Perinatal Administration of Caffeine in Mice Affects the Spatial Memory and Motor Coordination Later in Adult Life
Abstract
Data emerging that maternal consumption of moderate amount of caffeine will affect the development of the fetus and later the cognitive function of the brain. The aim of this study was to determine the effect of caffeine administration in pregnant mice on the learning and memory and motor coordination of offspring's at one and a half months of age. Two groups of animals were assigned, in which pregnant mice were given caffeine mixed with water (60 mg/kg/day) during their entire pregnancy and postnatally till weaning and a second group of pregnant mice which were given drinking water without caffeine. Only male offsprings were later chosen to be enrolled in the experiments. Learning and memory were assessed by Morris-Water maze test which consisted of 140 cm diameter circular swimming pool where the animals were trained to locate a submerged platform as it was the only escape from swimming. At the end a probe final test was performed where the platform was removed and the time spent in each quadrant of the maze was recorded. The latency to reach the platform reflects the learning and memory of the tested animals. Other tests were used to assess sociability, preference for social novelty, locomotion and anxiety. The results showed that the perinatally caffeine exposed offsprings reached the hidden platform with longer latency than the control animals in the water-maze test. Similarly, the distance swum to reach the platform was significantly higher in the perinatal caffeine exposed offspring than the control. No significant differences were calculated between the control and perinatally exposed mice to caffeine regarding social behavior and anxiety. Motor coordination measured by rotarod showed significant differences between the two groups. We concluded that moderate and chronic caffeine administration to the pregnant mice during their entire pregnancy and postnatally till the time of weaning can affect the hippocampal spatial memory and motor coordination, while anxiety and social behavior were not significantly affected.
Keywords
Caffeine, Brain development, Mice, Learning and memory, Motor coordination, Social behavior
Introduction
Caffeine is found in coffee, tea, cola drinks and more other food staffs [1]. It is the most widely consumed material by humans due to its psychostimulant effects. Its effects on enhancing memory and concentration were reported frequently [2-4]. Psychostimulants elicit their psychomotor-activating and reinforcing effects by increasing the central dopamine neurotransmission. Since the psychostimulant effect of caffeine is done by its ability to inhibit adenosine receptors, the role of adenosine in the control or modulation of the central dopamine neurotransmission were extensively debated [5]. Prolonged use of caffeine resulted into adenosine receptors expression changes [6] and may be involved in potentiation of the addictive and toxic effects of drugs of abuse [5,7]. Caffeine is a water and fat soluble material; it can cross easily many tissue barriers like the blood-brain-barrier and placenta to reach the brain and the growing fetus [8]. It can also be secreted in milk and even semen. The main mechanism of action of caffeine is its ability to antagonize the adenosine receptors [9,10]. Adenosine is known as a neuromodulator in the central nervous system which is released after prolonged neuronal stimulation and resulted in inhibition of neuronal circuits leading into neuron fatigue, and resting. Besides, serotonergic system development was shown to be defected in chicken with perinatal caffeine administration [11]. Silva, et al. [12] showed that moderate caffeine intake during pregnancy resulted in delayed migration of gamma-amino-butyric acid (GABA) neurons and an increased seizures susceptibility in mice pups.
It is well known that the mechanisms involved in brain development, including cell migration, synaptic formations and proper neuronal circuits wiring could be affected by external influences like drugs or environmental factors [13-15]. Ability of caffeine to cross the placenta makes it readily available in the growing fetal brain, and may eventually alter the normal development of the central nervous system. Moreover, data showed that perinatal caffeine exposure resulted into intra uterine growth retardation and miscarriage [16].
The aim of this study was to investigate the effect of perinatal caffeine exposure on the higher brain functions including learning and memory as well as behavioral changes and motor coordination. Our main finding showed defects in learning and memory in the mice exposed to perinatal caffeine with changes in anxiety and social behaviors.
Materials and Methods
Animals
Adult (two months old) BALB/c mice were randomly chosen to form two groups of 8 couples. In the beginning of the experiment, the normal daily water consumption of each mouse was measured. Caffeine administration was controlled by dissolving the required dose in the measured daily water requirement. The females of one group were given caffeine (caffeine 99%, C8H10N4O2, Alfa Aesar GmbH-Germany) mixed with drinking water (60 mg/kg/day) and after the 7th day of their pregnancy when they were separated from the males till the time of weaning of the offsprings. The off springs were raised with normal caffeine-free water till the age of 6 weeks. Complete behavioral and cognitive tests were conducted for these animals, and the results were compared to normal control mice subjected to the same protocol but not exposed to caffeine. Following the litter-based design [17], one mouse from each litter was randomly chosen to form the two groups (n = 8 in each group). Animals were fed ad libitum and weighed weekly. All experimental protocols were performed according to the National Institutes of Health (NIH) Guiding Principles in the Care and Use of Animals which are implicated by the guidelines of the Arabian Gulf University for laboratory animal's research.
The tests
Behavioral tests were conducted between 9:00 and 18:00. All behavioral tests were conducted and scored blindly. All sessions, except the rotarod, were videotaped and scored accordingly. Each parameter was scored by one observer for all mice to eliminate any potential inter-observer variability.
