Normal Fetal Fourth Ventricle Biometry Measured On Mri Mid-Sagittal Images
Abstract
Objective: The goal of this study was to present the measurement range of fetal fourth ventricle on MRI mid-sagittal images by gestational age in normal fetuses.
Methods: 207 pregnant women between 17th to the 38th week of gestation were imaged using MRI. The fourth ventricle height and angle were measured, and the curve estimation analyses for linear and box-plots were performed.
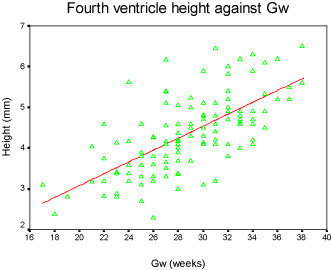
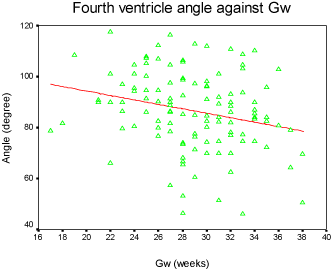
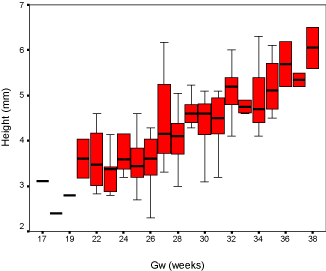
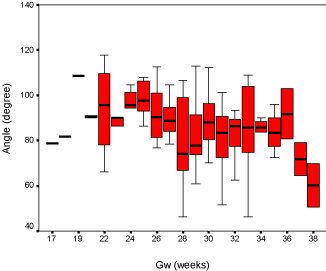
Results: Our discovery showed that the fourth ventricle height had a close relationship with GA, increasing slightly from 2mm to 7mm between 17 to 38 gestational weeks. The fourth ventricle angle ranged from 40 degrees to 120 degrees and demonstrated a moderate downward trend. Box-plots were graphed for clinical reference.
Conclusions: As the fetal fourth ventricle height and angle can be readily assessed by MRI, using the normative data provided in this study, fetal posterior fossa anomalies could be detected in an early stage.
Keywords
Fetus, Fourth ventricle, MRI
Introduction
In recent years, significant progress has been made in understanding of supratentorial brain [1-5] development and malformations. However, much less attention has been paid to the posterior fossa, especially the fetal fourth ventricle, which is an important landmark for evaluating the development of the posterior fossa [6-9]. Although the fourth ventricle can be estimated by both the fetal MRI and ultrasonography, only a few previous studies have done research on describing normal fetal biometric data of the fourth ventricle [10-12]. The potential of studying the normal appearance of the fetal fourth ventricle could help diagnosing the prenatal posterior fossa malformations where the size and the shape of the fourth ventricle usually change earlier [13,14].
Prenatal sonographic assessment of the fetal brain is the main technique of choice, yet it owns limitations which particularly in the area of examining of the posterior fossa [15]. With the development of rapid acquisition MR imaging, it is now possible to evaluate the fetal fourth ventricle in the late second trimester of pregnancy onwards. Direct measurement of the fourth ventricle from the fetus in utero would be more representative of normal development, especially in the middle sagittal MRI images. The purpose of the present study is to describe the normal appearance and the growth of the fourth ventricle of the fetus on fetal MRI mid-sagittal images.
Materials and Methods
A retrospective evaluation of 207 pregnant women with known gestational age (GA) between 17 and 38 gestational weeks were selected for this study, and they received MRI imaging between January and December 2015. GA was established based on the last menstrual period and confirmed by first-trimester sonographic estimation. Informed consent was obtained for all patients. The following inclusion criteria were applied:
1. Single fetus
2. Absence of a documented fetal abnormal chromosome
3. Fetuses without complicated CNS pathologies documented by ultrasound.
119 out of 207 cases met the criteria of the study. Most of them were previous diagnosed with various pathologies, including congenital diaphragmatic hernia, gastroschisis, urologic abnormalities, cystic adenomatoid malformation and tumors such as teratoma, lymphangioma where they were all unrelated to the region of the fetal brain etc.
Fetal MRI imaging was performed on a 1.5 Tesla superconductive unit (Philips Gyroscan Intera, Philips Medical Systems, Best, the Netherlands) with a five-element cardiac surface coil. Subjects were scanned in the supine or lateral decubitus position, without sedation. Multi-planar scout images were obtained with steady-state free precession (SSFP) sequence to localize the fetus and to determine whether coil repositioning was needed. The imaging protocol included T2-weighted, single-shot, fast spin echo (SSFSE) sequences (TE 80 ms, 100 ms or 140 ms, TR ranging from 6000 to 20000 ms, slice thickness 4.0 mm, 256*256 matrix), planned in axial orientations, perpendicular to the fetal brainstem. Additionally, coronal, and sagittal sequences were obtained, and each sequence was served as a scout image for the next sequence. The FOV was adjusted to fetal size and maternal body habitus, ranging from 180 to 240 mm.
