Sensitizing Breast Cancer Cells Using Standard Microwave Devices and Temperature Monitoring With Non-Invasive Thermochemical Thermometers
Abstract
Purpose: To review the effect of combined radiotherapy and hyperthermia in the treatment of recurrent breast cancer, two microwave antennas were compared through in vitro experimental tests, and the patient's skin temperature was monitored with calibrated thermo chemical thermometers. The main purpose of this research is to study the influence of shape of microwave antennas on the temperature transmission. The secondary goal is to select treatment procedures to ensure Good Clinical Practice (GCP) of hyperthermia using non-invasive thermometers.
Material and methods: Two "SIRETHERM 609 S" microwave devices (433 MHz) were used to treat two "retrospectively registered" patients with loco-regional recurrent breast carcinoma (T3 and T4 stages: TNM classification). In vitro studies were conducted by using the cube water phantom to verify the uniformity and stability of heating, and to compare antennae of different shapes. Two non-invasive thermo chemical thermometers (colour-coded) are calibrated using two alcohol "VWR Precision" and ImageJ image processing tool.
Results: The current stability of each power amplitude is studied, and the constancy of the transmitted generator frequency is confirmed. The radio sensitivity effect of hyperthermia has obtained consistent results, showing how to monitor the temperature with non-invasive thermometers and Good Clinical Practice (GCP) in hyperthermia using standard microwave equipment.
Conclusions: The feasibility of the SIRETHERM device as a palliative treatment for locally recurrent breast cancer has been proven, demonstrating how to use a non-invasive thermometer to monitor the temperature. The experience and lessons of how to conduct microwave hyperthermia in an appropriate way have been discussed. All these lessons are necessary for the Good Clinical Practice (GCP) of using microwave devices for hyperthermia. The radiation sensitivity of hyperthermia has not been displayed. For this reason, large-scale clinical research is required, which is not obvious.
Keywords
Antenna, Microwave, Non-invasive, Thermo chemical, Thermometer, Breast cancer
Introduction
Breast cancer is the most common type of cancer worldwide, accounting for 16% of all cancers (WHO). Recurrent breast cancer is usually aggressive, and the difficulty of treatment results in a short survival term from ablation [1]. Despite new treatment options, it is still a challenge for interdisciplinary treatment [2]. Hyperthermia induces an increase in the sensitivity of the tumor to subsequent combination [3] therapy whether it is radiotherapy [4-8], chemotherapy [9,10] or the "triple mode of radiotherapy and chemotherapy" [11-13]. Although, recurrent breast cancer is affected by effective treatment options.
The goal of any cancer treatment is to maximize the destruction of malignant cells while causing minimal or acceptable damage to normal tissues [14,15] the heat-killing process occurs in all stages of the cell cycle (G1, S, G2, M, G1), showing its necessity as the original treatment process in tumor treatment [16]. The ability to use heating to inhibit the DNA repair process, affect the blood flow and oxygenation of the tumor, and cause direct cytotoxicity to acidosis and nutrient-deficient cells [15].
Depending on the location (superficial or deep) and size of the tumor [17], several heating methods have been used (ultrasound, magnetic induction, high-frequency current fields, and microwaves). Therefore, a complete set of radiant heating devices have been used in hyperthermia [18]. Several studies have shown that the combination of radiotherapy and hyperthermia is very effective [19,20].
In the past few years, the prospects for successful introduction of hyperthermia in the field of radiation oncology have increased due to the technological realization of selective and controlled thermal application in optimized devices. Refer to the ESHO guidelines: The European Society of Hyperthermic Oncology [21], hyperthermia in clinical research is completely dependent on its thermal effect on the tumor, which depends on temperature and time. Since breast cancer is a disease with significant tumor cell heterogeneity and blood flow distribution in low-flow areas of the tumor, the temperature map indicates an uneven temperature distribution throughout the tumor [22].
Therefore, hyperthermia is very challenging in cancer treatment, especially when dealing with temperature monitoring. At all frequencies, the dielectric properties in tumor tissues are greater than those in normal tissues of the same kind, and the conductivity of each part of the tissue is different [23-25].
During hyperthermia, the tumor tends to become hotter than the surrounding normal tissue due to the slower blood supply and slower heat dissipation. Heating will reduce the blood flow in the tumor, leading to hypoxia and acidosis, and further increase the possibility of killing high temperature tumor cells [19]. The relationship between Thermal Skin Damage (TSD) and the time-temperature effect levels of breast cancer recurrence patients treated with a combination of hyperthermia and radiotherapy was studied [20]. For this reason, past treatment history is important for estimating risk factors for TSD. The current work is to deal with the causes of hyperthermia procedures to avoid or reduce TSD to maintain and prolong a good quality of life. Several studies have focused on evaluating the efficacy of re-radiotherapy combined with hyperthermia after surgery for locally recurrent breast cancer [26-28].
Some works are interested in descriptions of hyperthermia procedures and detailed information about the heating techniques used. Important developments in hyperthermia are treatment planning and non-invasive temperature monitoring. These tools will help make it easier and better to control its application and effectiveness [29].
Indeed, temperature measurement during hyperthermia needs to be accurate to prevent thermal toxicity. There are two groups of temperature monitoring techniques: The former includes invasive techniques based on the use of thermocouples and fibre-optic sensors; the second analyses non-invasive methods, i.e. magnetic resonance imaging, computerised tomography and ultrasound-based thermometry [30]. The last group is expensive and requires these instruments to be installed near the heating equipment. However, color-coded thermo chemical thermometers are very simple, non-invasive, and inexpensive.
In this study, two microwave devices have been used to induce heat sensitization of superficial tumors in local areas. Then, attempts to enhance the technology are shown; some improvements related to the treatment procedures have been revealed. The same microwave device as the last clinical study [5-7] is used. The current SIRETHERM device has the same frequency as the Lucite device [31,32].
However, there is very little information about the heating device SIRETHERM 609. Because it is inexpensive and primarily used in physiotherapy, especially in diathermy [33], it can be a good choice to become the standard equipment for hyperthermia today.
Due to the hot spots caused by the non-stability of heating, the choice of the procedure is believed to be of great importance in the Good Clinical Practice (GCP) of hyperthermia. Unfortunately, at present, there is limited data in the literature concerning the monitoring of temperature delivered by "SIRETHERM 609 S" microwave antennae, especially during hyperthermia treatment of recurrent breast carcinoma. In this context, hyperthermia guidelines have been included by the National Comprehensive Cancer Network (NCCN) as an option for the treatment of breast cancer recurrences [34].
The current focus of work is on in-vitro and clinical studies using two microwave "SIRETHERM 609 S" devices for inspection. Since temperature rise is the main parameter that characterizes the performance of the applicator [32], the goal is to evaluate the delivery temperature of the two devices. As far as we know, the in vitro temperature measurement provided by the "SIRETHERM 609 S" device and temperature monitoring using non-invasive thermochemical thermometers (color-coded) have never been performed before.
Materials and Methods
Microwave device "SIRETHERM 609 S" and applicators
The "SIRETHERM 609 S, Pyrodor applicator", manufactured by Siemens and Paul Scherrer Institute (Switzerland) is a thermotherapy device. It is an Electromagnetic "EM" radiant heating system: Its applicator operates in the 434MHz ISM frequency band. It provides a power range of (50 - 250) Watts. The device has low penetration (∼ 1.5 cm), sufficient for superficial treatments [6,9,32].
A single radiation aperture connected to the power amplifier can independently control 0-100% output power (power range 1-7).