Morris Water Maze test (MWM): Water maze measures spatial learning and memory [18]. The apparatus consisted of a circular swimming pool [140 cm diameter and 50 cm height, filled to a depth of 30 cm with water (26 °C-28 °C)] in which a submerged platform (8 cm diameter, 1 cm below surface) was hidden on a fixed location (50 cm from the edge of the pool). The animal could climb onto the platform to emerge from the water and escape from the necessity of swimming. During a series of trials, each mouse was trained to locate the platform. The maze was housed in a darkened room with visual cues and illuminated by sparse red light. Each mouse was given five acquisition sessions in first day (training day) of the experiment to learn the position of a hidden. On each session, the mice were released successively from four predetermined positions on the perimeter of the pool. Animals were given a maximum of 2 min to find the platform, and were allowed to remain on the platform for 30 s. Mice that failed to locate the platform were put onto it by the experimenter and allowed to stay there for 30 s. After 48 hours, three test sessions were done to determine the memory of each animal to locate the hidden platform. The position and movement of the animals in the pool, were captured and analyzed every 0.2 s, using a video-camera computer system, and ANY-maze video- tracking system [Stoelting Co., Wood Dale, IL, and USA]. Outcome measures were latency time and distance swum to reach the platform. Performance in each trial was averaged to yield one data point per mouse per test. Speed of swimming was measured as control between the groups. In the following day, a probe test was performed in which the platform was removed and each animal was allowed to swim for 120 s. The percentage of time spent by the animals in each quadrant of the pool was determined.
Three-chambers social apparatus (Crawley's sociability and preference for social novelty test): Sociability and preference for social novelty was assessed using the Three-chamber social apparatus, as described previously [19]. The apparatus comprised a rectangular, three-chambered box. Each chamber was 20 cm × 40 cm × 22 cm. The walls were made from clear Plexiglas. The dividing walls (made from the same material) had small square openings (5 cm × 3 cm) allowing access into each chamber. Each chamber contained a circular wire cage which was 11 cm high, with a bottom diameter of 9 cm and bars spaced 0.5 cm apart. The subject mouse was first placed in the middle chamber to habituate for 5 min. Session 1 was started when an unfamiliar male (stranger 1) that had no prior contact with the subject mouse was placed inside the wire cage in one of the side chambers while the subject mouse was allowed to explore the entire apparatus freely. The placement of stranger 1 in the left or right side chambers was systematically alternated between trials. Session 1 continued for 10 min, and the time spent in each chamber as well as the number of chamber entries were recorded. Immediately after the 10 min of session 1finished, session 2 started with a second unfamiliar mouse being placed in the wire cage inside the chamber that had been empty during the first session. The test mouse had a choice between the chamber containing the already investigated mouse (stranger 1), and the one containing the novel unfamiliar mouse (stranger 2). The same parameters recorded for session 1 were recorded for session 2. The apparatus was cleaned with 70% ethanol and water between subjects. Three trials were done for each mouse. Session 1 tests for sociability (or social motivation/affiliation), which is spending significantly more time in the chamber containing a mouse than in the empty chamber. Session 2 tests for preference for social novelty, which is spending significantly more time in the chamber containing the novel mouse than the one containing the already investigated mouse.
Elevated plus maze test: The elevated plus maze test is for anxiety-like behavior [20]; it consisted of two open arms (25 cm × 5 cm) and two enclosed arms of the same size with 15-cm-high walls. The arms were elevated 55 cm above the floor. To minimize the likelihood of animals falling from the apparatus, 3-mm-high walls surrounded the sides of the open arms. Arms of the same type were located opposite from each other. Each mouse was individually placed in the central square of the maze (5 cm × 5 cm), facing one of the closed arms and allowed to freely explore the apparatus. Mouse behavior was recorded during a 10-min test period. The number of entries into an arm and the time spent in the open and enclosed arms were recorded. Percentage of entries into open arms, time spent in open arms (s), and total number of entries was analyzed. Entering the open arms less frequently and spending less time in them were indicative of anxiety-like behavior. The apparatus was cleaned with 70% ethanol between subjects. An open- or closed-arm entry was defined as all four paws in an arm. The numbers of open- and closed-arm entries were combined to yield a measure of total entries, which reflected general activity during the10 min test.
Rotarod test: Motor coordination and balance were assessed using an accelerating rotarod. Mice were placed on a cylinder that rotates at pre-assigned speed which increased by 0.5 cm/s every 5 sec. Each mouse was tested for three trials. Latency to fall from the rotating rod was recorded with a maximum trial length of 300 s. The two groups of mice were tested within the same experiment to allow comparison of baseline motor performance. All testing was done on the same day.
Statistical analysis
All data are presented as Mean ± SEM. Between the groups differences were calculated by the t-test paired two samples for means. Statistical analysis was conducted using SPSS package (version PASW Statistic 18.0.3).