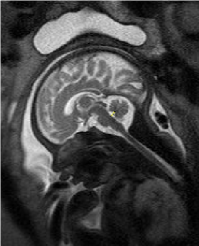
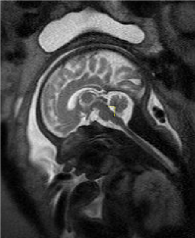
The 2D measurements were processed on Philips workstation (version 1.45S, National Institutes of Health, Bethesda, MD, USA) by a radiologist who has 10-year MRI processing experience to maintain consistency. The fourth ventricle dimensions were regarded as the greatest distance from the posterior part of the pons to the fastigium for the height; the degree between the superior medullary velum, fastigium and obex for the fourth ventricle angle (Figure 1 and Figure 2).
Statistical Analysis
The statistical analysis was performed using SPSS 17.0 software (SPSS, Release 17.0.0, SPSS Inc. the USA). The measurements of the fourth ventricle height and angle were analyzed by regression methods. A curve for GA was drawn to estimate the linear mode.
Results
The fourth ventricle appeared as a triangular shape filling with cerebral-spinal fluid, showing high intensity signal on fetal MRI T2 weighted images, and completely covered by the vermis. The fourth ventricle height and angle were charted by the linear model (Figure 3 and Figure 4), and demonstrated strong connection with GA accompanied with a slight increase from 2mm to 7mm between the 17th and 38th gestational weeks. The result for fourth ventricle angle was ranged from 40 to 120 degrees, showing an insignificant declining trend and also closely related with GA. Furthermore, the box-plot curves of the measurements were plotted over GA for reference in practice (Figure 5 and Figure 6).
Discussion
We plotted the fourth ventricular height and angle against GA between the 17th and 38th weeks of pregnancy, and we discovered that the height and the angle of the fourth ventricle grow in a linear fashion and correlate well with GA. The height of the fourth ventricle indicated an increasing trend, ranging from 2 mm to 7 mm. However, the fourth ventricle angle reflected a slight declining trend and ranges from 40° to 120°.
The fourth ventricle is a cavity filled with cerebral-spinal fluid, extending from the sylvian aqueduct to the foramen of Magendie and separates the brain stem from the cerebellum [15]. The fourth ventricle has a tent-like appearance, in which the lateral walls are formed by the cerebellar peduncles. The roof is formed by cerebellar peduncles, the medulla velum, and the cerebellar nodules; the floor is delineated by the surface of the pons and the medulla oblongata [15]. Previous fetal MRI studies have shown that from the second stage, the fourth ventricle has undergone significant changes, which might be due to the dynamic changes of the cerebrospinal fluid and the morphological changes of the configuration of the fourth ventricle [9], and the present study was very consistent with them.
Our results found that the fourth ventricle height has a remarkable increase between 17 and 38 gestational weeks. It is speculated that the fourth ventricle development closely follows the development of the surrounding structures, especially the vermis which developed completely after 18 weeks gestational age [16]. Although the fourth ventricle angle showed a slight decrease in 2D images, the volume of the fourth ventricle has a significant variation during the second trimester, and it was demonstrated by 3D measurements [9].
The fourth ventricle appears as a triangle on mid-saggital fetal MRI images. Any changes from the surroundings within the posterior fossa may cause the fourth ventricle height or/ and angle to increase or decrease [17,18]. Moreover, the shape of the fourth ventricle should be alerted where the fourth ventricle may lose the normal morphological appearance when affected by certain diseases. Generally, increasing or decreasing the fourth ventricle angle and height could indicate the possible presence of anomaly of the posterior fossa. Cystic malformations within the posterior fossa, for example, Dandy-Walker malformation, Blake's pouch cyst, posterior fossa arachnoid cyst, and etc. are the main diseases which may cause the fourth ventricle to change its shape [19]. Some diseases like Arnold-Chiari malformation often decrease the height of the fourth ventricle [20]. Recent studies have demonstrated that genetic diseases may also change the morphological appearance of the fourth ventricle [21].
Clinically, ultrasonography is the main tool for fetal cerebral development evaluation, however, minimal focus has been placed on normal anatomy and development of the fourth ventricle since it is difficult to measure for ultrasound technically [18]. Fetal MRI has more advantages than ultrasonography to delineate the posterior fossa structures, especially for the fourth ventricle. It is easy to measure the fourth ventricle height and angle on MRI mid-saggital images. Previous studies have proven that fetal MRI imaging was able to define the anatomy and pathology of the fetus more effectively while evaluating the posterior fossa [7]. In addition, Fetal MRI is superior to sonography in the measurement of the fourth ventricle by obtaining the sagittal images. Recently, Vatansever, et al. measured the posterior fossa by fetal MRI multi-dimensionally [9]. They measured 79 normal fetuses and made the conclusion that the fourth ventricular height and angle had significant difference against GA. The present study has the same results with them. 3D measurements may be more accurate to depict changes of the fourth ventricle. However, in practice, the 2D measurement of the fourth ventricle used in this study is faster and easier to implement than the 3D measurement.
