A Study of Nuclear Medicine Contrast Retention in Vascular Access Ports
Abstract
Vascular access ports were investigated to determine the retention rate of injected radiotracers during PET scan acquisition. A total of 30 ports were tested, including 3 different port models from a single manufacturer. Both 18F-fluorodeoxyglucose (FDG) and 18F-Sodium Fluoride (Na18F) contrast agents were used. Radioactivity counts and images acquired by PET was the primary means used to identify retention of radioactivity in those ports. In repeatable experiments, AngioDynamics™ port lines including Smart Port™ CT, BioFlo™ Port, and BioFlo™ Dual Port exhibited radioactive contrast retention ranged from 0.86 ± 0.12 to 1.38 ± 0.23%, following an injection of 10 mL saline. These port models tested satisfy the interest to be identified procedurally for potential use in clinical Nuclear Medicine PET contrast studies.
Keywords
FDG, NaF, PET, Ports, Radiopharmaceutical
PACS Numbers
87.57.uk, 87.57.U-, 87.19.uj
Introduction
A port is a small medical device implanted superficially beneath the skin, consisting of a reservoir and a rubbery septum where needles are inserted. The lower part of the portal includes an outlet tube. It is here that the catheter is connected that is then surgically inserted into either the jugular or subclavian veins. The distal end of the catheter is carefully led to the heart for blood sample extraction or drug infusion [1,2]. Vascular access ports are small in size. They generally go unnoticed by patients after implantation and offer the benefit of relieving the alternative traditionally attempted multiple needle sticks in small veins of the wrist each day [3].
In Radiology, physicians often prescribe a contrast agent to be injected in a patient during a Computerized Tomography (CT) scan. The choice of contrast depends on the diagnosis suspected or the interest to discern different anatomical or vascularized tissues [4]. Similarly, there are contrast agents used during Positron Emission Tomography (PET) scanning. For this procedure, Nuclear Medicine physicians are more interested in metabolic tissue uptake and perfusion. The PET scanner has proven to be a beneficial device for drug development and molecular imaging investigations in small animals [5]. To date, there have been many different opinions issued by physicians are to whether vascular access ports are an appropriate means of introducing radioactive contrast agents. For those who are skeptical about this application, questions are posed as to the amount of radiopharmaceutical uptake within the materials of the device. Hypothetically, it is rumored that undesirable uptake by a port may result in image artifacts, altered image resolvability, and therefore could lead to potential errors in diagnosis [6,7].
A group from The Netherlands has already investigated similar concerns with vascular access ports, though considering effects involving only hydrodynamics and temperature as key parameters for their efficiency in ridding contrast media. The group evaluated six different ports, reporting that port cavities are incompletely void of contrast media once primed with 30 mL saline solution in a laboratory environment [8].
In our study, we explore the corollaries of evaluating port voiding following injection and saline priming, but taking into consideration radiopharmaceutical contrast containment via PET imaging. We include a sample size of 15 ports, divided into 5 ports each from 3 different models. In the study, 15 ports were investigated with radioactive 18F-fluorodeoxyglucose (FDG) and 15 more ports with 18F-Sodium Fluoride (Na18F) contrast. The intent was to examine the results for each port type with respect to the remaining activity following multiple saline priming injections with PET imaging at each injection. Ports were chosen based on a variety of designs, all of which are currently marketed models from a single manufacturer. Prior to this research, PET radiotracers interaction with port and catheter material were unknown.
Materials and Methods
Radioactive fluoride (18F) and 18F-fluorodeoxyglucose (FDG) was first acquired from PETNET Solutions, Inc. (dba Siemens Medical Solutions USA, Inc.®) (Louisville, KY). Using published techniques to chemically alter 18F, the production of Na18F was made possible [9,10]. This procedure includes passing 18F-fluoride and diluted 0.1 mL H18O through a cation exchange (in the form of H+). The Na18F end product is complete after the exchange solution is eluted with approximately 12 mL saline and passed through a 200 nm filter.