Results
The birth weight was significantly lower in the pups of caffeine-administered mothers (1.44 ± 0.04 g versus control animals birth weight of 1.7 ± 0.06 g, t-test p < 0.05, t Critical = 2.365). At six weeks of age, and before testing, no body weight differences were recorded between the control (18.4 ± 0.5 g) and the perinatal caffeine-exposed (17.9 ± 0.3 g) mice (t-test, p = 0.365, t Critical = 3.365) (Table 1).
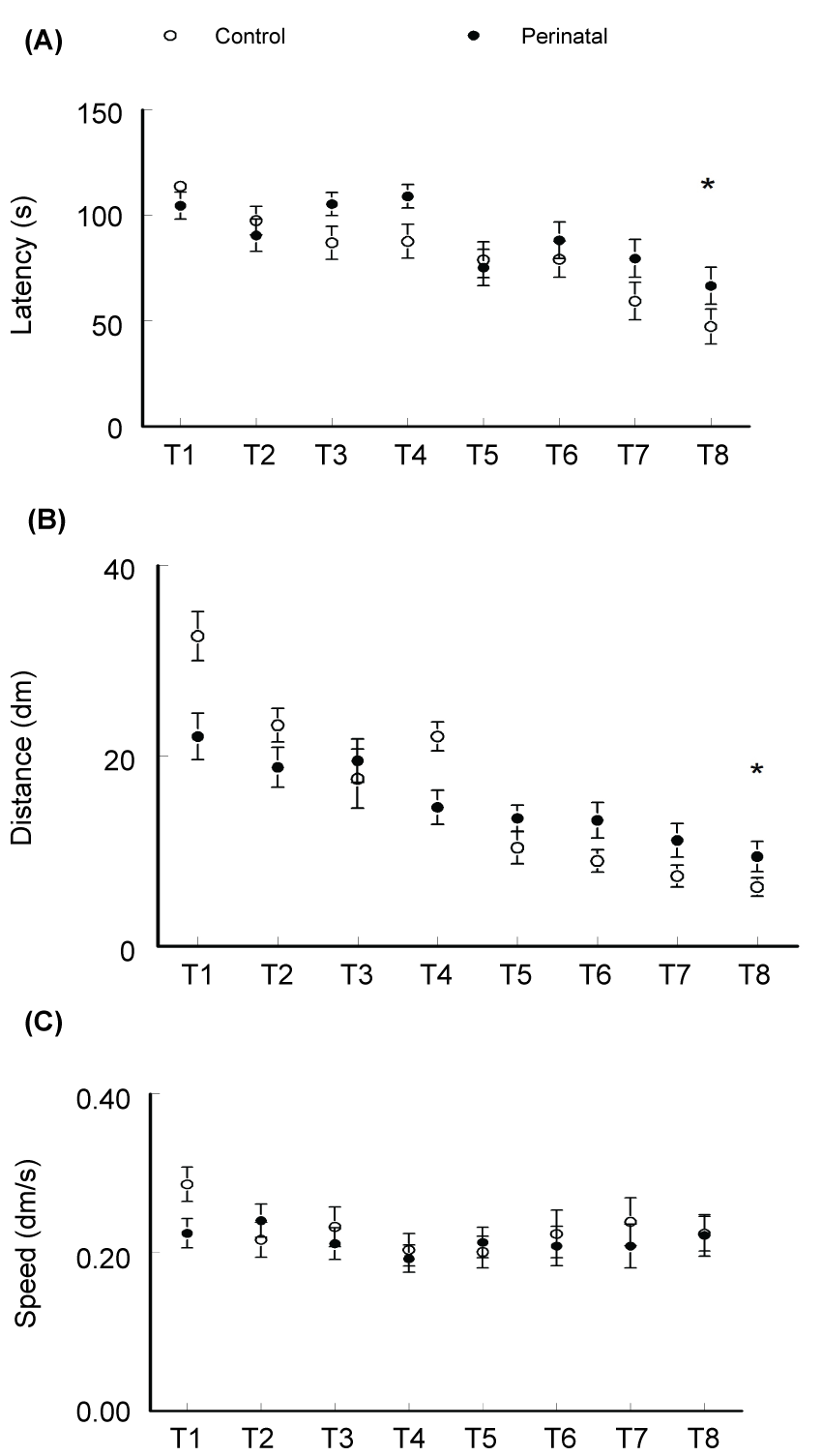
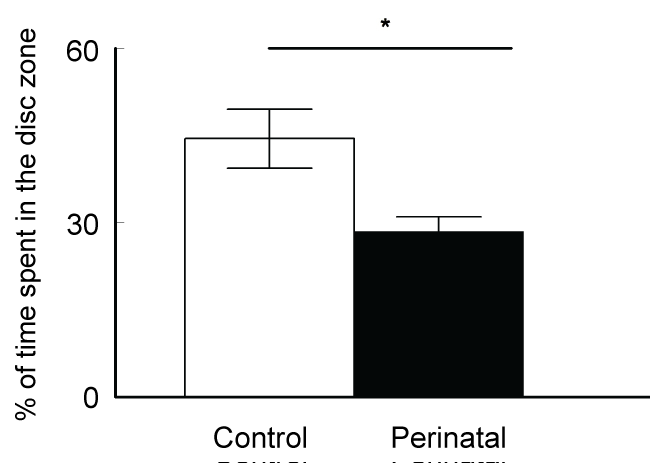
Testing the animals for memory in the water maze revealed significant differences between the groups. The latencies to reach the hidden platform in the maze with repeated testing represent a direct measure of learning and memory functions of the tested animal. In the last (8th) trial the control animals could reach the platform with an average latency of 47.3 ± 5.1 s compared to 66.6 ± 7.8 s of the caffeine-exposed group (t-test p < 0.05, t Critical = 1.697, figure 1A). Similarly, calculation of the distance swam by the animals to reach the platform showed significantly higher values measured in the caffeine-exposed mice (t-test p < 0.05, t Critical = 1.697, figure 1B). Figure 1C showed no significant differences between the two groups regarding the speed of swimming in the maze (t-test p = 0.46, t-Critical = 1.6955). This may indicate that the better achievement of the control animals in the water maze test was not due to differences in the speed of swimming. In the following day, the platform was removed and each animal was allowed to swim for 120 s. In this probe trial, the selective search strategy was measured. It showed that the control animals spend significantly more time in the target quadrant than would be expected by chance (25%) (t-test; p < 0.001, t-Critical two-trials = 1.997) while the caffeine-exposed group failed to spend significant time in the target quadrant (t-test, p = 0.162, t-critical two-trials = 1.998, figure 2). Statistical analysis showed significant differences between the two group (t-test p < 0.005, t Critical = 1.6694).
