In Vitro Callus Induction of Duroia Macrophylla Huber, Chemical Prospection and Biotechnological Potentialities of Its Extracts
Abstract
The Amazonian wild tree species Duroia Macrophylla (Rubiaceae) produces triterpenes and alkaloids, some of them showed antituberculosis and antitumoral activities. This paper describes the in vitro establishment and callus induction of Duroia Macrophylla leaf explants, preparation of callus extracts and the chemical analysis to verify the presence of alkaloids and terpenes in it. Young leaf explants were established in vitro with 50% of asepsis, being the best disinfestation method with immersion of 5 h at biocide solution (fungicidal and bactericidal), 3 min in 70% ethanol and 15 min in sodium hypochlorite 2%. Once established, the explants in vitro were induced to callus formation process by adding the growth regulators kinetin and 2,4-dichlorophenoxyacetic acid, on Murashige and Skoog medium. In vitro callus were extracted with hexane and methanol and the presence of secondary metabolites was assessed by thin layer chromatography and 1H-NMR analysis. Preliminary chemical analysis suggests that both callus extract of D. macrophylla did not contain alkaloids, but terpenes. Thus, more studies are needed to induce the production of alkaloids in D. macrophylla callus, where the use of elicitors, or other methods, may induce the production of these metabolites in callus, as alkaloids and terpenes showed antituberculosis and antitumoral activities, what are potential of (social and economics) uses with bioprospection activities. This is the first report of D. macrophylla callus induction and the chemical prospection of its extracts.
Keywords
Callogenesis, Plant cell culture, Phytochemical analysis, Rubiaceae, Bioprospection, Plant biotechnology
Introduction
The plants are one of the most important sources of organic substances, with a variety of chemical classes that arouse scientific interest because of their biological and chemical properties. In the search for new drugs, plants have been an important source for new bioactive substances [1].
In the Amazon, several plants with medicinal potential have been found and studied. Among them is the Duroia Macrophylla Huber species, Rubiaceae family. Previous studies showed the presence of an indole alkaloid obtained from the leaves, which showed antitumor activity [2]. Also eight indole alkaloids and two triterpenes were isolated from dichloromethane and methanol extracts of leaves and branches, which have demonstrated activity against Mycobacterium tuberculosis [3,4].
However, several factors may compromise the use of plants for pharmaceutical purposes like, for example, the heterogeneity of individuals, due to the existing genetic and biochemical variabilities [5] and exposure to different environmental interactions. In the case of D. macrophylla, substances of interest are produced in small quantities and they cannot be found easily in different individuals (pers. commun.).
According to Fumagali, et al. [6], the in vitro production of metabolites by tissue culture may offer advantages compared to in vivo production: plant tissue cultures in vitro can simulate the ideal environment for the production of metabolites for each type of species, obtaining constant production of metabolites in tissues and in plants grown in vitro, without interference from interactions between environmental and seasonal factors with the plant.
The culture of cells and tissues in vitro is an area of research that is growing exponentially, because the benefits of the technique are very promising as the constant and large-scale production of metabolites of interest [6], and also promotes the sustainability of plant resources.
As the plant D. macrophylla produces the active metabolites in small quantities and not all the extracts obtained from different plant collection presented the same metabolites (unpublished data), so, this paper proposes the in vitro establishment and callus induction of D. macrophylla leaf explants. Then, the hexane and methanol callus extracts were chemically analyzed, in order to identify the presence of alkaloids and terpenes. The results were compared with those obtained from wild plant extracts.
Material and Methods
Plant material
D. macrophylla leaves were collected from two individuals located in the "Reserva A. Ducke", 26 km NE of Manaus, AM-010 highway. Leaf explants were chosen because they have meristematic tissues in the conducting vessels, providing greater capacity to express totipotency [7], and are not lignified, being more appropriate explants for calli culture [8]. Samples were collected in the period between March 2013 and April 2014. The plant materials of the two individuals were identified and voucher specimens were deposited in the Herbarium of the National Institute of Amazonian Research - INPA (herbarium number: 249228 and 261554).