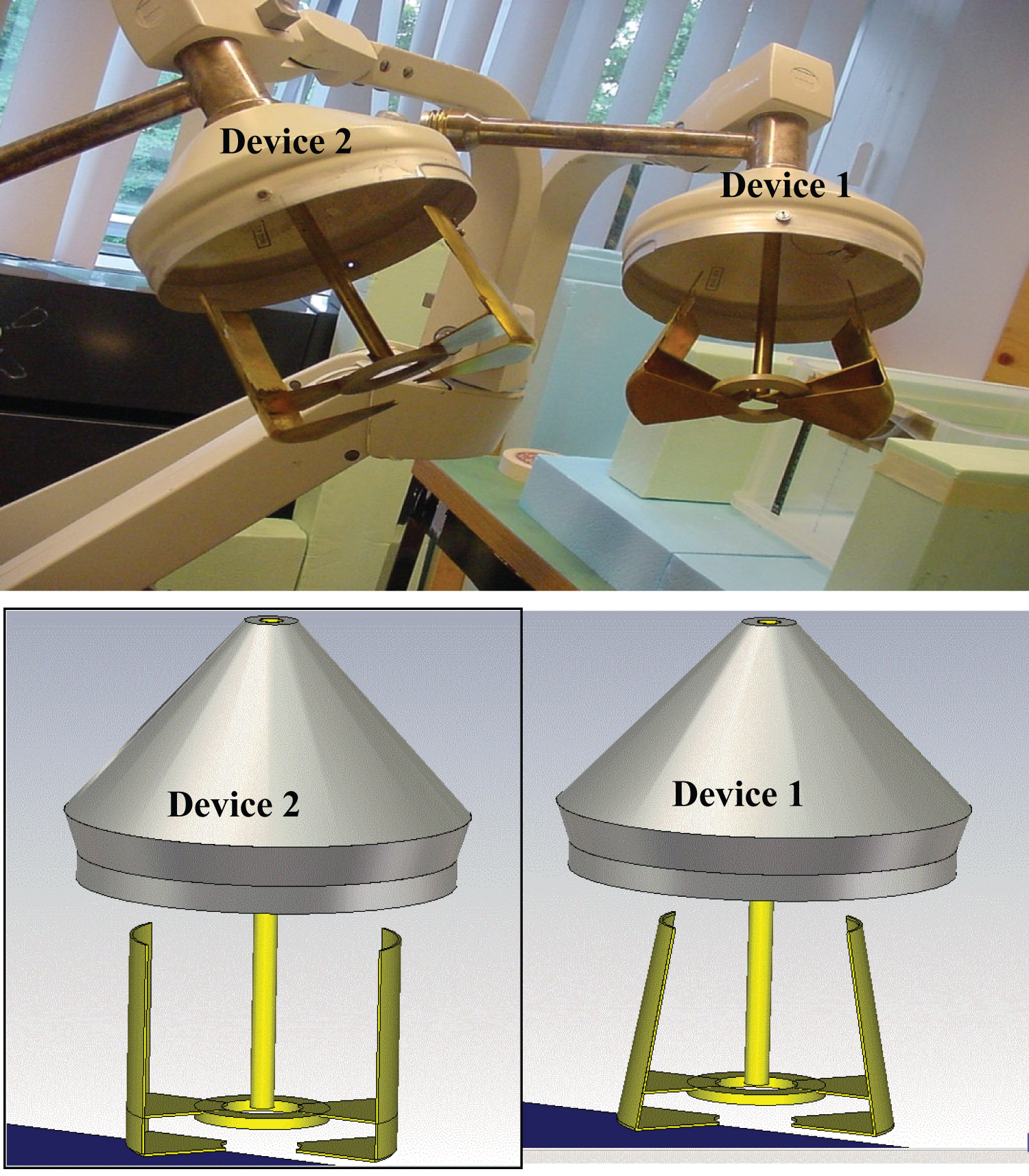
Two "SIRETHERM 609 S" were used. Two different antennas (or applicators) have the same size but two different shapes. The first has a trapezoidal shape, but the second has two parallel copper plates, corresponding to devices 1 and 2, respectively, as shown in Figure 1. The radiation aperture of both antennas is 10 cm × 10 cm.
Both devices 1 and 2 are used for in-vitro experimental testing and superficial hyperthermia treatments. Seven power settings (1-7) are provided, corresponding to the heating range.
This kind of equipment is mainly used for physiotherapy, and the price is low, which is the focus of our research.
Experimental set up of in-vitro studies
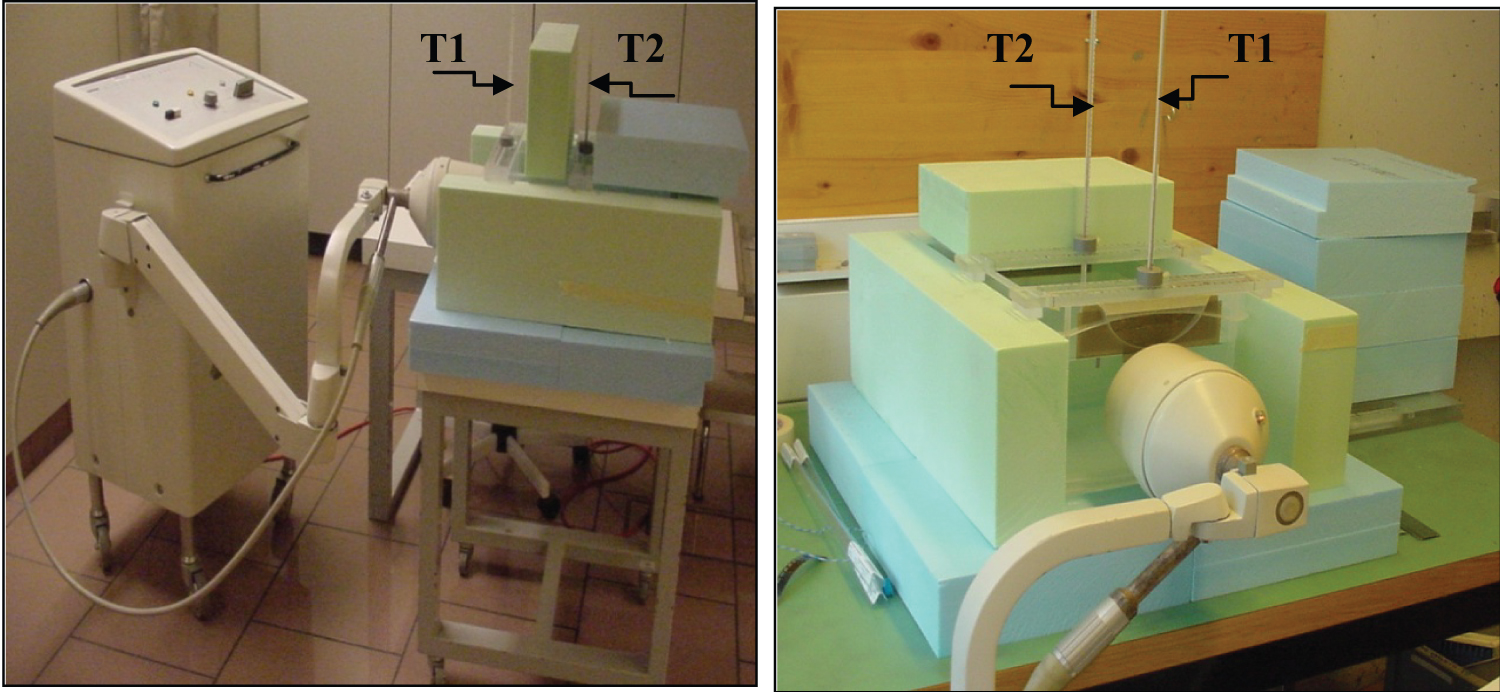
A 15 cm × 36 cm × 26 cm cube phantom filled with water is used to measure temperature distribution and calibrate thermo chemical thermometers. As shown in Figure 2, the phantom is isolated by insulating polystyrene. Here, it is biased to mention that 0.6% salt water is usually used for the 434 MHz microwave frequency. The choice of water is only for price reasons.
All the tests are established under the same isolation conditions. This experimental study does not consider the heat loss from the water through conduction. In-vitro measurements are performed in the radiotherapy simulator room. The heating method and technical equipment of the cube phantom are the same as those of the patient. The temperature measurement is monitored by two alcohol "VWR precision" and thermo chemical (℃/℉) thermometers. One alcohol-thermometer is placed in the center of the cube phantom and the other is close to the applicator as shown in Figure 2. The thermochemical thermometer is placed on the front of the cube phantom near the second alcohol thermometer.
Calibration of the thermochemical thermometer
The temperature measurement is carried out by placing two alcohol "VWR precision" and a thermo chemical (℃/℉) thermometer, as shown in Figure 2. Since the calibration test does not require heating with microwave device, the cube phantom is filled with water heated in a 'water-bath' at a temperature close to 45 ℃ and the temperature drop is observed every 5 minutes. To scan the thermo chemical thermometer temperature, a photo is taken for each measurement. The temperature of the alcohol thermometer is manually scanned. The measurement is performed under the condition that the average room temperature of 24 ℃ and the humidity does not exceed 60%.
Calibration is based on visual readings using the "Image J" software package [35] (Version 1.71: Based on Image J Release 1.36). This comparison took a long time and the visual reading was about the same (± 0.5 ℃). In addition, during hyperthermia, the temperature needs to be read quickly and intuitively.
In order to read the approximate temperature value on the thermo chemical thermometer intuitively (Figure 3), first the green is used as the reference temperature value and compared with other colors. In general, the color before green is blue (called pre-green) and the color after green is red or orange (called post-green). In order to read the temperature easily, the pre-green is ignored and the post-green is considered, which depends on the shade of greens and red. If the post-green is green, the second green value is increased by half a degree Celsius, as shown in the left part of Figure 3. If the two reds are regarded as post-green, the first red value plus the value of one-tenth of a degree Celsius are considered, as shown in the right part of Figure 3. The error of the temperature visual reading here can reach 0.5 ℃elsius.