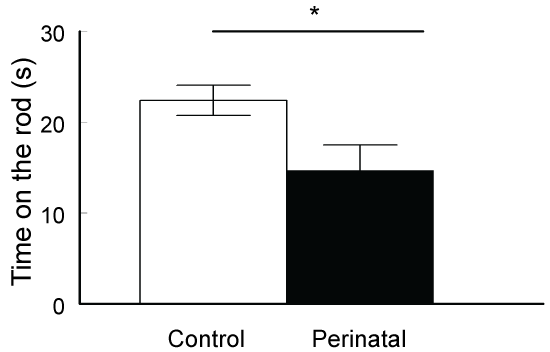
Motor coordination and balance were tested by the rotarod. The time the animal could stay on a rotating rod was calculated. The control mice stayed on the rotating rod before falling (22.4 ± 1.7 s) significantly longer time than the perinatally exposed animals with caffeine (14.7 ± 2.8 s; t-test p < 0.05, t Critical = 1.6838, figure 3).
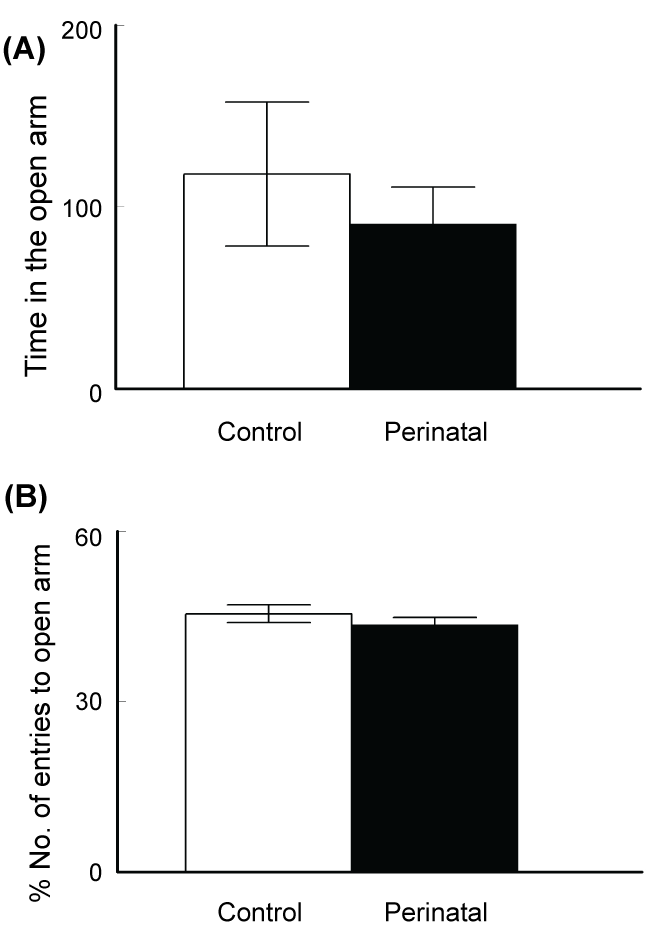
Testing for anxiety like behavior in the elevated plus maze test revealed no significant differences between the two groups. Both, the time spent in the open arm (118 ± 39.6 s for the control and 90.6 ± 20.1 for the caffeine-exposed mice, t-test p = 0.28, t Critical = 1.795; figure 4A) and percentage number of entries to the open arm (control: 45.5 ± 1.5%, caffeine exposed group: 43.5 ± 1.3%, t-test p = 0.18, t Critical = 1.771; figure 4B) were not significantly different between the two groups.
