Obtainment of Polyhydroxyalkanoates (PHAs) from Microalgae Supplemented with Agro-Industry Residue Corn Steep Liquor
Abstract
In this study, it was possible to evaluate the production of polyhydroxyalkanoates (PHAs) from the microalgae Chlorella vulgaris and Tetradesmus obliquus, using 1% and 0.25% (v/v) of agro-indusytial Corn Steep Liquor residue, a product from corn processing. The biomass was subjected to mechanical extraction using 4% sodium hypochlorite + chloroform, and the extract was further characterized using Fourier Transform Infrared Spectroscopy (FTIR) analysis. It was possible to observe stretching common to PHAs described in the literature, such as the strong vibration of the carnonyl ester group (C=O) at 1735 cm-1 and 1720 cm-1, and the presence of methyl (CH3) and methylene (CH2) groups, for C. Vulgaris and T. obliquus, respectively. Therefore, these indications suggest that both microalgae are potential producers of polyhydroxyalkanoate biopolymers, and have possible applications in the production of bioplastics. This is the first report on the production of polyhydroxyalkanoates from these microalgae, especially the isolated strain, Tetradesmus obliquus.
Keywords
Bioplastic, Polyester, Polyhydroxyalkanoate, Corn Steep Liquor, Chlorella vulgaris, Tetradesmus obliquus
Introduction
Polyhydroxyalkanoates (PHAs) are biopolymers produced by a range of microorganisms, including microalgae, and will be great replacements for conventional petroleum-derived plastics as they have properties similar to synthetic polymers and are biodegradable [1], capable of forming plastic membranes. Bioplastics obtained from renewable biomass have already been produced from first-generation raw materials such as sugar beet, cornor second-generation raw materials such as lignocellulose materials. Currently, more attention is being paid to production from microorganisms, theso-called third generation, which do not compete with human, animal, fresh water or arable land [2,3]. The microbial production of PHAs has been reported for decades, however it has been gaining a lot of attention in recent years, due to the wide variety of applications of these biopolymers in several sectors. However, there is a concern with its production, due to the high costs of organic substrates used for PHA production by heterotrophic bacteria. Thus, the use of photosynthetic microorganisms such as microalgae and cyanobacteria can become quite advantageous, since these microorganisms do not require costly nutritional sources and have minimal nutritional requirements such as in organic sources (CO2, N, P) and light [4].
Materials and Methods
Microalgae and growing conditions
Two microalgae were used in the experiment and both were cultivated in standard Bold's Basal medium [5]. Chlorella vulgaris UTEX 1803 was cultivated at 27 ± 1 ℃, constant lighting (3800 lux), constant aeration and supplemented with 1% corn steep liquor (v/v) [6]. Tetradesmus obliquus was grown at 27 ± 2 ℃, constant illumination (3000 lux), constant aeration and supplemented with 0.25% corn steep liquor (v/v) [7]. C. Vulgaris is a commercial strain (University of Texas, Austin) and T.obliquus was isolated from Açude of Apipucos (Recife, Pernambuco, Brazil, coordinates 8°1'13.08" S; 34°55' 56.51" W).
Treatment of corn steep liquor
Corn Steep Liquor was kindly provided by the company Ingredion (Cabo de Santo Agostinho, Pernambuco, Brazil), was treated in accordance with Liggett and Koffler [8], with slight modifications. Initially, Corn Steep Liquor was centrifuged at 8000 rpm for 10 minutes to remove solid particles and then concentrated NaOH was added to adjust pH 8 and autoclaved at 121 ℃ for 20 minutes. The agroindustrial residue was then centrifuged again and the supernatant was used on the cultures.
Biopolymer extraction
1.0 g of lyophilized biomass of C. Vulgaris (CHLOB) and T. Obliquuos (TETRAB) were subjected to motor agitation with a solution of hypochlorite (4%) + chloroform (v/v), then the samples were centrifuged (3000 rpm, 10 minutes, temperature environment) and the organic phase was collected, evaporated, degreased with hexane and subjected to spectroscopic analysis.
Fourier Trasnform Infrared Spectroscopy (FTIR)
Samples of PHAs were qualitatively analyzed with Fourier transform infrared spectroscopy (FTIR, Shimadzu, model IR-Tracer 100) between 4000 and 600 cm-1 using ATR accessory with a zinc selenide crystal.