In-vitro Experimental Tests
Uniformity of heating
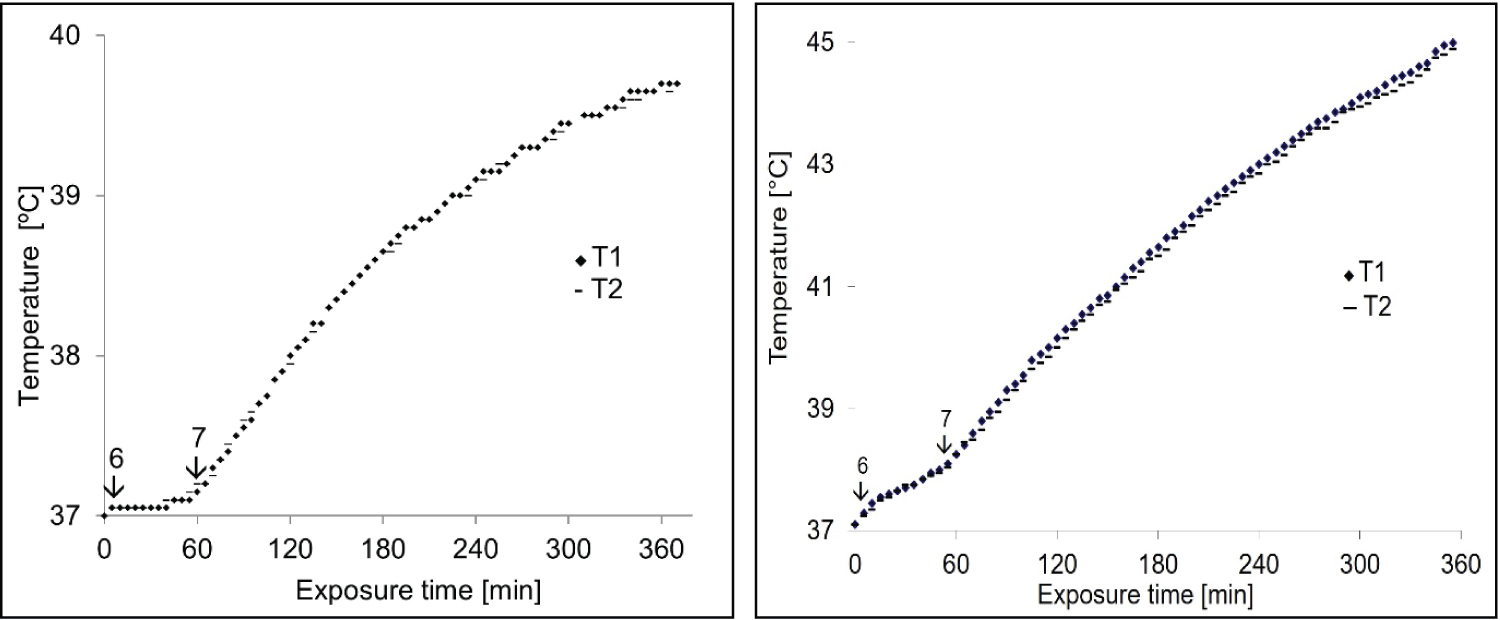
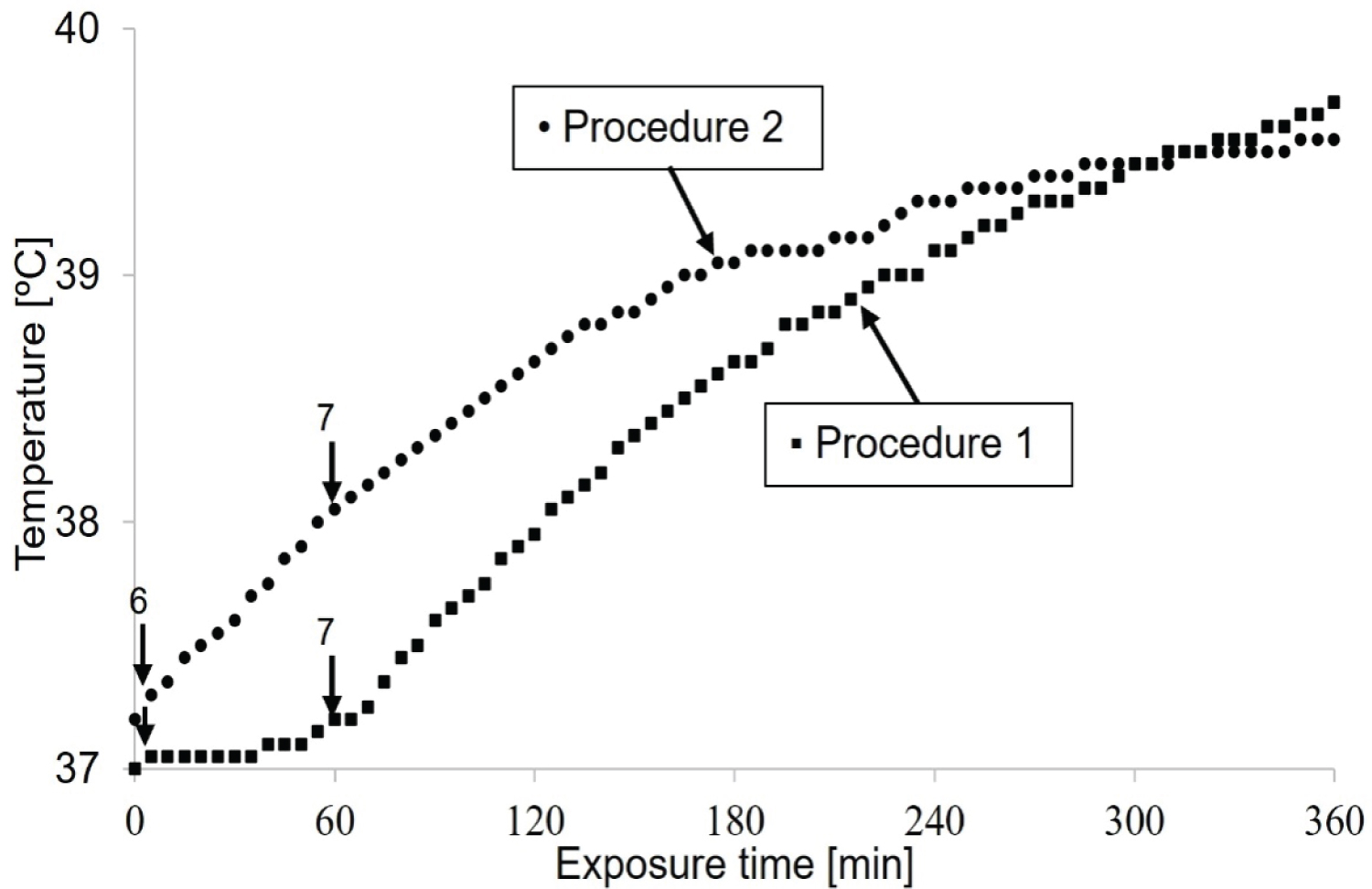
To verify the uniformity of heating, as predicted in Figure 2, the temperature is measured in the same bath at the closest distance to the applicator (T1) and the center of the cube phantom (T2). The heat treatment started from the lowest power level of the devices (1-5) until the normal body temperature (37 ℃) was reached. Then, the amplitude 6 and 7 is turned on until the heating stabilizes, and stopped after 6 hours (Figure 4).