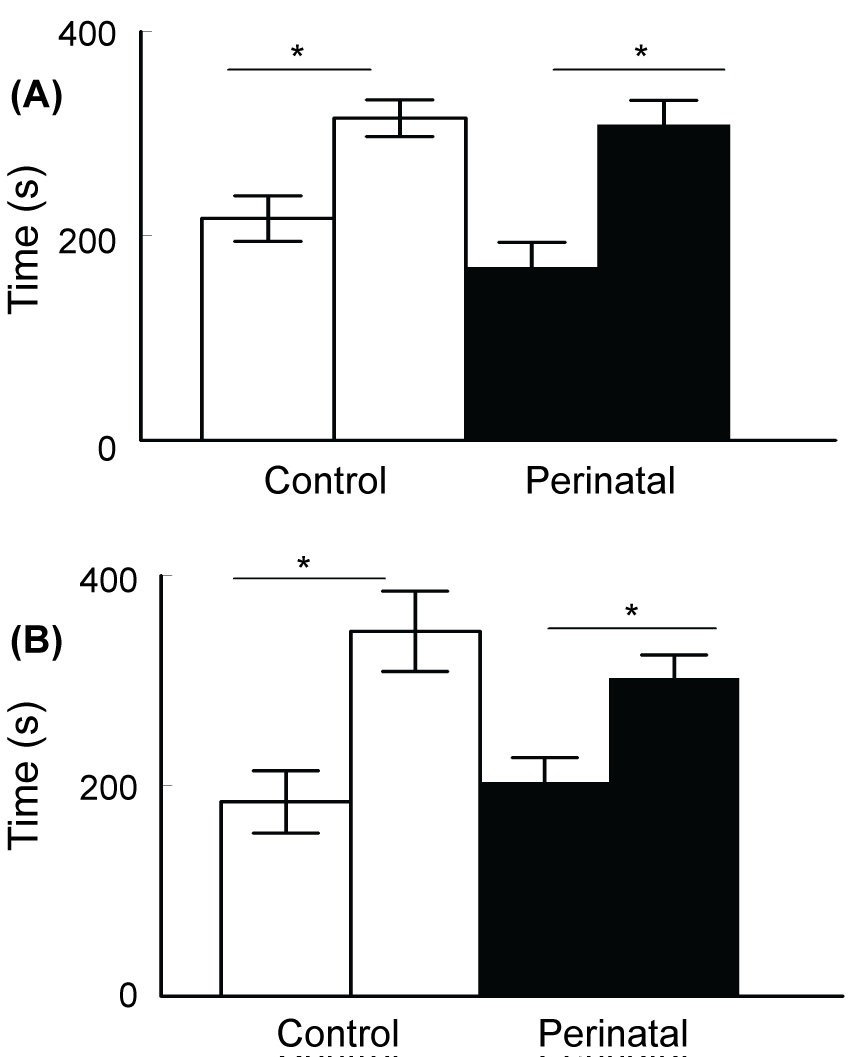
In the three-chambers social apparatus, the control and caffeine-exposed groups of mice demonstrated normal sociability by spending more time in the chamber containing the mouse (315.4 ± 17.9 s and 308.7 ± 24.1 s respectively) than in the chamber containing an empty cage (217 ± 22.4 s and 169.3 ± 24.1 s) in session 1 (Figure 5A; t-test p < 0.005, t Critical = 1.771). In session 2, both groups demonstrated normal preference for social novelty by spending more time in the chamber containing the novel mice than in the chamber containing the already investigated mouse (Figure 5B; t-test p < 0.005, t Critical = 1.771).
Discussion
Caffeine is a non-selective adenosine receptor antagonist, with reported similar affinities for A1 and A2A and lower affinity for A3 receptors. These receptors are highly expressed in the brain and striatum respectively [21,22]. Caffeine is well documented for its effects on cognitive functions in different ages in adult animals [23,24]. In addition, chronic caffeine was shown to prevent memory disturbances associated with aging [25,26] and experimental models of Alzheimer's disease [27]. However, it was also reported that caffeine during brain development is associated with low birth weight, intrauterine growth retardation and miscarriage [28-30]. Recent data suggested that caffeine even in low doses may alter the fetal brain development [16]. Silva, et al. [12] demonstrated impairment of hippocampal GABAergic neurons migration and increased hippocampal excitation with increased seizure susceptibility [12]. Altered hippocampal neuronal network and imbalance between the GABAergic and Glutamatergic neurons could be the cause of changes in spatial memory tests [31]. Some other studies however, indicated that maternal caffeine intake has little effects on adenosine receptor development in the rat brain [32]. Opposite results were reported by others [33].
In addition to this controversy, most of the research on caffeine effects was done to evaluate its acute or chronic administration on adult animals. Very limited research was done to study the consequences of perinatal caffeine exposure on the brain function later in adult life. Few reports demonstrated no significant effects in human children who were examined at 5-7 years of age only [34].
In this study, pregnant female mice were administered caffeine in drinking water throughout pregnancy and during breast feeding. The born pups were significantly of lower birth weight compared to the control animal's pups. This difference in weight disappeared later in adulthood. These result confirms other previously published data [28-30,35]. In humans, lower birth weight correlates with enhanced seizure susceptibility [36] and cognitive deficits [37].