In vitro establishment
Young and adult leaves of D. macrophylla were collected and small fragments of approximately 1.0 × 0.5 cm were taken away from central areas of the leaves, containing primary or secondary veins. Various explants pre-desinfestation procedures were made; first of all they were washed in distilled water and then immersed in 70% etanol. Then, the explants were immersed in a solution containing biocidal (2 g L-1 Mancozeb® and 100 mg L-1 streptomycin) in different times (Table 1). After the pre-disinfestation treatments, the explants were exposed to disinfestation treatment with the combination of the disinfectants agents: 70% ethanol, 2% hypochlorite sodium and 4% calcium hypochlorite (Table 2), in biological safety cabinet (Esco, model Labculture Class II A2, Singapore). Between 15 and 20 replicates were utilized on each treatment, with an explant in each test tube (plot). Between each immersion on different disinfestants agents and at the end of the disinfestation process, explants were subjected to three washes with sterile distilled water, and then inoculated in Murashige and Skoog (MS) (1962) [9] culture medium supplemented with 30 g L-1 sucrose and 7 g L-1 agar.
Inoculated explants were incubated in a growth chamber at 28 ± 2 °C, relative humidity of about 60% in diffuse light (shading) intensity of 50 μmol m2 s-1 and 16 h photoperiod for 30 days. The different disinfestation treatments were evaluated considering the percentage of alive explants and contamination (fungi and/or bacteria). It was utilized analysis of variance with Tukey's test at the 5% level of probability, for statistical analysis.
Callus induction
The explants that remained alive and aseptic after the disinfestation treatments, were transferred to the MS medium added 30 g L-1 sucrose, 7 g L-1 agar, 4 mg L-1 2,4-dichlorophenoxyacetic (2,4-D) and 2 mg L-1 kinetin (KIN) to the callus induction process. The incubation environment was kept equal to the growth chamber after disinfestation.
Preparation and analysis of callus extracts
The callus obtained in in-vitro process were united and dried in a lyophilizer. The lyophilized callus (macerated with pestle) were subjected to extraction with hexane, using ultrasound for 20 minutes (Unique, Ultra Cleaner model, Brazil), filtered, and this process repeated twice more. The callus cake was dried in flow hood and then extracted with methanol using ultrasound for 20 minutes, filtered and the process repeated twice more. The extracts were concentrated in rotary evaporator (Fisatom, model 550, Brazil) and were analyzed by Thin Layer Chromatography (TLC) (Marcherey, Nagel, MN and Merck, Germany) and 1H Nuclear Magnetic Resonance (NMR) at 300 MHz (Bruker, model Fourier-300, Germany) for analysis of metabolites present.
Results
In vitro establishment
Alive aseptic explants were only achieved when young leaves were used. The best results were found with the pre-disinfestation treatment 02 of D. macrophylla young leaves (TPDJ 02) and the disinfestation treatments 05 and 06 of D. macrophylla young leaves (DMJ 05 and DMJ 06) (Table 3).
Callus induction
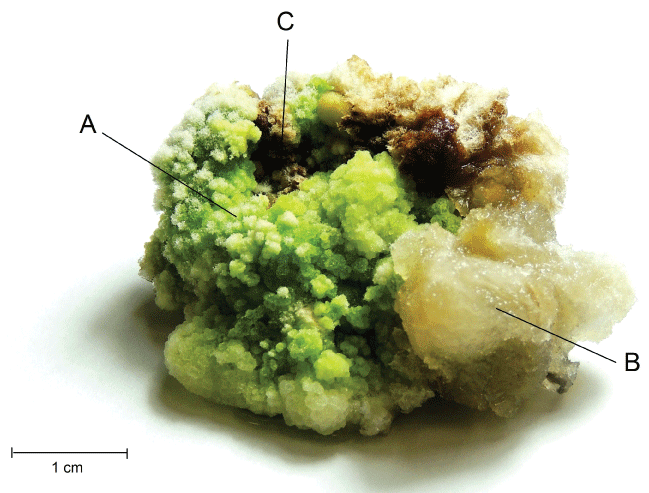
Alive and aseptic explants begin to exhibit the callus formation at the end of leaf segments, after two weeks of inoculated in MS medium with 4 mg L-1 of 2,4-D and 2 mg L-1 of KIN. The transformation of the leaf segments tissues in callus was complete after five weeks, presenting multiform aspect, where, in the same batch, there were evidenced some friable, other compact and even callus with chlorophyll (Figure 1).