Comparison between the devices 1 and 2
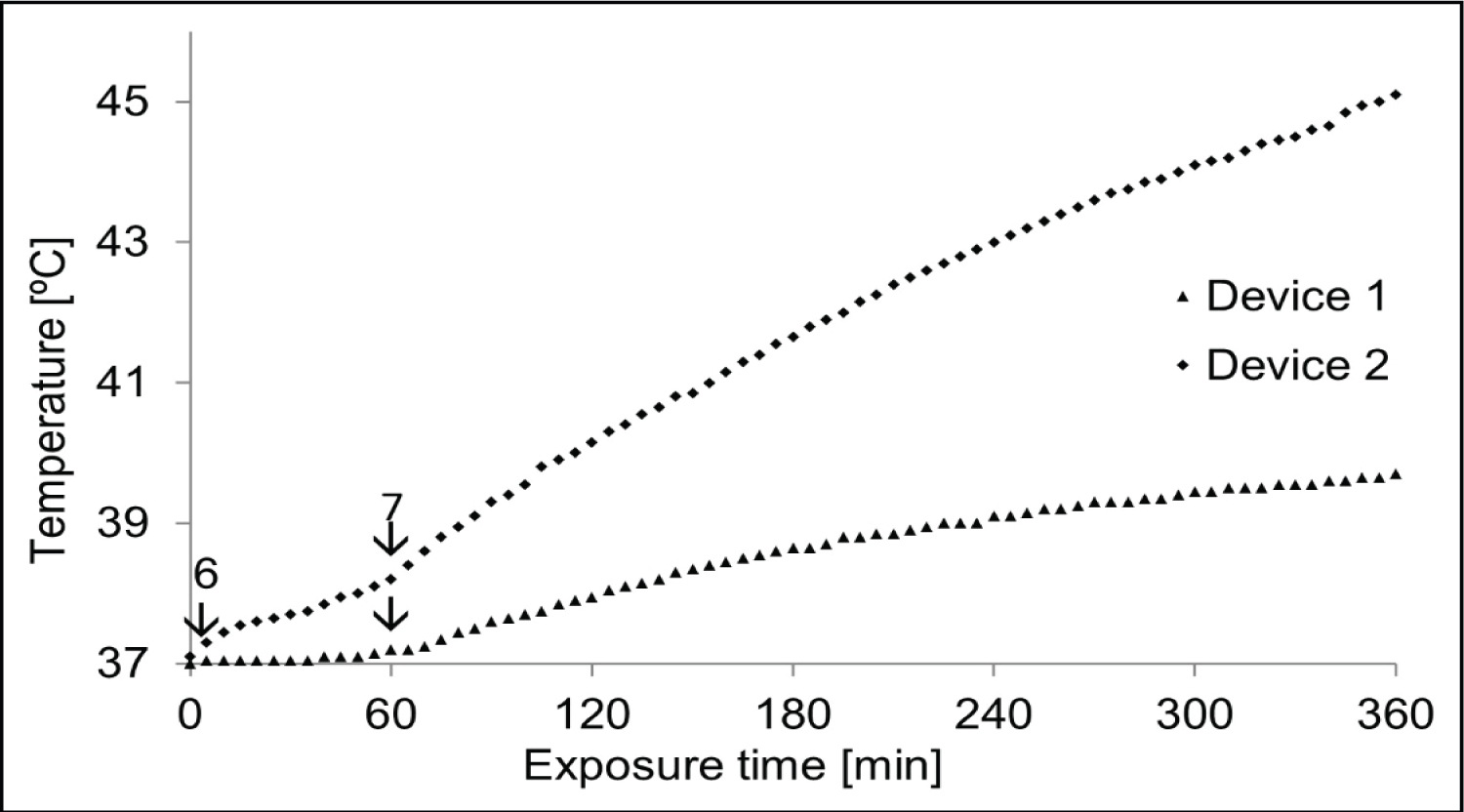
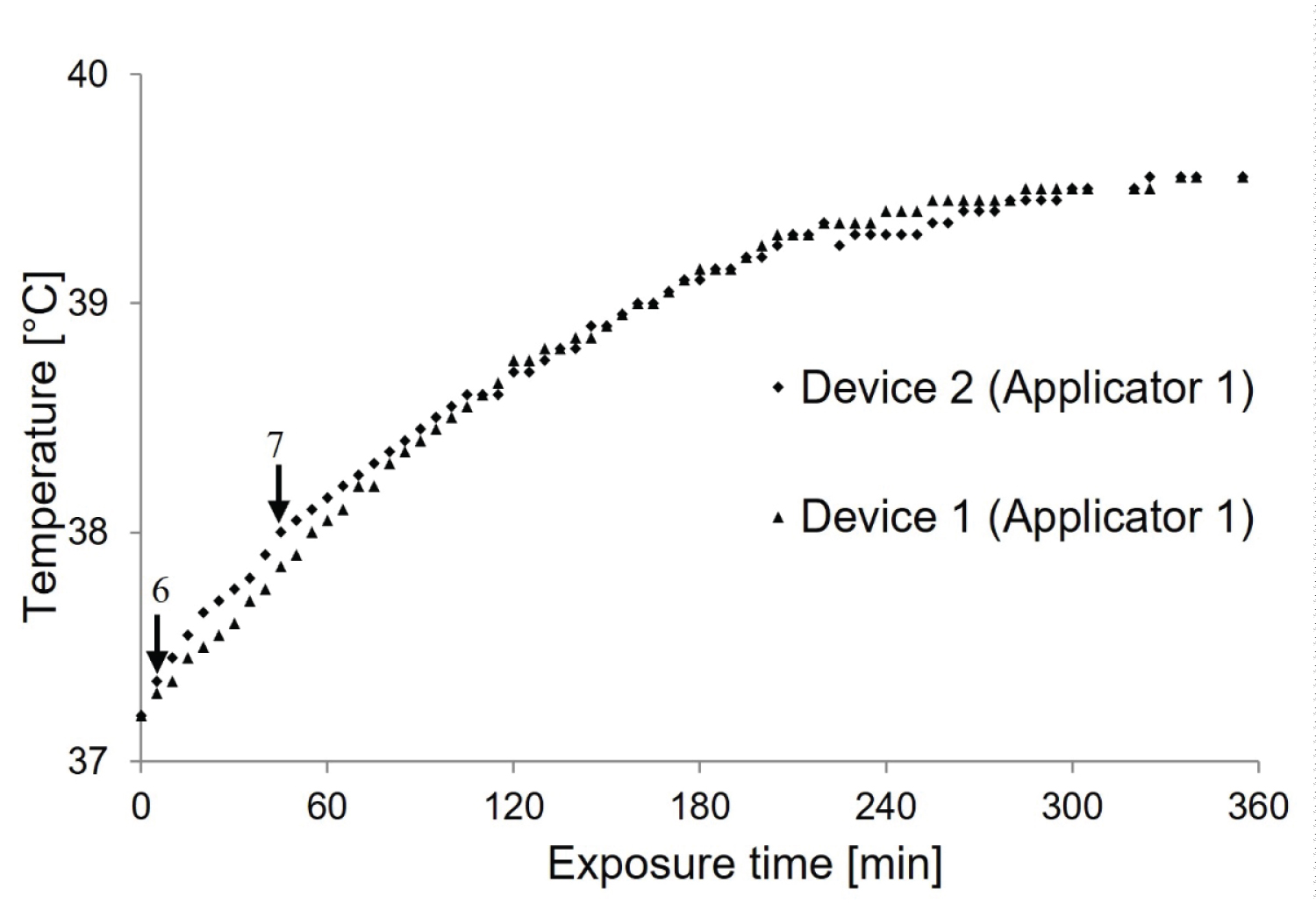
A comparison between device 1 and device 2 was performed, maintaining the same heating procedure (Figure 5).
The superposition of the temperature-time curves of the two devices confirms that the heat intensity of device 1 and device 2 are different. Notice that the second device heats more than the first device. To determine the cause of this additional heating, the relative amplitude of each power and the frequency of the two generators were measured. Figure 5 demonstrates the difference between parallel plate (device 2) and trapezoidal (device 1) antennas in terms of electric field distribution, temperature transfer and thermal dose distribution.
Measurement of the generator frequency and the relative amplitude provided by the two generators at each power amplitude
An oscilloscope (Lecroy) with a bandwidth of approximately 500 MHz and 1M 𝛀 resistor was used. To upgrade the bandwidth at the 1 GHz frequency, a resistor of about 50 𝛀 was added, that broadly covers the frequencies generated by the two devices.
The oscilloscope signal obtained through the 10 cm tip, placed on the applicator of the electromagnetic power from devices 1 and 2 has been registered. The results show that the power provided by the generator is between 50 and 250 W. Therefore, it is necessary to take a turn with a small grass 5 times the initial distance. The distance between the spire and the applicator is significantly greater than the maximum current amplitude supported by the oscilloscope. The purpose is to be able to read the highest power amplitude 7.