Imaging for this study was conducted using a Siemens® (Knoxville, TN) Model R4 Micro-PET scanner. The scanner functions with submillimeter spatial resolution and is used routinely for radiobiological investigations. The integrated Model R4 Micro-PET scanner is a dedicated 3rd-generation rodent PET scanner, capable of obtaining nominally high spatial resolution of 2 mm3 at its center field of view during data acquisition. The bore-hole has a dimension of 148 mm and 78 mm at transaxial and axial directions. Micro-PET data were acquired by using Micro-PET manager software for 10 min with an energy window of 350-650 keV and a timing window of 6 ns. All data were formatted to a image pixel size of 0.85 × 0.85 × 1.21 mm3 and reconstructed with a two-dimensional Ordered Subset Expectation Maximization (OSEM2D) reconstruction algorithm. The image pixel size was 0.85 × 0.85 × 1.21 mm3 (X, Y and Z). Data were then analyzed in Siemens Healthcare GmbH® (Erlangen, GERMANY) Model Inveon™ Research Workplace (IRW) software for total radiation counts.
While the IRW software are able to quantify numeric photon detections from the decay of 18F, a Biodex Medical Systems (Shirley, NY) Model Atomlab™ 500 dose calibrator was also incorporated into the study to assay remaining activity after scanning. Recommended procedures for insuring scanner calibration and quality assurance were performed prior to imaging.
Various vascular access ports were evaluated separately and identically in this study. Ports were supplied by AngioDynamics™ (Latham, NY). Models include the Smart Port™ CT, BioFlo™ Dual Port and BioFlo™ Port (Pl), as illustrated in Figure 1. Five (n = 5) ports for each type were tested with FDG contrast, with five (n = 5) more ports tested with NaF contrast.
Prior to commencement, each port was primed with 2.0 mL saline and heated at 98.6 ℉ for 1 day prior to use. For the experiment, each port was secured to a piece of cardboard. Independently and consecutively they were investigated. Once a port was chosen to be evaluated, it was injected with 0.074-0.111 GBq (2-3 mCi) of contrast, thus serving as a positive control. One additional port was set aside as a negative control at all times. The port was then positioned inside the bore of the scanner for image acquisition. Figure 2 depicts the set-up employed for these investigations.
Once the port was scanned, it was flushed with 10 mL of saline and imaged again. The saline used for the study was Baxter® (Deerfield, IL) Model BSCl050124 isotonic irrigation fluid, composed of 0.9% sodium chloride. There was no time delay between flushes and imaging. Additional saline volumes were cumulatively injected into the septum of the port. With 10 mL saline already introduced by this time, an additional 20 mL saline followed for the next scan, where a cumulative total of 30 mL saline was introduced. The process continued for scans with 60 mL and 120 mL of saline injected, all with the intent to force the radioactive contrast out of the port. PET scans were conducted following each flush, with the negative control port kept aside. IRW software permitted the recording of decay counts measured, by integrating the total number of counts from 12 consecutive slices encompassing the port at center. Residual activity in the port was also measured using a Biodex Medical Systems (Shirley, NY) Model Atomlab™ 500 Plus well counter for confirmation. The activity retention rate was calculated as 100% × (Counts in each flushed sample - Counts of negative control)/(Total counts of positive control).
Results
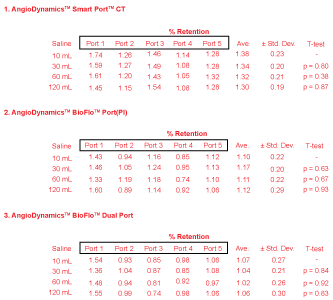
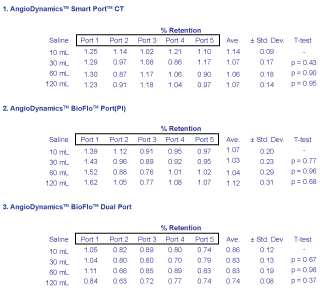
Figures 3 and Figure 4 demonstrate results obtained from PET scanning the BioFlo™ Port (Pl) with FDG or Na18F injections, and saline flushes to follow. It is immediately observable that for all 30 ports, whether injected with FDG or NaF, less than 2% radioactive contrast was measured to remain after just 10 mL saline flush injection. Depending on which region in the field-of-view PET counts were obtained, reproducibility measured by IRW software ranged from ± 0.8-3.1%. When comparing the magnitude of system reproducibility with respect to the nominal retention rates measured, it is easily observable that measured retention of radioactivity found within the port was insignificant.
For FDG, there was no stark difference between in uptake with regard to port model. The average retention ranged from 1.02 ± 0.30% to 1.38 ± 0.30% for all models.