Calli extracts
The calli were multiplicated to increase the total weight of the plant material, for chemical extraction. The calli formed on DMJ 02 treatments (TPDJ 01) and DMJ 03 (TPDJ 01) were subcultured five times, and then lyophilized, weighed and named DMC 01 (2.52 g) and DMC 02 (2.38 g), respectively. The calli formed on DMJ 05 treatments (TPDJ 02) and DMJ 06 (TPDJ 02) were subcultured twice, then lyophilized, weighed and named DMC 03 (1.50 g) and DMC 04 (1.88 g), respectively.
The calli were extracted with hexane and methanol, yielding eight extracts at first. During drying process on rotary evaporator all methanolic extracts showed a precipitate, which was separated from the supernatant. The masses and yields of the extracts obtained from D. macrophylla calli are shown in table 4.
In the Thin Layer Chromatographic (TLC) analysis, the system hexane/ethyl acetate (EtOAc) 9:1 showed to be the best to split the spots for the hexane extracts, while pure methanol showed to be the best elution system for methanolic extracts.
The revelation of the plates in ultraviolet light at 254 nm did not show spots in any of the extracts analyzed, and in 365 nm showed blue fluorescent spots, suggesting the presence of substances with chromophores. There was no evidence of the presence of alkaloids in any hexane and methanolic extracts of callus D. macrophylla when revealed with Dragendorff reagent. Plates stained with ceric sulphate showed the possible presence of terpenes in all extracts, where purple colored spots were observed after heating (Rf of methanolic extract spots were ≅ 0.75/Rf of hexane extract spots were ≅ 0.14; 0.51; 0.75).
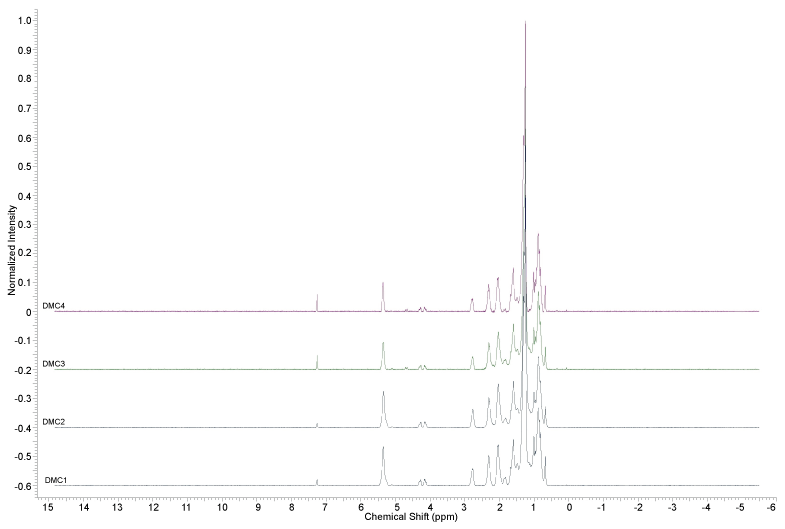
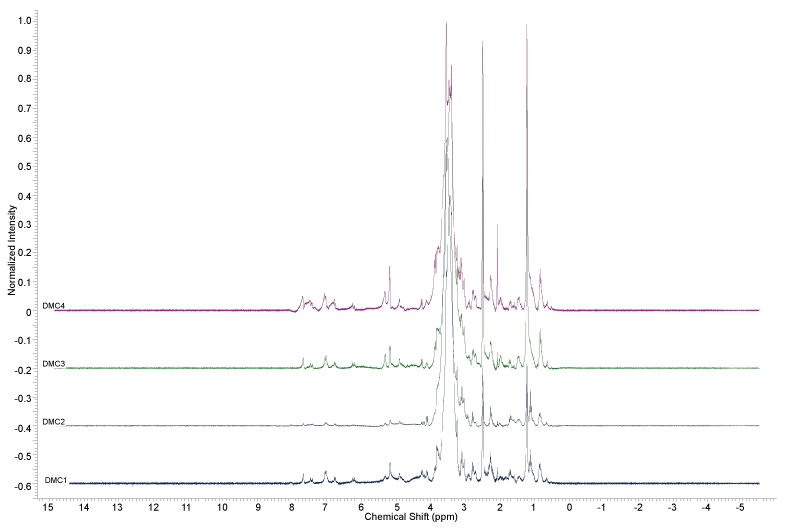
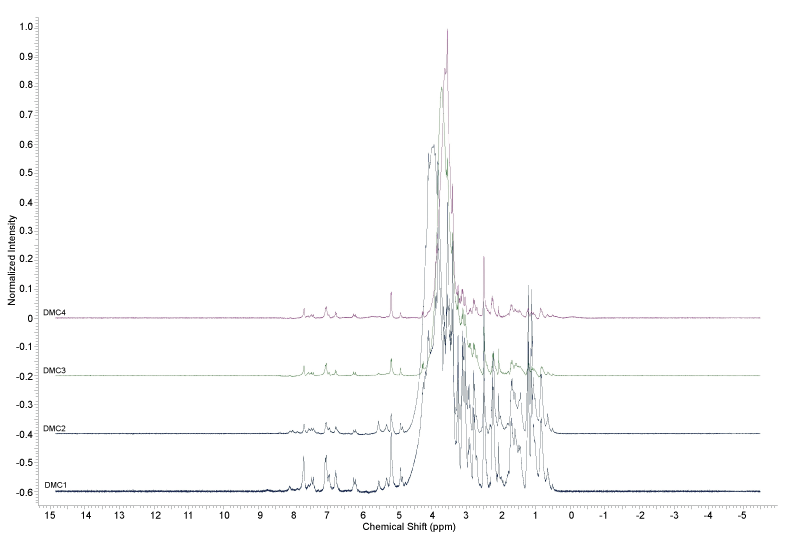
The 1H-NMR analysis of D. macrophylla callus extracts (Figures 2, Figure 3 and Figures 4) did not indicate the presence of indole alkaloids, due to the absence of signals above ϒH 8.0. The signals indicating the presence of aromatic substances (ϒH between 6 and 8) were only observed in the methanol extracts.
Discussion
Tissue culture