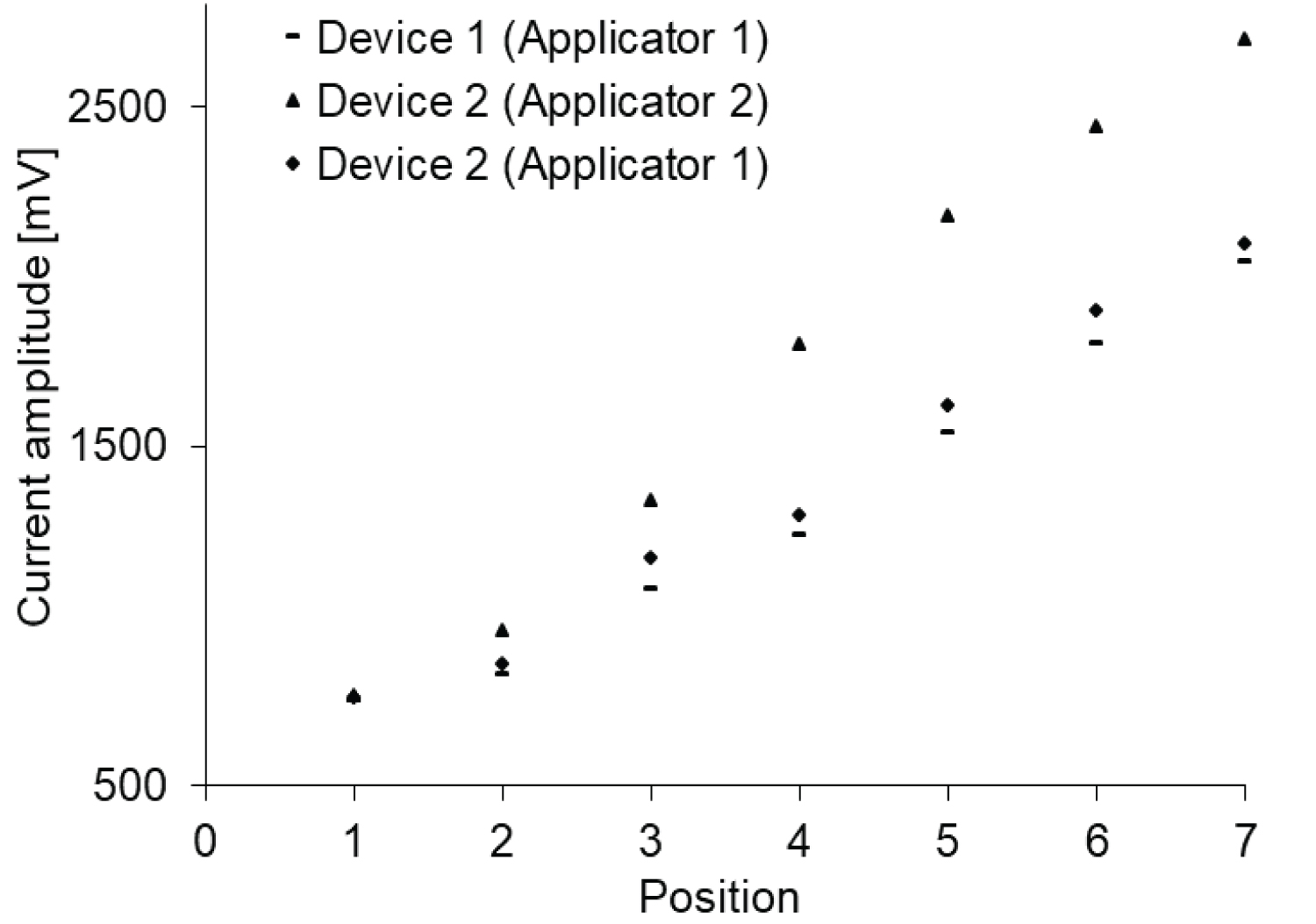
The oscilloscope signals of the two devices have been verified. A similar behavior of the two oscilloscope signals is observed. In addition, the relative current measurements for each power amplitude are also derived. First, the current amplitude of each device with its own applicator (device 1 with trapezoidal applicator and device 2 with parallel copper plates) is measured. Secondly, the applicator of device 2 is changed through the applicator of the device 1, and the relative amplitude is compared as shown in Figure 6.
Choosing the right procedure for treatment
When there are microwave devices with different power amplitudes (1-7), it is more suitable to find the best treatment procedure before starting a clinical trial. Therefore, an in vitro test was established using a cube model heated by device 1. The only difference is the heating power level. Two treatment procedures were considered in this study:
Procedure-1 involves heating water at a temperature below 37 ℃ with a power level of 5, and after reaching the required temperature (37 ℃) in a short time, the level 6 is used. After one hour, the amplitude 7 is turned on until the end of heating, as shown in Figure 7.
Procedure-2 involves measuring temperature by slowly and gradually changing the power amplitude every (5-10) minutes. Water was heated starting from amplitudes 1-5. At amplitude 6, the temperature reached 37 ℃. After heating for one hour, the amplitude 7 was selected until the end of heating, as predicted in Figure 7.
Comparison of the two antennae under the same power amplitude
The second device with parallel copper plates provides higher current amplitude than the first device with a trapezoidal shape. There is evidence for further comparison with applicators 1 and 2 fixed to device 1. To consolidate the additional idea that the antenna shape is responsible for the heating of device 2 compared to device 1, the temperature measurement is established using device 1 with its applicator and device 2 with the applicator of device 1 (Figure 8).
Since the Good Clinical Practice (GCP) of hyperthermia is ensured when moving slowly from power amplitude 1 to the highest amplitude (7), the procedure is suitable for this study when the temperature reaches approximately 37 ℃.
When the temperature reaches 38 ℃, the power amplitude 6 is turned on and then 7 is applied and maintained to a steady state.
Hyperthermia using both devices "Interference":
For large breast cancer areas, a full coverage with only one applicator is impossible. Adding a separate heating area to cover all tumors will result in a significant decrease in radio sensitivity. For these reasons, the two EM applicators of devices 1 and 2 can be combined into a large multi-element array, and the individual power control of each element can achieve two-dimensional power steering by adjusting the power of a single element [36-38].
In this study, two calibrated thermo chemical thermometers were considered, facing the applicators 1 and 2 and measuring the temperature T1 and T2, respectively.
Clinical Cases
A retrospective registration study was conducted on two patients (patient 1: 76-years and patient 2: 84-years) (Table 1) with recurrent breast cancer after surgery. Both patients received superficial radiation therapy after microwave hyperthermia. The two selected patients underwent almost the same treatment. Prior approval is obtained from the competent ethics committee and the Federal Agency for Therapeutic Products. During the trial, adverse reactions and amendments are always reported to the Swissmedicethics committee. In addition, both patients signed a special informed consent form before treatment.
One or two "SIRETHERM 609 S" devices (for patient 1) are used twice a week for local hyperthermia below 44 ℃ and in almost all cases immediately before external electron beam therapy. The overlapping thermal field covers the entire radiation field, according to the position of the applicator as shown in Table 1. Hyperthermia never stops. When the patient feels hot, the applicator is only moved 1 or 2 cm, or the power is reduced by changing the amplitude. Note that after surgery on certain parts of the body, the heat sensation will disappear.
The temperature distribution of the treatment area is continuously monitored and photographed by one or two calibrated thermo chemical thermometers (℃/℉) facing the treatment area. In addition, visual inspection of the temperature color-coded image is used to guide the correct centering of the heating area and avoid TSD caused by hot spots.
After 40-60 minutes of uninterrupted hyperthermia, irradiation is performed using a precise linear accelerator (15 MeV) and clinical dose (2 Gy) is used. A total dose of 32-40 Gy is given to patients within 4-5 weeks (twice a week). The average duration of hyperthermia for the second patient was 60 minutes, and for the first patient who received interference during treatment, it was approximately 40 minutes.
During the treatment, the patients maintain the same posture, lying on their backs. The frequency is always maintained at 433 MHz. The power amplitude is changed from 1 to 7 keeping the applicator in the same position.
Results
In-vitro studies
The applied current distributions of the two devices both oscillate at a single frequency of 433 MHz. With reference to the work of Otasowie [39], the size of the antenna is proportional to the wavelength and ultimately depends on the generator itself. Therefore, the constancy of the frequency is ensured. It can be concluded that the two generators provide the same frequency.
As shown in Figure 8, after heating for 4 hours, the steady state remained stable at a temperature of about 39.5 ℃.
A comparison between these two microwave devices with different antenna shapes revealed evidence of the effect of shape on temperature transfer (Figure 8).
Clinical cases
The temperature-time curves of the two microwave devices during the entire treatment process were drawn for two patients. Table 1 provides more detailed information about the different sessions, programs, and devices.
