-[34]) composite score will be used. A total of 39 patients are currently enrolled, the final data collection is scheduled for April 2017.
Discussion
The aim of this review was to assess the available RCTs that investigated the effects of statins on the positive, negative and cognitive symptoms of schizophrenia. Until January 1st 2017, four RCTs have investigated the effect of statins in the treatment of schizophrenia spectrum disorder. None of these trials have found significantly beneficial results for add-on statin treatment in comparison to placebo. No significant effects were found on positive, negative and cognitive symptoms at the end of the treatment. The only significant reduction on PANSS positive score was found after 6 weeks of pravastatin treatment, while this effect was not found after a longer treatment period (12 weeks). Three larger RCTs investigating statins in schizophrenia are still ongoing, and further results are expected around the end of 2020.
The four completed RCTs did not describe any side serious adverse events. There were limited differences in side effects of statin use compared to placebo; muscle soreness was more frequently reported by pravastatin users compared to placebo. However, it was not described whether it was significant.
Currently evidence for efficacy for positive, negative and cognitive symptoms in schizophrenia of statin treatment is lacking for various reasons. Firstly, although evidence suggests that inflammatory processes could play a role in the psychopathology of schizophrenia, this has not yet been proven [11]. In addition, promising studies on inflammatory markers in the blood and liquor of schizophrenia spectrum disorder patients have not yet established consistent markers of disease, which makes it difficult to evaluate effects of anti-inflammatory treatment on inflammation in the CNS [10]. Secondly, it is still uncertain whether statins' inflammatory properties could be sufficient in decreasing inflammation in the CNS [25]. Lastly, Ghanizadeh, et al. [25] described the possibility of pharmacological interactions between risperidone and lovastatin. Pharmacological interaction between antipsychotics and statins could possibly decrease the (anti-inflammatory) effects of statin treatment.
Although the results of the four completed RCTs did not show any significant differences, add-on statin therapy could still be a promising treatment for schizophrenia for various reasons. First, several methodological shortcomings were observed in the included trials. Every RCT was performed within a relatively small time frame (6-12 weeks), while studies describing effects of non-statin anti-inflammatory medication (e.g. NAC) on schizophrenia patients only showed clear evidence for efficacy after six months [35]. Second, as described by Sayyah, et al. [22], Ghanizadeh, et al. [25] and Vincenzi, et al. [26], small or medium effects would be expected for add-on statin treatment; as sample sizes in the four clinical trials were relatively small (n = 30-49), these studies may have been underpowered. Increased sample sizes and studies with longer duration could be more beneficial in demonstrating effects of adjuvant statin therapy. Third, statins differ in their lipophilicity, e.g. their potential to cross the BBB and their neuroprotective capabilities. Sierra, et al. [21] has recently stated that simvastatin presents the best characteristics as potential neuroprotectant, when compared to other statins in vitro. Pravastatins and lovastatins limited potential to cross the BBB could make them less suitable candidates for suppressing inflammation in the CNS compared to simvastatin and fluvastatin. Fourth, although the effects of statins on inflammation in the CNS might still be unknown, the adverse side effects of statins are well studied. Large scale evidence from RCTs points to the enormous effect of statins in lowering cholesterol, while side-effects from appropriately dosed medication seems to be minimal. This is in contrast to other anti-inflammatory drugs such as aspirin, which needs to be combined with gastric protection to prevent gastrointestinal bleedings, and estrogen, which needs to be combined with progesterone when it is used for more than 2 months [16]. Lastly, cardiovascular risk factors in schizophrenia patients should not be underestimated. Cardiovascular death occurs more frequent in schizophrenia patients compared to non-psychiatric persons, and is the most common cause of death in this group [36]. Previous studies have already stated that schizophrenia patients are less likely to receive appropriate dosage of cardiovascular medication compared to non-schizophrenia persons, despite of increased risk factors such as metabolic syndrome, smoking, weight increase and a sedentary lifestyle [37]. Consensus guidelines for early detection and prevention of CVD in schizophrenia would be a good first step towards decreasing mortality in schizophrenia patients [38]. Given the fact that schizophrenia spectrum disorder patients are often characterized by high levels of cardiovascular risk factors statin treatment combining anti-inflammatory with cardio protective properties may provide a double-hit effect in the treatment of psychosis.