The difference between the contamination results of adult and young leaf explants probably was due to the time of natural exposure of adult leaves to environmental and microorganisms interactions in their habitat, which is larger than of young leaves. The largest amount of contamination in mature leaves has been observed in studies with endophytic microorganisms of plants: Pimentel, et al. (2006) [10], in research with fungi of Ilex paraguariensis, found that the amount of endophytic fungi in adult leaves is higher than in young leaves; Oki, et al. (2009) [11] also observed that the wealth of endophytic in Baccharis dracunculifolia (Asteraceae) increases as the age of the leaves is higher.
Among the treatments with young leaves explants, the fungicide agent was the main factor for the better performance of the pre-disinfection process. The optimum time for the fungicidal action on this study was approximately 5 hours. Times longer than 8 hours showed greater asepsis, however dead tissues. Shorter times than 4 hours caused contamination by fungi in all assays.
According to Esposito-Polesi (2011) [12], one way to overcome the problem of contamination in explants is the application of fungicides and antibiotics, which can act on explant surface contamination. Fungicides are usually quite toxic, and their use should be controlled to do not cause damage to the plant tissues. The ideal use of fungicides is applying directly on the mother plant, where the correct application of fungicide is of utmost importance to the greater disinfestation efficiency. However, Duroia Macrophylla is wild (living in natural habitat in the Amazon forest), where treatment with biocide solution becomes unviable. Therefore, it was decided to directly treat the explants with prolonged biocide solution immersion, which contains Mancozeb as fungicide in its composition.
Several studies have used immersion of explants in fungicidal agents to obtain aseptic cultures: Andrade, et al. (2005) [13] obtained better results in disinfestation of Mangifera indica explants with 30 minutes of immersion in fungicide; Naranjo, et al. (2014) [14] also used in the pre-disinfestation the immersion of Psychotria ipecacuanha (Rubiaceae) leaf explants in fungicide for 2 hours; Medina and Casas (2014) [15], in the disinfestation process of Thymus moroderi (Lamiaceae) used immersion in fungicide for 15 minutes; Regalado, et al. (2015) [16] obtained good results in the disinfestation of Asparagus brachyphyllus and Asparagus pseudoscaber (Asparagaceae) rhizomes using immersion in the fungicide (Benomyl) for 20 minutes.