Results and Discussion
Fourier Transform Infrared Spectroscopy (FTIR) analysis of PHA samples
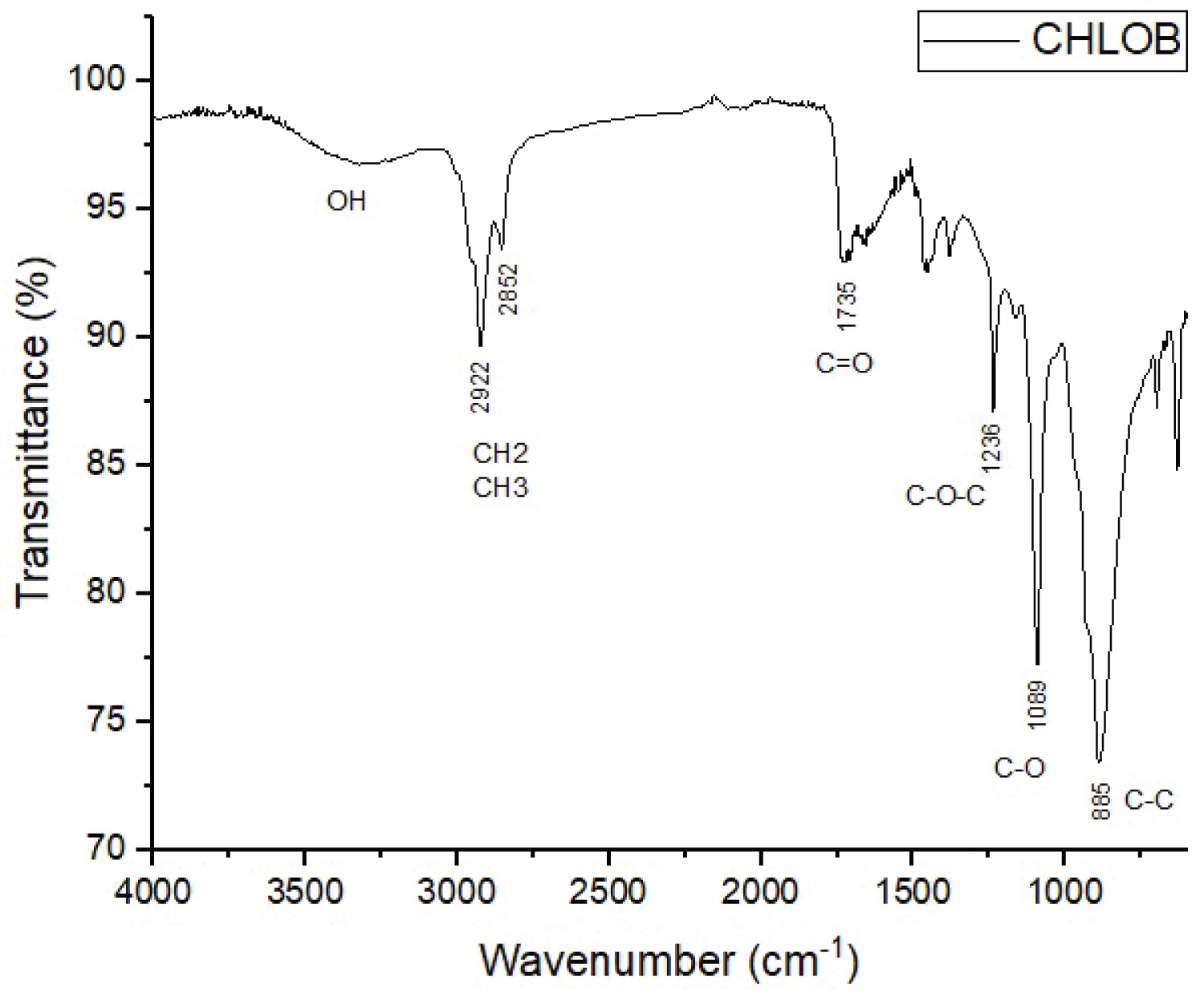
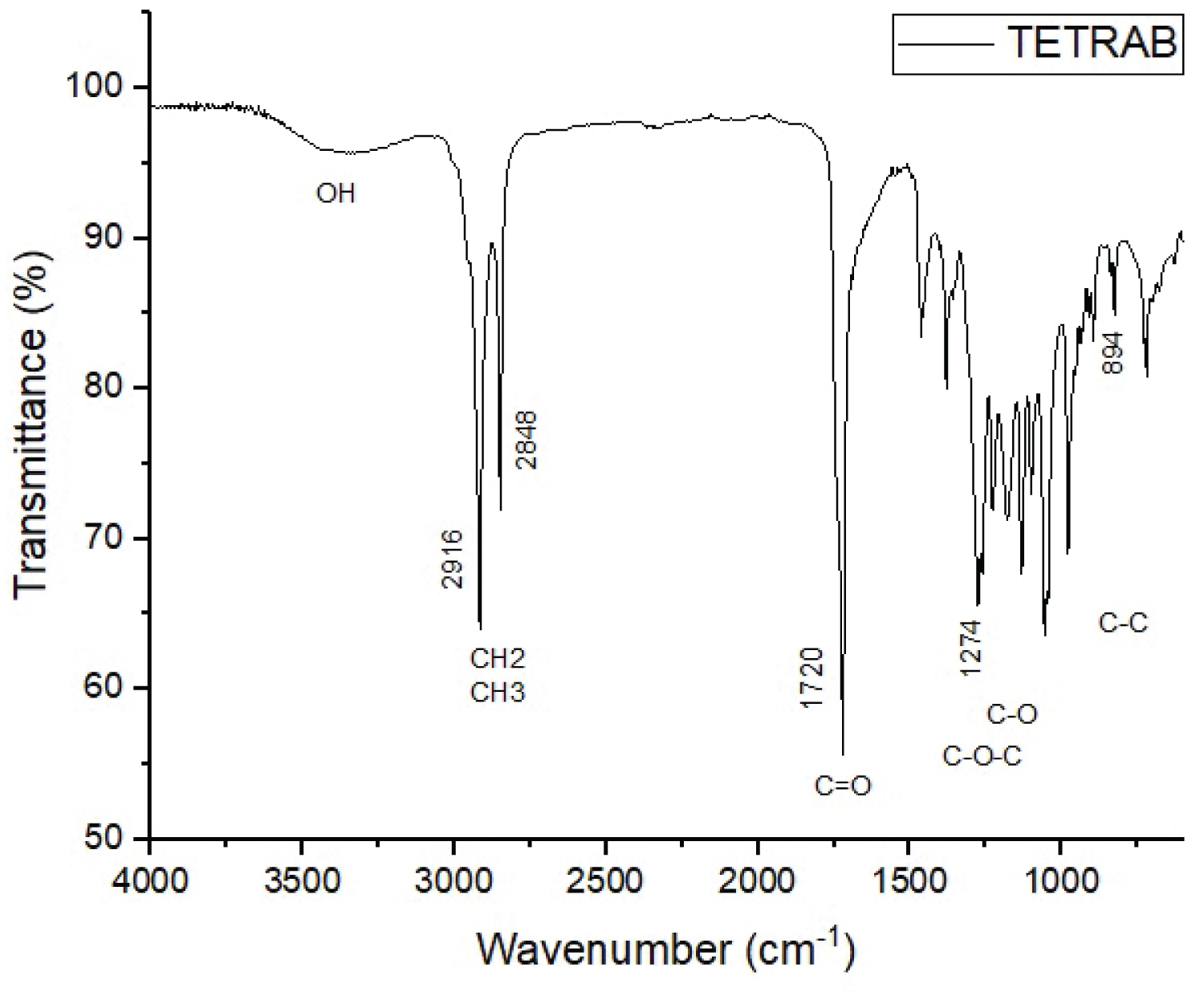
Figure 1 and Figure 2 show the spectra of the obtained PHA samples. In the figures it is possible to observe how transmittance bands at 1735 and 1720 cm-1 are attributed to strong vibration of the carbonyl ester group (C=O), strongly indicative of medium chain length PHAs (mcl-PHAs), corroborating with Giaquinto, et al. [9] who say that PHBs have bands corresponding to the carbonyl ester group ranging between 1720-1650 cm-1. Other characteristic signs of PHAs are also observed in the spectra, such as the presence of elongations of the –OH group within the carboxyl group, ranging between 3400-3200 cm-1, methyl groups (CH3) and methylene group (CH2), with spectra ranging between 2961-2854 cm-1, flash bands between 1466-1000 cm-1 are attributed to strong vibration of the C-O group, and other characteristic bands present that are attributed to the C-C group [10,11]. All these indicatives presented as microalgae studied in this work have the ability to synthesize biopolymers capable of forming plastic membranes. Roja, et al. [11] analyzing the elongations obtained in the FTIR of 4 types of algae (Chlorella sp., Oscillatoria salina, Leptolyngbya valderiana and Synechococcus elongatus) obtained wave number of the C=O group varying between 1625-1644 cm-1. Costa, et al. [12] evaluating different methods of extraction of the biopolymer obtained from Spirulina sp. obtained a strong vibration of the carbonyl ester group (C=O) ranging from 1735-1745 cm-1, corroborating the present study.