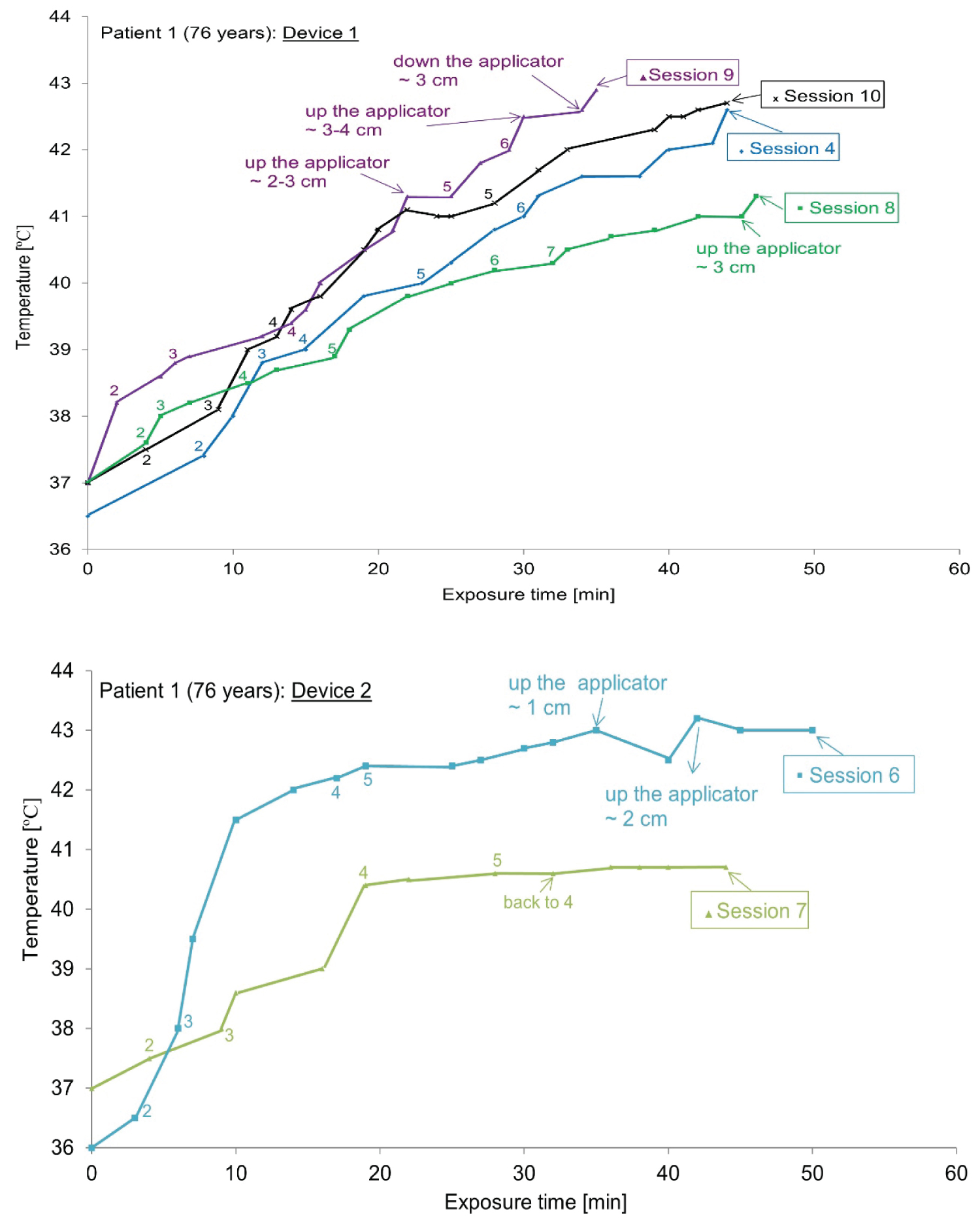
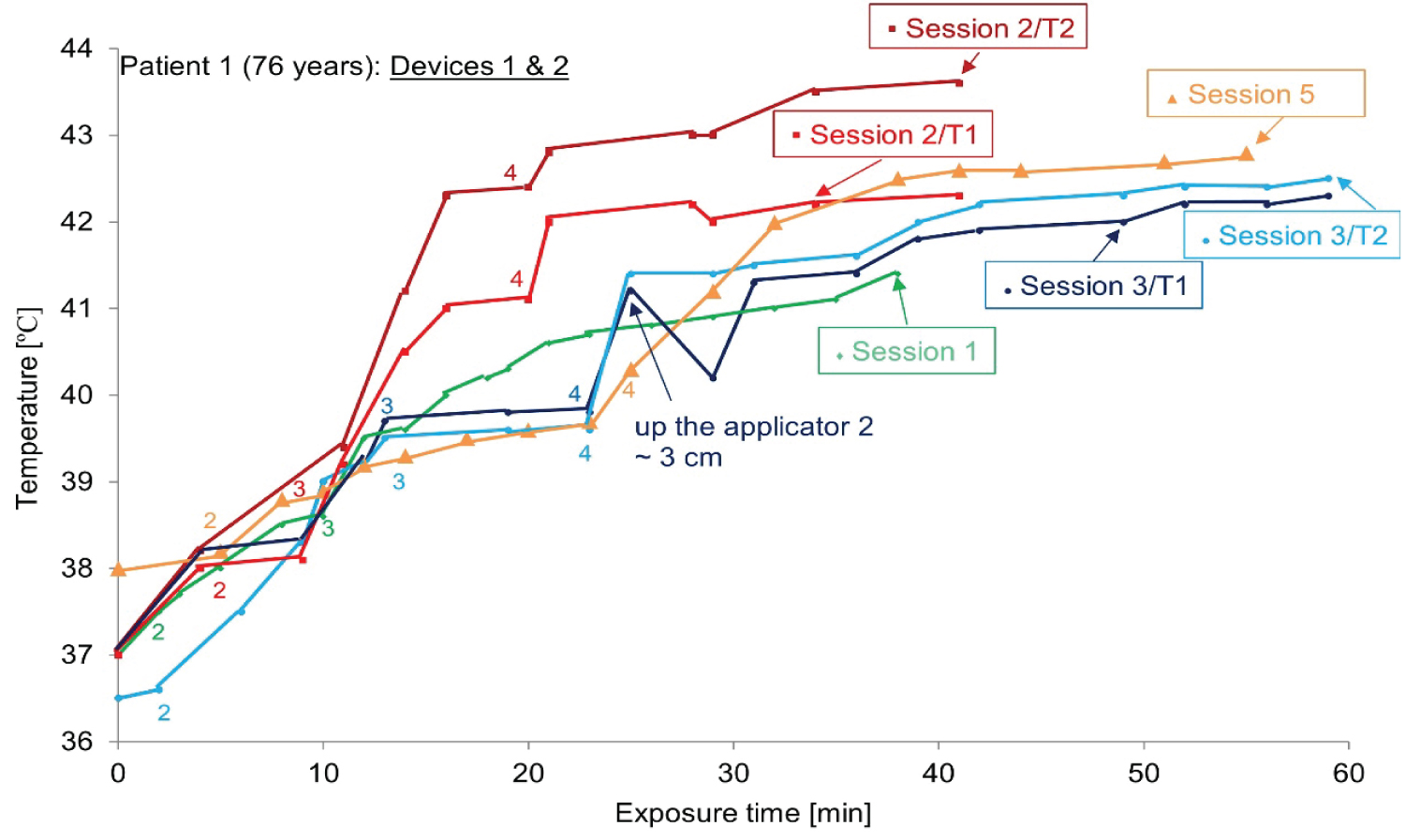
The case of ‘Patient 1’: To avoid TSD and take into account the heat sensation of patient 1, sometimes only one device is used (1 or 2, depending on the doctor's prescription).The temperature-time curve during the whole treatment process is drawn in Figure 9 for patient 1. A calibrated thermo chemical thermometer facing the applicator is used to measure skin temperature (T1 for the applicator 1 and T2 for the applicator 2).
The manual scan of the skin temperature of patient 1 was recorded, and the average value was 36 ℃ to 44 ℃ as shown in Figure 9. As shown in Figure 10, when using both devices to cover the large area, the treatment temperature (42-43 ℃) and steady state for each session are reached after approximately 10 minutes.