The best disinfestation treatments, among the best pre-disinfestation group (TPD02), were those who had greater exposure time to ethanol 70%, they being DMJ05 and DMJ06 treatments. It is known that 70% ethanol has germicidal characteristics with greater action in fungi, in addition to their surfactant action. Its initial application facilitates the action of sodium hypochlorite, which has mainly effect on bacteria [7,17].
The plant tissue time exposure in ethanol is usually of a few tens of seconds, but according to the consistency of the tissue, it can reach to few minutes. D. macrophylla leaves, because they are leathery and have a thick epidermis cuticle layer, provides greater difficulty for the contact of pest control agents with their plant tissues. According to Grattapaglia and Machado (1998) [7], when the explant is protected by other tissue layers, greater concentrations and longer exposure times of disinfection agents may be used. Despite the positive results, it is still necessary to perform more disinfestation assays to establish the species in vitro, since the best results were of 50% of the aseptic explants. According to Golle, et al. (2013) [18], one of the main difficulties of in vitro micro propagation, besides the rooting, is to obtain a sufficient number of aseptic cultures. This often occurs due to the presence of microorganisms associated with the explants and the relative inefficiency of disinfestation procedures of woody species, which are normally successfully employed in herbaceous.
Callus induction
Callus formed in D. macrophylla explants presents varied types (multiform), but all are considered undifferentiated cells mass. Werner, et al. (2009) [19] also showed multiform callus from Caesalpinia echinata (Fabaceae) species. According to Termignoni (2010) [20], it is possible to observe variations in the callus tissues distribution pattern, where the division plans and directions taken by growing cells (dependent on endogenous nutritional and hormonal gradients) are going to define the histological patterns in callus tissues.
To obtain callus with uniform cells, the balance of cytokinin and auxin in the medium should be altered. Pereira, et al. (2007) [21], in a study with in vitro Uncaria guianensis species (Rubiaceae), obtained callus with different colors and consistencies depending on the auxins present in the medium; Almeida, et al. (2010) [22] found better development and visual aspect of Mussaenda erythrophylla (Rubiaceae) in vitro callus with the presence of auxin NAA (naphthalene-1-acetic acid) in the medium.
In this paper, the medium for the callus formation process has been selected based on previous studies of the research group with other amazonian woody species and also from literature studies with Coffea arabica (Rubiaceae), where larger percentages of leaf explants callus were obtained by using 4.18 mg L-1 of 2,4-D (auxin) combined with 2 mg L-1 of kinetin (cytokinin), in a ratio of 2:1 [23].
Calli extracts
The analyzes realized with the extracts obtained from D. macrophylla in vitro calli indicated that they did not produce the alkaloids obtained from in vivo leaves and branches extracts in a previous study [24]; since it was not observed any spot on the TLC assays of the samples stained with Dragendorff reagent, and also no signal between 8 and 10 ppm was observed in the 1H NMR analysis, where the signals of the hydrogens attached to the nitrogen atoms of the indole alkaloids previously isolated from the plant can be found [24].
The metabolites productions by in vitro tissue culture do not have always the same proportions as the in vivo production, even searching for the best in vitro plant conditions. The production of certain substances can be reduced or even absent in in-vitro cultures as compared with the in vivo production. Many factors can influence in the production of metabolites, for example, the culture conditions or the type of the culture tissues (calli, seedlings, embryos). In the undifferentiated cell culture the production of specific metabolites can be compromised by the absence of some enzymes that normally would be produced in the vegetal development phase [25]. In these cases, the production of these substances can be induced by the introduction of metabolic precursors in the medium. According to Moreno, et al. (1995), for alkaloids, the utilization of secoiridoids precursors in the medium can be efficient. In studies with Catharanthus roseus, Moreno, et al. [26] showed remarkable difference in alkaloids production in vitro through the use of secoiridoids in the medium.