Conclusion
The results show that the use of maize for the production of polyhydroxyaalkanoates from the microalgae Chlorella vulgaris and Tetradesmus obliquus is possible. In the spectroscopic analysis (FTIR) it was possible to observe the main characteristic elongation of the PHAs, with a strong vibration of the est carbonyl group (C=O), in addition to the methyl (CH3) and methylene (CH2) groups. Therefore, microalgae have a strong industrial potential in the production of plastic biofilms.
Acknowledgements
The authors acknowledge supply of Corn Steep Liquor by Ingredion, Cabo de santo Agostinho - Pernambuco, Brazil and the financial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (Process: 302284/2021-4).
Conflicts of Interest
None.
References
- Costa SS, Miranda AL, Morais MG, et al. (2019) Microalgae as source of polyhydroxyalkanoates (PHAs) - A review. International Journal of Biological Macromolecules 131: 536-547.
- Ge S, Champagne P (2017) Cultivation of the marine macroalgae chaetomorphalinum in municipal waste water for nutrient recovery and biomass production. Environ Sci Technol 51: 3558-3566.
- Qiu S, Wang L, Champagne P, et al. (2019) Effects of crystalline nanocellulose on waste water-cultivated microalgal separation and biomass composition. Applied Energy 239: 207-217.
- Afreen R, Tyagi S, Singh GP, et al. (2021) Challenges and perspectives of polyhydroxyalkanoate production from microalgae/cyanobacteria and bacteria as microbial factories: An assessment of hybrid biological system. Front Bioeng Biotechnol 9: 624885.
- Bischoff HW, Bold HC (1963) Phycological studies IV, Some soilalgae from Enchanted Rock and related algal species, University of Texas Publication 6318: 95.
- Silva PEC, Barros RC, Albuquerque WWC, et al. (2018) Invitro thrombolytic activity of a purified fibrinolytic enzyme from Chlorellavulgaris. Journal of Chromatography B 1092: 524-529.
- Souza ATV, Silva PEC, Barros PDS, et al. (2016) Otimização da Produção de Enzimas Fibrinolíticas pela Microalga Scenedesmussp. XII Seminário Brasileiro de Tecnologia Enzimática (ENZITEC).
- Liggett RW, Koffler H (1948) Corn steep liquor in microbiology. Bacteriol Rev 12: 297-311.
- Giaquinto CDM, Souza GKM, Caetano VC, et al. (2017) Evaluation of the mechanical and thermal properties of PHB/canola oil films. Polímeros 27: 201-207.
- Tanikkul P, Sullivan GL, Sarp S, et al. (2020) Biosynthesis of medium chain length polyhydroxyalkanoates (mcl-PHAs) from palm oil. Case Studies in Chemical and Environmental Engineering 2: 100045.
- Roja K, Ruben Sudhakar D, Anto S, et al. (2019) Extraction and characterization of polyhydroxyalkanoates from marine green alga and cyanobacteria. Biocatalysis and Agricultural Biotechnology 22: 01358.
- Costa SS, Miranda AL, Assis DJ, et al. (2018) Efficacy of Spirulina sp. polyhydroxyalkanoates extraction methods and influence on polymer properties and composition. Algal Research 33: 231-238.
Corresponding Author
Páblo Eugênio da Costa e Silva, Centro de Tecnologias Estratégicas do Nordeste, Av. Prof. Luís Freire, 1 - Cidade Universitária, Recife - PE, 50740-545, Brazil, Tel: 558-199-505-6544.
Copyright
© 2022 Silva PEC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.