Conclusion
This review identified four RCTs which have not shown significant beneficial effects of statins in the treatment of positive, negative and cognitive symptoms in schizophrenia spectrum disorder patients. However, given the established safety of statins, their valuable effects on cardiovascular symptoms in at-risk individuals and their anti-inflammatory properties make statins theoretically an interesting adjuvant therapy for schizophrenia. Larger and longer RCTs are required to assess the potential of statins more accurately to reduce both specific schizophrenia symptoms and cardiovascular mortality in patients with a schizophrenia spectrum disorder.
References
- Rössler W, Joachim Salize H, Van Os J, et al. (2005) Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol 15: 399-409.
- Bumb JM, Enning F, Leweke FM (2015) Drug repurposing and emerging adjunctive treatments for schizophrenia. Expert Opin Pharmacother 16: 1049-1067.
- Brown HE, Roffman JL (2016) Emerging Treatments in Schizophrenia. Harv Rev Psychiatry 24: e1-e7.
- Garcia-Bueno B, Bioque M, Mac-Dowell KS, et al. (2014) Pro-/Anti-inflammatory Dysregulation in Patients With First Episode of Psychosis: Toward an Integrative Inflammatory Hypothesis of Schizophrenia. Schizophr Bull 40: 376-387.
- Brown AS, Hooton J, Schaefer CA, et al. (2004) Elevated Maternal Interleukin-8 Levels and Risk of Schizophrenia in Adult Offspring. Am J Psychiatry 161: 889-895.
- Benros ME, Nielsen PR, Nordentoft M, et al. (2011) Autoimmune Diseases and Severe Infections as Risk Factors for Schizophrenia: A 30-Year Population-Based Register Study. Am J Psychiatry 168: 1303-1310.
- Knight JG, Menkes DB, Highton J, et al. (2007) Rationale for a trial of immunosuppressive therapy in acute schizophrenia. Mol Psychiatry 12: 424-431.
- Laan W, Selten JP, Grobbee DE, et al. (2007) Non-steroidal anti-inflammatory drugs and the risk of psychosis. Eur Neuropsychopharmacol 17: 309-311.
- Laan W, Smeets H, de Wit NJ, et al. (2009) Glucocorticosteroids Associated With a Decreased Risk of Psychosis. J Clin Psychopharmacol 29: 288-290.
- Miller BJ, Buckley P, Seabolt W, et al. (2011) Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol Psychiatry 70: 663-671.
- Norbert Müller, Elif Weidinger, Bianka Leitner, et al. (2015) The role of inflammation in schizophrenia. Front Neurosci 9: 372.
- Koola MM (2016) Cytokines in Schizophrenia: Hope or Hype? Indian J Psychol Med 38: 97-100.
- Fillman SG, Sinclair D, Fung SJ, et al. (2014) Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry 4: e365.
- Körschenhausen DA, Hampel HJ, Ackenheil M, et al. (1996) Fibrin degradation products in post mortem brain tissue of schizophrenics: a possible marker for underlying inflammatory processes. Schizophr Res 19: 103-109.
- Stefansson H, Ophoff RA, Steinberg S, et al. (2009) Common variants conferring risk of schizophrenia. Nature 460: 744-747.
- Sommer IE, Van Westrhenen R, Begemann MJH, et al. (2014) Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: An update. Schizophr Bull 40: 181-191.
- Begemann MJH, Schutte MJL, Slot MIE, et al. (2015) Simvastatin augmentation for recent-onset psychotic disorder: A study protocol. BBA Clin 4: 52-58.
- Jain MK, Ridker PM (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 4: 977-987.
- Collins R, Reith C, Emberson J, et al. (2016) Review Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388: 2532-2561.