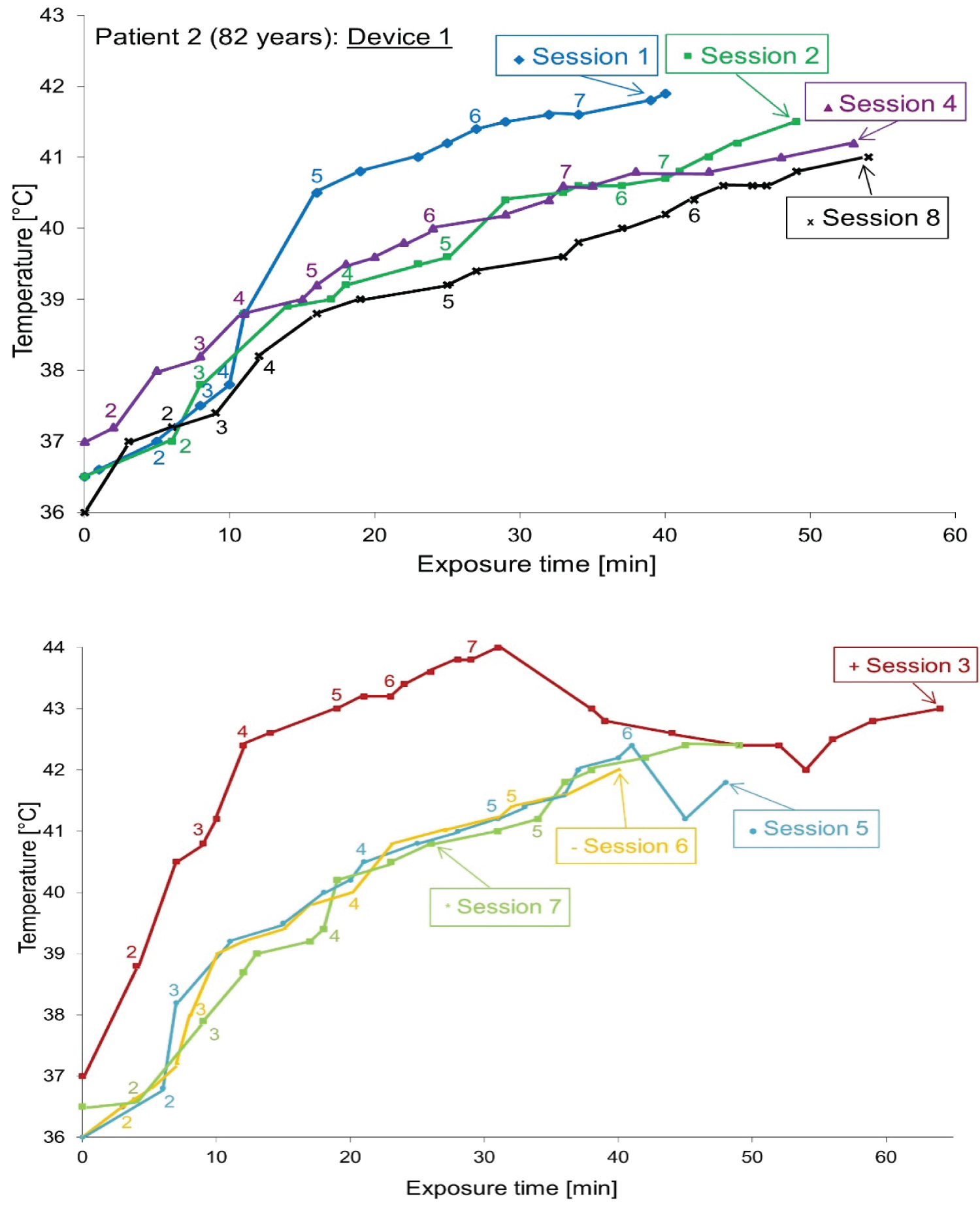
The case of ‘Patient 2’: Despite the ulcers, patient 2 had very limited breast cancer, and the heat sensation was even less than that of patient 1. Therefore, there is only one device for hyperthermia, and a thermo chemical thermometer facing the applicator to measure skin temperature. Figure 11 plots the temperature-time curve for patient 2 during the entire treatment process.
Discussion
In vitro studies
Uniformity of heating, constancy of frequency and stability of amplitudes: The temperature time curve (Figure 4) provides information about the temperature distribution. These curves confirm the uniformity of the thermal dose deposition of the two devices throughout the cube phantom. The stability of heating is ensured by an uncertainty of approximately ± 0.1 ℃.
No difference was observed in the transfer temperature of the two devices at different amplitudes. However, for device 2, parallel copper plates, the transferred temperature increased over time. For this reason, better thermal isolation is needed to obtain linear temperature and time dependence and reach a steady-state point. Knowing that the uniformity of power deposition is better determined with more than two temperature sensors. The reason why, one alcoholic thermometer is placed in the center of the cube phantom and the other one is in the front of it.
Choosing a treatment program: an indicator of Good Clinical Practice (GCP): The analysis of Figure 7 confirms that the procedure 2 is associated with Good Clinical Practice (GCP) regarding hyperthermia, when heating slowly and gradually. Higher temperatures are achieved, and heating stability is ensured.
Since the resistivity of the human body is smaller than that of water, it can quickly reach a steady state at a higher temperature [23,24]. Otherwise, the temperature of muscle and fat must never exceed 44 ℃ [23]. In addition, muscle is the tissue with the largest dielectric constant, its relative permittivity is and the effective conductivity is [S.m-1] at 433 MHz [23].
In summary, the shape of the antenna is increased by the temperature transmission. Similarly, our research results are consistent with the finding of Rietveld, et al. and concluded that the use of cone applicator provides a better invasive temperature in breast cancer treatment areas with surface hyperthermia without any negative side effects.
As demonstrated by Otasowie [39], open waveguide is an inefficient energy radiator due to aperture impedance mismatch; it can be improved by simply flaring the end of the waveguide. This flaring forms a horn antenna.
Clinical Cases
The temperature supported by the two patients maintained a constant behaviour throughout the exposure time. During the heating process of about one hour, the temperature of normal tissues should not exceed 43 ℃. The course of hyperthermia is described in Table 1.
As shown in Figure 9 (device 1 (up) and device 2 (down)), the temperature evolution related to the exposure time has the same profile for device 1 but device 2 gives a large variation between sessions. The treatment temperature (~43 ℃) is reached after about 10 minutes. Approximately, the temperatures increase when device 2 and device 1 are used. Later, an appearance of excessive heat and hot spots in patient 1 while using device 2 which explain the effect of antenna shape.
In addition, according to the simulation results by using CST Studio software (Dassault systems, Vélizy-Villacoublay, France) [40], the evaluation of the SAR distribution of the two antennas shows that the maximum values of the antenna with two parallel plates for 1g and 10g tissues are 2.53 W/kg and 1.63 W/kg, respectively. The value of trapezoidal second antenna for 1g is displayed as 1.81 W/kg, and the value for 10 g is displayed as 1.43 W/kg.
As shown in Figure 9 (patient 1) and Figure 10 (patient 2), the temperature changes related to exposure time have the same distribution for device 1, but device 2 gives changes between sessions. Here, the instability of the heat transferred by device 2 is confirmed. In vitro studies are considered a reliable method as the basis for selecting the best hyperthermia treatment procedures. Compared with the initial tumor shape, even in the presence of normal tissue limitations, the results after treatment are even more impressive (the case of patient 2, no sensation of heat).
Using one or two devices, a detailed heat map of the hyperthermia process was drawn for patients 1 and 2. For all sessions, the curve tends to have a typical continuous temperature graph, which is related to the exposure time, including the initial build-up and plateau period.
Compliance of this work with the guidelines of the European Hyperthermic Society
The European Hyperthermic Society guidelines were recently released (2017), and the work described in this article is established previously. Then, it is difficult to follow recently collected guidelines when conducting clinical trials in advance. However, present studies meet some specific requirements of the clinical research protocol, even for technical tests (A) [41] or clinical trials (B) [21], applying hyperthermia to the defined clinical goals.
The four requirements and guidelines for the proper clinical use of applicators [21,41] have been followed. The criteria are as follows: 1) The radiation aperture can produce an effective surface HT under the aperture (according to the test conditions). 2) The radiating elements of the applicator array are powered in an incoherent manner to avoid cross-coupling effects between elements. 3) The third requirement does not apply here. 4) The definition of the treatment area of the array applicator is like the definition of the treatment area of a single applicator.
The European Hyperthermic Society strongly recommends including a temperature measurement point under each individual power supply element of the array applicator. This point is particularly suitable for respect and is described in detail in this article. Therefore, clinical temperature monitoring represents the highest interest in our work. Regarding the recommendation: "The minimum number of temperature measurement locations is five, and the reading cycle is at least once per minute." The goal of the present work is to heat the entire target volume to a sufficiently high thermal dose at the end of the treatment, instead of focusing only on measuring the temperature. The instantaneous temperature of a specific part. As a conclusion, the guaranteed points of surface hyperthermia that comply with the European society's recommendations on Good Clinical Practice (GCP) are pointed out in the guidelines.
As recommended by the European Hyperthermic Society, the heat distribution of the applicator is at the same frequency as in clinical use under standardized operating conditions. In addition, all measurements were performed under the same clinical trial conditions. At the beginning of each experiment, the cube phantom should be balanced with normal body temperature.
In the current work, no water bolus is used: the manufacturer does not provide a water bolus that is coupled with the applicator hole. The temperature-controlled water circulation bolus has the function to couple electromagnetic energy into the patient's body and control the skin surface temperature. For Electromagnetic (EM) heating, the thermometer should minimize the interaction with the EM field, even if they have a slight error in reading the temperature.
The reason for using thermo chemical thermometers is that they have minimal interference and are suitable for EM heating technology. The temperature is only measured on the surface. In addition, the measured temperature is combined with the patient's feedback on signs of discomfort in the form of subjective pain complaints.