The male offsprings were tested at age of six weeks. The main difference was reported in the Morris-water maze learning and memory test. Forty eight hours after training trials, the test trials showed significantly higher values of latency to reach the hidden platform in the group of animals which were exposed perinatally to caffeine when compared to the control animals. Same results were confirmed by measurement of the distance swam to reach the platform. No significant difference was recorded between the two groups concerning the swimming speed in the water maze. This may indicate that the difference reported in the latency and distance to reach the platform was not due to a difference in the speed of swimming but rather due to the difference in the formed memory of the platform position. In the probe test, the platform was removed and the time spent by each animal in the different quadrants of the swimming pool was calculated during 2 minutes. Good memory is correlated with more than chance time (25%) spent by the animals in the platform quadrant. The perinatally administered mice showed significantly less time in the platform quadrant when compared to the control animals. The learning and memory test in our experiment showed that there was clearly negative impact of perinatal caffeine exposure on the cognitive function of the brain later in adult life. The hippocampus is involved in spatial memory formation. On basic cellular level, the hippocampal long-term potentiation (LTP) and depression (LTD) are the two principle mechanisms involved in this function. However, in spite of all the recorded changes in the learning and memory in this study, the LTP induction was shown to be similar to the controls animals [12]. LTP represents memory mechanism at cellular level, and normal LTP induction in presence of learning and memory defects may suggest that synaptic plasticity is not entirely LTP induction phenomenon but rather a change in the amplitude of synaptic plasticity range which extends from maximum potentiation (LTP) to maximum depression (long-term depression; LTD) of the synapses [38,39]. Caffeine Administration in this study was stopped after weaning. However, the early effects of caffeine exposure during pregnancy and breast feeding on neuron migration and integration had already caused the long-term sequelae in hippocampal network. Early defects of developmental programs usually leads to long-term alterations in GABAergic circuits, represented by neuronal loss and cognitive deficits in adult animals [40,41]. These results raise the suspicion of weather this significant effect of caffeine exposure during pregnancy is applicable on other species or human beings. As a matter of fact, data indicates that A2AR-related pathways can be potentially affected by caffeine in primates [42].
Our results also showed that perinatal caffeine exposure of mice resulted into impairment of motor coordination measured by rotarod test. These animals couldn't stay on the rotating rod the same time as the control animals. Research data showed that high dose of caffeine consumption resulted into reduction in the perception of effort [43] and lowering the extent of pain sensation [44,45]. It was also demonstrated that high dose of caffeine could affect skeletal muscle contraction either through the stimulation of Ca2+ release from the sarcoplasmic reticulum [46,47] or by reducing the K+ efflux [48]. In addition, it was found that a derivative of caffeine, theobromine upregulates cerebral brain-derived neurotrophic factor (BDNF) and facilitates motor learning in mice [49]. Most of the data suggested that caffeine or its derivatives is usually causing hyper motility when given to adult animals. Contradictory results emerged about the effect of perinatal administration of caffeine on the motor activity. While young offspring rats treated with caffeine during pregnancy exhibited alteration in locomotion, some reports showed decreased motor activities [50,51], while others reported increased motor activity which may even persisted up to ageing [52]. It seems that the changes or alterations depend strictly on the timing of caffeine administration and its dosage [16,53]. Some researchers showed improved motor activity after perinatal caffeine administration and this improvement lasted to adulthood and was due to increased level of vigilance and anxiety [50,54]. However, in our animals we did not see any significant increase in anxiety levels compared to the control animals. Moreover, some other reports demonstrated a decrease in anxiety-related behavior in perinatally administered caffeine rats [55]. We also did not find any significant alteration in the social behavior of our perinatally caffeine-exposed mice.
Being easily penetrating most of the biological membranes, caffeine reaches the brain, passes in breast milk and through the placenta to the fetus. A substance which has such ability may affect prominently several structures, or functions of the body. Especially important is its effect on fetus during pregnancy and on the neonatal period. The brain starts to develop early in an embryo. Neurogenesis, neuronal cell multiplications and cell migrations are all processes that take place early during intrauterine life. These processes are highly organized and any interference may have serious consequences on the normal development of the brain. In addition, postnatal period during the first few months to the first or even the second year of life is characterized by the formation of neural circuits and synaptic connections. A chemical known to antagonize receptors or enhance the release of some neurotransmitters may affect the processes of CNS development and organization. Caffeine with its well recognized antagonistic activity to the adenosine receptors and a possible modulating effect on the release of another inhibitory neurotransmitter, the gamma-amino-buteric acid (GABA) may have a potential effect on the development of CNS synapses and eventually brain function.
Human neonate who were born with apnea due to prematurity are frequently treated with caffeine. These patients showed no considerable consequences when they were followed and examined for about 5 years [34,56,57].
With the absence or lack of data concerning the long term follow up of such patients or babies born with history of caffeine consumption during pregnancy, the safety in such situations may be not very certain and needs further long-term follow up studies.