- Fünfschilling U, Saher G, Xiao L, et al. (2007) Survival of adult neurons lacking cholesterol synthesis in vivo. BMC Neurosci 8: 1.
- Sierra S, Ramos MC, Molina P, et al. (2011) Statins as neuroprotectants: A comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis 23: 307-318.
- Sayyah M, Boostani H, Ashrafpoori M, et al. (2015) Effects of Atorvastatin on Negative Sign in Chronic Schizophrenia: a Double Blind Clinical Trial. Iran J Pharm Res 14: 1269-1274.
- Kay SR, Opler LA, Lindenmayer JP (1989) The Positive and Negative Syndrome Scale (PANSS): Rationale and standardisation. Br J Psychiatry 155: 59-65.
- Andreasen NC (1983) Scale for the Assessment of Negative Symptoms. Br J Psychiatry 49-58.
- Ghanizadeh A, Rezaee Z, Dehbozorgi S, et al. (2014) Lovastatin for the adjunctive treatment of schizophrenia: A preliminary randomized double-blind placebo-controlled trial. Psychiatry Res 219: 431-435.
- Vincenzi B, Stock S, Borba CPC, et al. (2014) A randomized placebo-controlled pilot study of pravastatin as an adjunctive therapy in schizophrenia patients: Effect on inflammation, psychopathology, cognition and lipid metabolism. Schizophr Res 159: 395-403.
- Chaudhry IB, Husain N, Drake R, et al. (2014) Add-on clinical effects of simvastatin and ondansetron in patients with schizophrenia stabilized on antipsychotic treatment: pilot study. Ther Adv Psychopharmacol 4: 110-116.
- Flanagan J (1982) Measurement of quality of life: current state of the art. Arch Phys Med Rehabil 63: 56-59.
- Robbins TW, James M, Owen AM, et al. (1994) Cambridge Neuropsychological Test Automated Battery (CANTAB): A Factor Analytic Study of a Large Sample of Normal Elderly Volunteers. Dementia 5: 266-281.
- Keefe RSE, Poe M, Walker TM, et al. (2006) The Relationship of the Brief Assessment of Cognition in Schizophrenia (BACS) to Functional Capacity and Real-world Functional Outcome. J Clin Exp Neuropsychol 28: 260-269.
- Barnes TRE (1989) A rating scale for drug-induced akathisia. Br J Psychiatry 154: 672-676.
- Gerlach J, Korsgaard S, Clemmesen P, et al. (1993) The St. Hans Rating Scale for extrapyramidal syndromes: reliability and validity. Acta Psychiatr Scand 87: 244-252.
- Overall J, Gorham D (1962) The Brief Pyschicatric Rating Scale (BPRS). Psychol Rep 10: 799-812.
- Nuechterlein KH, Green MF, Kern RS, et al. (2008) The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. Am J Psychiatry 165: 203-213.
- Berk M, Copolov D, Dean O, et al. (2008) N-Acetyl Cysteine as a Glutathione Precursor for Schizophrenia-A Double-Blind, Randomized, Placebo-Controlled Trial. Biol Psychiatry 64: 361-368.
- Shulman M, Miller A, Misher J, et al. (2014) Managing cardiovascular disease risk in patients treated with antipsychotics: a multidisciplinary approach. J Multidiscip Healthc 7: 489-501.
- Blackburn R, Osborn D, Petersen I, et al. (2014) Patterns of statin prescribing for the primary prevention of cardiovascular disease in people with severe mental illness. Pharmacoepidemiol Drug Saf 23: 200-201.
- Azad MC, Shoesmith WD, AI Mamun M, et al. (2016) Cardiovascular diseases among patients with schizophrenia. Asian J Psychiatr 19: 28-36.
Corresponding Author
Lyliana G Nasib, Department of Psychiatry, University Medical Center Utrecht (UMCU), PO box, 3508 GA Utrecht, Netherlands, Tel: +318-8755-1460.
Copyright
© 2017 Postma TS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.