The presence of terpenes in D. macrophylla calli extracts can be inferred from the TLC revelation of the samples with ceric sulphate and the presence of signals with chemical shifts between ϒH 0.7 and 1.2 in the 1H-NMR spectra. These signals can be an indication that D. macrophylla callus are producing the same terpenes, or similar ones, that the in vivo plant, already isolated by Martins, et al. (2013,2014) [3,4]. However, larger amount of extracts are required to conduct chemical fractionation and then identify the chemical structure of the substances.
Conclusions
Produce technical processes with arboreal species poorly studied and little literature such as Duroia Macrophylla becomes a challenge for Science & Technology. The production of triterpenes and alkaloids with activity on Mycobacterium tuberculosis and an alkaloid with antitumoral activity are some of the attractions of Duroia Macrophylla as potential drugs. The emphasis on production of calli is the search/attempt to obtain mass of the active ingredient for a new product; a new source of active metabolites and thus obtain new raw materials in larger quantities that the plant produces for subsequent large-scale production to purposes of drugs derived from the Amazonian Brazilian biodiversity. The in vitro establishment of Duroia macrophylla callus from young leaf explants was possible with the combination of a pre-disinfestation with immersion on biocide solution (fungicide and bactericide) for 5 hours and disinfestation with the combination of 70% ethanol and NaClO 2%, reaching 50% of efficiency. It needs adapt the disinfestation protocol for a higher yield of aseptic explants. The in vitro callus induction of Duroia Macrophylla leaf explants is possible from the combination of 2,4-D and KIN, forming multiform callus. D. macrophylla callus, under this condition, produces terpenes, but do not produce alkaloids. Some substances found in plant extracts were not found in the calli extracts. Thus, further studies are needed to induce the production of alkaloids in D. macrophylla callus, where the use of elicitors, or other methods, such as the changing medium composition, may induce the production of these metabolites in the callus. This study was based on plant biotechnology, is the first one for Duroia Macrophylla Huber, values the Amazonian biodiversity and develops techniques to a tree with still unknown spatial distribution.
Terpenes have the potential to become raw material for antituberculosis drugs, from the Amazonian tree species D. macrophylla. That is, more broadly, the Brazilian plant biodiversity can be the matrix for the establishment of bases that can be aggregated and aggregators of Science & Technology and with specific use values in their final product.
Acknowledgment
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico: CT-Agro/CNPq, PPBio/CNPq, REPENSA/CNPq/FAPEAM, CT-Amazônia/CNPq, INCT-CENBAM/CNPq, to Coordenacão de Aperfeicoamento de Pessoal de Nível Superior: Pro-Amazônia/CAPES and Fundacão de Amparo à Pesquisa do Estado do Amazonas - FAPEAM, for financial support. The SSZ author thanks CNPq and PPG-Botany/INPA for granted scholarship and financial support.
References
- Giordani RB, Pagliosa LB, Henriques AT, et al. (2008) Investigacão do potencial antioxidante e anticolinesterásico de Hippeastrum (Amaryllidaceae) Antioxidant and anticolinesterasic effects of Hippeastrum species (Amaryllidaceae). Quim Nova 31: 2042-2046.
- Nunez CV, Vasconcelos MC (2012). Novo Alcaloide Antitumoral de Duroia macrophylla. Patent: Innovation privilege, n. PI10201203380, Instituto Nacional da Propriedade Industrial.
- Martins D, Carrion LL, Ramos DF, et al. (2013) Triterpenes and the antimycobacterial activity of Duroia macrophylla Huber (Rubiaceae). BioMed Research International.
- Martins D, Fachin-Espinar MT, Oliveira TA, et al. (2014). Estudio químico y biológico de Duroia macrophylla. J Pharm Pharmacogn Res 2: 158-171.
- Morais TP, Luz JMQ, Silva SM, et al. (2012) Aplicacões da cultura de tecidos em plantas medicinais. Rev Bras Plantas Med 14: 110-121.
- Fumagali E, Goncalves RAC, Machado MFPS, et al. (2008) Producão de metabólitos secundários em cultura de células e tecidos de plantas: O exemplo dos gêneros Tabernaemontana e Aspidosperma. Rev Bras Farmacogn 18: 627-641.
- Grattapaglia D, Machado MA (1998). Micropropagacão. In: Cultura de tecidos e transformacão genética de plantas. EMBRAPA, Brasília, Distrito Federal 1: 183-260.