Conclusion
We show that perinatal administration of caffeine to mice affects significantly their performance in learning and memory tests as well as in motor coordination. We did not observed any long term significant changes in these animals concerning anxiety and social behavior. These data demonstrated different consequences of caffeine administration to pregnant animal on their offspring than the effects seen when administered to adult animals. Long-term follow up and longitudinal clinical studies may be needed to investigate thoroughly the consequences of caffeine consumption by the mothers during pregnancy and breast feeding on their babies.
References
- Jack E James (1997) Understanding caffeine: A Biobehavioral Analysis. Sage Publications 2: 240.
- Shukitt-Hale B, Miller MG, Chu YF, et al. (2013) Coffee, but not caffeine, has positive effects on cognition and psychomotor behavior in aging. Age (Dordr) 35: 2183-2192.
- Leite MR, Marcondes Sari MH, de Freitas ML, et al. (2014) Caffiene and diphenyl diselenide improve long-term memory impaired in middle-aged rats. Exp Gerontol 53: 67-73.
- Eskelinen MH, Ngandu T, Tuomilehto J, et al. (2009) Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis 16: 85-91.
- Ferré S (2016) Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology (Berl) 233: 1963-1979.
- Varani K, Portaluppi F, Gessi S, et al. (2000) Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: functional and biochemical aspects. Circulation 102: 285-289.
- Golden LE, Sassoon P, Cáceda R (2015) A case report of late onset psychosis with dementia and aspirin and caffeine addiction. Schizophr Res 168: 591-592.
- Fredholm BB, Bättig K, Holmén J, et al. (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51: 83-133.
- McLellan TM, Caldwell JA, Lieberman HR (2016) A review of caffeine's effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev 71: 294-312.
- Fredholm BB, Chen JF, Cunha RA, et al. (2005) Adenosine and brain function. Int Rev Neurobiol 63: 191-270.
- Li XD, He RR, Qin Y, et al. (2012) Caffeine interferes embryonic development through over-stimulating serotonergic system in chicken embryo. Food Chem Toxicol 50: 1848-1853.
- Silva CG, Métin C, Fazeli W, et al. (2013) Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci Transl Med 5.
- Salisbury AL, Ponder KL, Padbury JF, et al. (2009) Fetal effects of psychoactive drugs. Clin Perinatol 36: 595-619.
- Crandall JE, Hackett HE, Tobet SA, et al. (2004) Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb Cortex 14: 665-675.
- Berghuis P, Rajnicek AM, Morozov YM, et al. (2007) Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316: 1212-1216.
- Mioranzza S, Nunes F, Marques DM, et al. (2014) Prenatal caffeine intake differently affects synaptic proteins during fetal brain development. Int J Dev Neurosci 36: 45-52.
- Holson RR, Pearce B (1992) Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol 14: 221-228.
- Morris RG, Garrud P, Rawlins JN, et al. (1982) Place navigation is impaired in rats with hippocampal lesions. Nature 297: 681-683.
- Oksana Kaidanovich-Beilin, Tatiana Lipina, Igor Vukobradovic, et al. (2011) Assessment of Social Interaction Behaviors. J Vis Exp 2473.
- Munekazu Komada, Keizo Takao, Tsuyoshi Miyakawa (2008) Elevated plus Maze for Mice. J Vis Exp 1088.
- Ferré S (2008) An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem 105: 1067-1079.
- Karcz-Kubicha M, Antoniou K, Terasmaa A (2003) Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology 28: 1281-1291.
- Angelucci ME, Vital MA, Cesário C, et al. (1999) The effect of caffeine in animal models of learning and memory. Eur J Pharmacol 373: 135-140.
- Costa MS, Botton PH, Mioranzza S, et al. (2008) Caffeine prevents age-associated recognition memory decline and changes brain derived neurotrophic factor and tirosine kinase receptor (TrkB) content in mice. Neuroscience 153: 1071-1078.
- Costa MS, Botton PH, Mioranzza S, et al. (2008) Caffeine improves adult mice performance in the object recognition task and increases BDNF and TrkB independent on phospho- CREB immunocontent in the hippocampus. Neurochem Int 53: 89-94.
- Sallaberry C, Nunes F, Costa MS, et al. (2013) Chronic caffeine prevents changes in inhibitory avoidance memory and hippocampal BDNF immunocontent in middle aged rats. Neuropharmacology 64: 153-159.
- Espinosa J, Rocha A, Nunes F, et al. (2013) Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J Alzheimers Dis 34: 509-518.
- Robert L Brent, Mildred S Christian, Robert M Diener (2011) Evaluation of the reproductive and developmental risks of caffeine. Birth Defects Res B Dev Reprod Toxicol 92: 152-187.
- Bakker R, Steegers EA, Obradov A, et al. (2010) Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: the Generation R Study. Am J Clin Nutr 91: 1691-1698.
- Giannelli M, Doyle P, Roman E, et al. (2003) The effect of caffeine consumption and nausea on the risk of miscarriage. Paediatr Perinat Epidemiol 17: 316-323.
- Kabir ZD, McCarthy DM, Bhide PG, et al. (2013) Cup of Joe: a brain development "no"? Sci Transl Med 5.