Conclusion
Indeed, present studies give an overview of the good procedure of superficial hyperthermia treatment of loco-regional recurrent breast carcinoma as one of indicators of the Good Clinical Practice (GCP). In this context, studies focused on antennae comparison, to show that the shape of antenna plays a major role in the increase of temperature intensity, were carried out. Part of this research has demonstrated the effectiveness of the antenna shape for temperature transmission and temperature monitoring using non-invasive thermo chemical. The use of "SIRETHERM 609 S" microwave device (433 MHz) for hyperthermia requires more research on treatment procedures and the shape of the antenna. Hyperthermia can also be used in low-income countries with universal equipment.
From the current work, the lessons learned to fully comply with the ESHO guidelines are as follows:
• Medical physicist should follow the Good Clinical Practice (GCP) in superficial hyperthermia.
• The detailed information of the clinical case must be provided, including its precise diagnosis and a description of the depth and location of the tumor.
• The number of the Ethics Committee protocol that approved such research study should be included.
• The tissue equivalent phantom for technical tests and a water bolus at the surface of tumor must be used.
• The use of computational modelling for optimization and verification is essential.
• The number of patients should be as high as possible, despite in palliative treatment it is not obvious.
If medical physicists solve these problems, the challenge of hyperthermia to make a valuable contribution to treat cancer will be solved.
Acknowledgement
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Funding Statement
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics Statement
Prior approval is obtained from the competent ethics committee and the Federal Agency for Therapeutic Products. During the trial, adverse reactions and amendments are always reported to the Swiss medic ethics committee. In addition, both patients signed a special informed consent form before treatment.
References
- Akbari ME, Marzieh R-R, Nahid N, et al. (2018) Effect of patho-biological factors on the survival of recurrent breast cancer cases. Asian Pac J Cancer Prev 19: 949-953.
- Gerber B, Freund M, Reimer T (2010) Recurrent breast cancer: Treatment strategies for maintaining and prolonging good quality of life. Dtsch Arztebl Int 107: 85-91.
- Wust P, Hildebrandt B, Sreenivasa G, et al. (2002) Hyperthermia in combined treatment of cancer. Lancet Oncol 3: 487-497.
- Linthorst M, Van Rhoon G, Van Geel A, et al. (2012) The tolerance of reirradiation and hyperthermia in breast cancer patients with reconstructions. Int J Hyperthermia 28: 267-277.
- Notter M (2015) Experiences in re-irradiation and wIRA-hyperthermia in recurrent breast cancer. Symposium Moderne Hyperthermie 8-9.
- Notter M (2014) Thermography controlled wIRA-hyperthermia and low dose re-irradiation in recurrent breast cancer. Oncothermia Journal 10: 78.
- Notter M, Piazena H, Vaupel P (2016) Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: A retrospective study of 73 patients. Int J Hyperthermia 33: 227-236.
- Refaat T, Sachdev S, Sathiaseelan V, et al. (2015) Hyperthermia and radiation therapy for locally advanced or recurrent breast cancer. Breast 24: 418-425.
- Issels RD, Lindner LH, Verweij J, et al. (2010) Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol 11: 561-570.
- Issels RD (2008) Hyperthermia adds to chemotherapy. Eur J Cancer 44: 2546-2554.
- Jones EL, Prosnitz LR, Dewhirst MW, et al. (2004) Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res 10: 4287-4293.
- Datta NR, Ordóñez SG, Gaipl US, et al. (2015) Local hyperthermia combined with radiotherapy and or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev 41: 742-753.
- Zagar TM, Higgins KA, Jones EF, et al. (2010) Durable palliation of breast cancer chest wall recurrence with radiation therapy, hyperthermia, and chemotherapy. Radiother Oncol 97: 535-540.
- Arruebo M, Vilaboa N, Sáez-Gutierrez B, et al. (2011) Assessment of the evolution of cancer treatment therapies. Cancers (Basel) 3: 3279-3330.
- Van der Zee J (2002) Heating the patient: A promising approach? Ann Oncol 13: 1173-1184.
- Maluta S, Kolff MW (2015) Role of hyperthermia in breast cancer loco regional recurrence: A review. Breast Care (Basel) 10: 408-412.
- Cheng KS, Roemer RB (2009) Optimal power deposition patterns for ideal high temperature therapy/hyperthermia treatments. Int J Hyperthermia 20: 57-72.
- Szasz O, Szasz MA, Minnaar C, et al. (2017) Heating preciosity-Trends in modern Oncological hyperthermia. Open Journal of Biophysics 7: 116-144.
- Di Dia A, Garibaldi E, Delmastro E, et al. (2016) Radiotherapy in association with hyperthermia: Outcome and toxicity in the treatment of superficial recurrent/metastatic tumours. Phys Med 32: 18-19.
- Bakker A, Kolff MW, Holman R, et al. (2017) Thermal skin damage during reirradiation and hyperthermia is time-temperature dependent. Int J Radiat Oncol Biol Phys 98: 392-399.
- Trefna HD, Crezee H, Schmidt M, et al. (2017) Quality assurance guidelines for the application of superficial hyperthermia: I. Clinical requirements. Int J Hyperthermia 33: 471-482.
- Wahab AA, Salim MI, Ahamat MA, et al. (2016) Thermal distribution analysis of three-dimensional tumor-embedded breast models with different breast density compositions. Med Biol Eng Comput 54: 1363-1373.
- Joines WT, Zhang Y, Li C, et al. (1994) The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Med Phys 21: 547-550.
- Gabriel S, Lau RW, Gabriel C (1996) The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol 41: 2251-2269.
- Lazebnik M, McCartney L, Popovic D, et al. (2007) A large-scale study of the ultra wide band microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys Med Biol 52: 2637-2656.
- Linthorst M, Van Geel AN, Baaijens M, et al. (2013) Re-irradiation and hyperthermia after surgery for recurrent breast cancer. Radiother Oncol 109: 355-359.
- Welz S, Hehr T, Lamprecht U, et al. (2005) Thermoradiotherapy of the chest wall in locally advanced or recurrent breast cancer with marginal resection. Int J Hyperthermia 21: 159-167.
- Müller AC, Eckert F, Heinrich V, et al. (2011) Re-surgery and chest wall re-irradiation for recurrent breast cancer: A second curative approach. BMC Cancer 11: 197.
- Wust P, Hildebrandt B, Sreenivasa G, et al. (2002) Hyperthermia in combined treatment of cancer. The Lancet Oncology 3: 487-497.
- Saccomandi P, Schena E, Silvestri S (2013) Techniques for temperature monitoring during laser-induced thermotherapy: An overview. Int J Hyperthermia 29: 609-619.
- Van Rhoon GC, Rietveld PJ, Van der Zee J (1998) A 433 MHz Lucite cone waveguide applicator for superficial hyperthermia. Int J Hyperthermia 14: 13-27.
- Rietveld PJ, Van Putten WL, Van der Zee J, et al. (1999) Comparison of the clinical effectiveness of the 433 MHz Lucite cone applicator with that of a conventional waveguide applicator in applications of superficial hyperthermia. Int J Radiat Oncol Biol Phys 43: 681-687.
- Kitchen SS, Partridge CJ (1991) A review of microwave diathermy. Physiotherapy 77: 647-652.
- Kouloulias V, Triantopoulou S, Uzunoglou N, et al. (2015) Hyperthermia is now included in the NCCN Clinical Practice Guidelines for breast cancer recurrences: An analysis of existing data. Breast Care (Basel) 10: 109-116.
- (2021) Image J: An open platform for scientific image analysis. Assessed September.
- Curto S, Prakash P (2015) Design of a compact antenna with ?ared ground plane for a wearable breast hyperthermia system. Int J Hyperthermia 31: 726-736.
- Stauffer PR, Maccarini P, Arunachalam K, et al. (2010) Conformal microwave array (CMA) applicators for hyperthermia of diffuse chest wall recurrence. Int J Hyperthermia 26: 686-698.
- Johnson JE, Neuman DG, Maccarini PF, et al. (2006) Evaluation of a dual-arm Archimedean spiral array for microwave hyperthermia. Int J Hyperthermia 22: 475-490.
- Otasowie PO (2012) Microwave antenna performance metrics (Chapter-5) Data acquisition applications, Zdravko Karakehayov 107-122.
- CST Studio software (2021) (Dassault systems, Vélizy-Villacoublay, France), Assessed September.
- Trefna HD, Crezee H, Schmidt M, et al. (2017) Quality assurance guidelines for the application of superficial hyperthermia:II. Technical requirements for heating devices. Strahlenther Onkol 193: 351-366.
Corresponding Author
Leila Ounalli, Research Laboratory on Energy and Matter for Nuclear Science Development, (Nuclear Safety and Security department, CNSTN 2020 Ariana, Tunisia.
Copyright
© 2022 Ounalli L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.