Several limitations may exist in the present study. Most importantly, we measured some fetuses with abnormal findings which are generally not related with disturbances of the fourth ventricle. The possibility of further changes of structural abnormalities of the fetal in the later GA could not be excluded. Moreover, the fetal gestational age may not be determined accurately, since we relied on the definitions provided by the first trimester ultrasonography scan, variations may exist and affect the age correction. Furthermore, we would like to acknowledge the possibility of the inadvertent inclusion of some subtle abnormal fetuses which affect the measurement and cannot be detected by ultrasound and/or MRI.
In conclusion, we measured the fetal fourth ventricle height and angle between the 17th and 38th week of gestation. The results could be helpful for accessing normal shape of fetal fourth ventricle and might serve as a reference in cases of suspected abnormalities of the posterior fossa.
References
- Tilea B, Alberti C, Adamsbaum C, et al. (2009) Cerebral biometry in fetal magnetic resonance imaging: New reference data. Ultrasound Obstet Gynecol 33: 173-181.
- Rutherford MA (2009) Magnetic resonance imaging of the fetal brain. Curr Opin Obstet Gyn 21: 180-186.
- Grossman R, Hoffman C, Mardor Y, et al. (2006) Quantitative MRI measurements of human fetal brain development in utero. NeuroImage 33: 463-470.
- Gholipour A, Estroff JA, Barnewolt CE, et al. (2011) Fetal brain volumetry through MRI volumetric reconstruction and segmentation. Int J Comput Radiol Surg 6: 329-339.
- Triulzi F, Parazzini C, Righini A (2006) Magnetic resonance imaging of fetal cerebellar development. Cerebellum 5:199-205.
- Gandolfi Colleoni G, Contro E, Carletti A, et al. Prenatal diagnosis and outcome of fetal posterior fossa fluid collections. Ultrasound Obstet Gynecol 39: 625-631.
- Shekdar K (2011) Posterior fossa malformations. Semin Ultrasound CT MR 32: 228-241.
- Hatab MR, Kamourieh SW, Twickler DM (2008) MR volume of the fetal cerebellum in relation to growth. J Magn Reson Imaging 27: 840-845.
- Vatansever D, Kyriakopoulou V, Allsop JM, et al. (2013) Mmultidimensional analysis of fetal posterior fossa in health and disease. Cerebellum 12: 632-644.
- Baumeister LA, Hertzberg BS, McNally PJ, et al. (1994) Fetal fourth ventricle: US appearance and frequency of depiction. Radiology 192: 333-336.
- Scott JA, Habas PA, Kim K, et al. (2011) Growth trajectories of the human fetal brain tissues estimated from 3D reconstructed in utero MRI. Int J Dev Neurosci 29: 529-536.
- Scott JA, Hamzelou KS, Rajagopalan V, et al. (2012) 3D Morphometric analysis of human fetal cerebellar development. Cerebellum 11: 761-770.
- Kazan-Tannus JF, Dialani V, Kataoka ML, et al. (2007) MR volumetry of brain and CSF in fetuses referred for ventriculomegaly. AJR Am J Roentgenol 189: 145-151.
- Kyriakopoulou V, Vatansever D, Elkommos S, et al. (2014) Cortical overgrowth in fetuses with hydrocephalus. Cerebral Cortex 24: 2141-2150.
- Błaszczyk E (2004) An evaluation of the size of the fourth ventricle in human foetuses. Folia Morphol (Warsz) 63: 241-243.
- Bromley B, Nadel A, Pauker S, et al. (1994) Closure of the cerebellar vermis: Evaluation with second trimester US. Radiology 193: 761-763.
- Bolduc ME, DU Plessis AJ, Evans A, et al. (2011) Cerebellar malformations alter regional cerebral development. Dev Med Child Neurol 53: 1128-1134.
- Goldstein I, Makhoul IR, Tamir A, et al. (2022) Ultrasonographic nomograms of the fetal fourth ventricle: Additional tool for detecting abnormalities of the posterior fossa. J Ultrasound Med 21: 849-856.
- Poretti A, Boltshauser E, Huisman TA (2016) Pre- and Postnatal neuroimaging of congenital cerebellar abnormalities. Cerebellum 15: 5-9.
- Wong AM, Bilaniuk LT, Zimmerman RA, et al. (2012) Prenatal MR imaging of dandy-walker complex: midline sagittal area analysis. Eur J Radiol 81: e26-e30.
- Loureiro T, Ferreira AF, Ushakov F, et al. (2012) Dilated fourth ventricle in fetuses with trisomy 18, trisomy 13 and triploidy at 11-13 weeks' gestation. Fetal Diagn Ther 32: 186-189.
Corresponding Author
Xiaobing Li, Department of Radiology, Shanghai Jiao Tong University Affiliated Sixth People's Hospital south campus, 6600 Nanfeng Rd, Shanghai, China, 201499, Tel: 00-86-021-57412834
Copyright
© 2022 Pan H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.