- Adén U, Herlenius E, Tang LQ, et al. (2000) Maternal caffeine intake has minor effects on adenosine receptor ontogeny in the rat brain. Pediatr Res 48: 177-183.
- Guillet R, Kellogg C (1991) Neonatal exposure to therapeutic caffeine alters the ontogeny of adenosine A1 receptors in brain of rats. Neuropharmacology 30: 489-496.
- Klebanoff MA, Keim SA (2015) Maternal Caffeine Intake During Pregnancy and Child Cognition and Behavior at 4 and 7 Years of Age. Am J Epidemiol 182: 1023-1032.
- Rhee J, Kim R, Kim Y, et al. (2015) Maternal Caffeine Consumption during Pregnancy and Risk of Low Birth Weight: A Dose-Response Meta-Analysis of Observational Studies. PLoS One 10: e0132334.
- Sun Y, Vestergaard M, Pedersen CB, et al. (2008) Gestational age, birth weight, intrauterine growth, and the risk of epilepsy. Am J Epidemiol 167: 262-270.
- Jefferis BJ, Power C, Hertzman C (2002) Birth weight, childhood socioeconomic environment, and cognitive development in the 1958 British birth cohort study. BMJ 325: 305.
- Kamal A, Biessels GJ, Duis SE, et al. (2000) Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: interaction of diabetes and ageing. Diabetologia 43: 500-506.
- Kamal A, Biessels GJ, Gispen WH, et al. (1998) Increasing age reduces expression of long term depression and dynamic range of transmission plasticity in CA1 Weld of the rat hippocampus. Neuroscience 83: 707-715.
- Ramamoorthi K, Lin Y (2011) The contribution of GABAergic dysfunction to neurodevelopmental disorders. Trends Mol Med 17: 452-462.
- Grantyn R, Henneberger C, Jüttner R, et al. (2011) Functional hallmarks of GABAergic synapse maturation and the diverse roles of neurotrophins. Front Cell Neurosci 5: 13.
- Hendry SH, Schwark HD, Jones EG, et al. (1987) Numbers and proportions of GABA immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci 7: 1503-1519.
- Doherty M, Smith PM (2005) Effects of caffeine ingestion on rating of perceived exertion during and after exercise: a meta-analysis. Scand J Med Sci Sports 15: 69-78.
- Gliottoni RC, Meyers JR, Arngrimsson SA, et al. (2009) Effect of caffeine on quadriceps muscle pain during acute cycling exercise in lowversus high caffeine consumers. Int J Sport Nutr Exerc Metab 19: 150-161.
- Stadheim HK, Nossum EM, Olsen R, et al. (2015) Caffeine improves performance in double poling during acute exposure to 2,000-m altitude. J Appl Physiol 119: 1501-1509.
- Mohr T, Van Soeren M, Graham TE, et al. (1998) Caffeine ingestion and metabolic responses of tetraplegic humans during electrical cycling. J Appl Physiol (1985) 85: 979-985.
- Tarnopolsky M, Cupido C (2000) Caffeine potentiates low frequency skeletal muscle force in habitual and nonhabitual caffeine consumers. J Appl Physiol (1985) 89: 1719-1724.
- Mohr M, Nielsen JJ, Bangsbo J (2011) Caffeine intake improves intense intermittent exercise performance and reduces muscle interstitial potassium accumulation. J Appl Physiol (1985) 111: 1372-1379.
- Yoneda M, Sugimoto N, Katakura M, et al. (2017) Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice. J Nutr Biochem 39: 110-116.
- Hughes RN, Beveridge IJ (1991) Behavioral effects of exposure to caffeine during gestation, lactation or both. Neurotoxicol Teratol 13: 641-647.
- Peruzzi G, Lombardelli G, Abbracchio MP, et al. (1985) Perinatal caffeine treatment: behavioral and biochemical effects in rats before weaning. Neurobehav Toxicol Teratol 7: 453-460.
- Nakamoto T, Roy G, Gottschalk SB, et al. (1991) Lasting effects of early chronic caffeine feeding on rats' behavior and brain in later life. Physiol Behav 49: 721-727.
- Rivkees SA, Wendler CC (2011) Adverse and Protective Influences of Adenosine on the Newborn and embryo: implications for Preterm white matter injury and embryo protection. Pediatr Res 69: 271-278.
- Hughes RN, Beveridge IJ (1986) Behavioral effects of prenatal exposure to caffeine in rats. Life Sci 38: 861-868.
- Pan HZ, Chen HH (2007) Hyperalgesia, low-anxiety, and impairment of avoidance learning in neonatal caffeine-treated rats. Psychopharmacology (Berl) 191: 119-125.
- Schmidt B, Roberts RS, Davis P, et al. (2007) Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 357: 1893-1902.
- Schmidt B, Anderson PJ, Doyle LW, et al. (2012) Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 307: 275-282.
Corresponding Author
Amer Kamal, Department of Physiology, College of medicine and medical Sciences, Arabian Gulf University, Manama, Bahrain, Tel: +973-1723-9767, Mobile: +973-366-22801, Fax: +973-172-71090.
Copyright
© 2017 Alsayed A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.