- Pierik RLM (1990). Cultivo in vitro de las plantas superiores. Martins: Nijoff, 326.
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Phisiologia Plantarum 15: 473-497.
- Pimentel IC, Kuczkowski FR, Chime MA, et al. (2006) Fungos endofíticos em folhas de erva-mate (Ilex paraguariensis A. St.-Hill.). Floresta 36: 123-128.
- Oki Y, Soares N, Belmiro MS, et al. (2009) Influência dos fungos endofíticos sobre os herbívoros de Baccharis dracunculifolia (Asteraceae). Neotrop Biol Conserv 4: 83-88.
- Esposito-Polesi NP (2011) Microrganismos endofíticos e a cultura de tecidos vegetais: quebrando paradigmas. Rev Bras Biocien 9: 533-541.
- Andrade SEM, Pinto ACQ, Faleiro FG, et al. (2005) Desenvolvimento e avaliacão de protocolos para descontaminacão de explantes de mangueira visando à micropropagacão. Bol. Pesq. Desenvolv. 153, 1 ed. EMBRAPA Cerrados, Planaltina, 24.
- Naranjo EJ, Urrea AI, Atehortua L (2014) Avances en la propagación vía embriogénesis somática de Psychotria ipecacuanha (Brot.) Stokes, planta medicinal en peligro crítico. Rev Colombiana Biotecnol 16: 86-92.
- Medina AM, Casas JL (2014) In vitro multiplication and essential oil composition of Thymus moroderi Pau ex. Martinez, an endemic Spanish plant. Plant Cell Tissue Organ Culture 120: 99-108.
- Regalado JJ, Carmona-Martin E, Castro P, et al. (2015) Micropropagation of wild species of the genus Asparagus L. and their interspecific hybrids with cultivated A. officinalis L., and verification of genetic stability using EST-SSRs. Plant Cell Tissue Organ Culture 120: 501-510.
- Sousa GC, Clemente PL, Isaac VLR, et al. (2007) Contaminacão Microbiana na Propagacão in vitro de Cattleya walkeriana e Schomburgkia crispa. Rev Bras Biociênc 5: 405-407.
- Golle DP, Reiniger LRS, Bellé RA, et al. (2013) Desinfestacão superficial de explantes isolados de ramos semilenhosos e herbáceos de Eugenia involucrata (Myrtaceae). Cerne 19: 77-82.
- Werner ET, Cuzzuol GRF, Pessotti KV, et al. (2009) Controle da calogênese do pau-brasil in vitro. Rev Árvore 33: 987-996.
- Termignoni RR (2005) Cultura de tecidos vegetais. UFRGS, Porto Alegre, Rio Grande do Sul, 182.
- Pereira RCA, Pinto JEBP, Reis ES, et al. (2007) Influência de diferentes auxinas na inducão e cinética de crescimento de calos de Uncaria guianensis J. F. GMel. (Unha de gato). Plant Cell Culture Micropropag 3: 69-77.
- Almeida JL, Diniz JDN, Oliveira AB, et al. (2010) Propagacão in vitro de Mussaenda (Mussaenda erythrophylla cv. Rosea). Pesq Agropec Trop 40: 206-212.
- Maciel ALR, Pasqual M, Pereira AR, et al. (2003) Embriogênese somática indireta em explantes foliares de Coffea arabica L. CV. Obatã. Ciência e Agrotecnologia 27: 107-116.
- Martins D (2014) Estudo químico e biológico de Duroia macrophylla Huber (Rubiaceae) [thesis]. Universidade Federal do Amazonas, Program of Biotechnology 231.
- Sierra MI (1991). Aspects if indole alkaloid accumulation in Tabernaemontana tissue cultures: differentiation, peroxidades and stability [thesis]. Leiden Universiteit.
- Moreno PRH, Van Der Heijden R, Veerporte, R (1995) Cell and tissue cultures of Catharanthus roseus: a literature survey. Plant Cell Tissue Organ Culture 42: 1-25.
Corresponding Author
Cecilia Veronica Nunez, Bioprospection and Biotechnology Laboratory, National Institute of Amazonia Research - INPA, Av. André Araújo, 2935, Petrópolis, CEP 69067-375, Manaus-AM, Brazil.
Copyright
© 2016 Zanca SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.