Similar results were found for NaF testing. For NaF radioactive contrast, the average retention was measured to be 0.74-1.14 ± 0.31% for all models regardless of port type. As a combined set for all 30 ports including both FDG and NaF contrast, the average retention ranged from 0.74-1.38 ± 0.31%. A few results were measured above 1.0%. However, these were statistically negligible in comparison to the maximum retention of only 1.0% after just one injection of 10 mL saline. T-test p-values validated the results using the GraphPad Software, Inc. (La Jolla, CA) QuickCalcs routine.
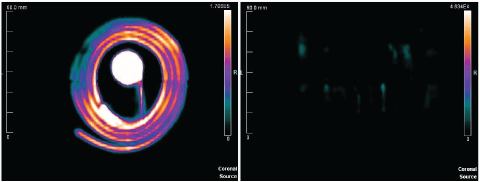
Figure 4 provides a more qualitative visualization of radioactive contrast uptake based on PET scan images within the software. First, an image is provided detailing the positive control. This image represents the first scan of the port after having been injected with the contrast agent of choice for that study. No saline contrast injections were followed prior to that scan. Second, the first 10 mL of saline is used to flush the port and catheter system. The images reveal the distinct radiological uptake present both before and after, which is expected similarly under clinical situations (Figure 5).
There is something to be said about the retention differences that do appear in data analysis. A slight pattern appears when one considers the retention of each port separately. If we consider first the AngioDynamics™ Smart Port™ CT Port results, we find that after 10 mL the retention on average was 1.38 ± 0.23% for FDG. These retentions are similar to those of the BioFlo™ Port and BioFlo™ Dual Port, but larger with theirs at 1.10 ± 0.22% and 1.07 ± 0.27% respectively. Likewise, the BioFlo™ Port exhibited slightly larger retention than the BioFlo™ Dual Port. This pattern of retention being greater for first the SmartPort™ CT Port, then BioFlo™ Port, and finally the BioFlo™ Dual Port continued for NaF contrast injections. Their results following a 10 mL saline flush were found to be 1.14 ± 0.09%, 1.07 ± 0.20%, and 0.86 ± 0.12%. It is possible that the construction design and composition differences between all three ports resulted in slight disparities in retention, albeit still negligible. However, it is more noteworthy to identify that there is an identical composition for the BioFlo™ Port and BioFlo™ Dual Port. The mere difference between them is construction design, where the BioFlo™ Dual Port has two septa. We propose that there is a construction design benefit exhibited by some ports with more than one septum to rid radioactive contrast more efficiently.
Discussion
This testing involved specific models from a single manufacturer and with two commonly used PET contrast agents. It is not recommended to use these results to conclude similar flow dynamics in ports with other radiotracers [12,13]. Likewise, not all ports are made of the same materials. In fact, many ports have a different construction designs altogether. Some are made of composite plastics and some are made partially of metal. Given these variables, it is possible that retention rates for dissimilar designs by different makes or models may yield different results. However, it is expected that for similar material construction, that there may be similar physical properties of surface tension and capillary action. Regarding surface tension, it is suggested here that the force of adhesion of FDG or Na18F as it comes in contact with a different material that make up the inside of the port in the reservoir, as well as with the proximal end of the catheter connected to it, is likely the same for similar materials. Capillary action is also proposed to be comparable for similar materials, since there is expected to be no change in the ability of the contrast to flow through the port without some other change in external forces; i.e. magnetic fields of a Magnetic Resonance Imaging (MRI) machine.
In this investigation, we intended to examine typical port designs that have typical material construction. We were able to prove that with saline flushes following the injection of radio labeled contrast for PET scans, that retention rates are very low. For untested port makes and models and for other contrast chemicals, we note that if remarkable activities remain even after saline flushing, such contrast could give rise to image artifacts that affect diagnostic reading, in the form of false positives or negatives, and even further affect treatment options for patients [14,15].
Although currently market ports from a variety of manufacturers largely involve the same general shape, design features and plastic or metal composition differences may cause retention above the values observed here. Therefore, the reader is cautioned not to attempt clinical studies with vascular access ports of a different make or model than was examined here. Since the contrast retention volume is directly related to discoverable artifacts in PET imaging, these results may not be reciprocal for other PET contrast forms.
Conclusion
A total of 30 ports were tested, including 3 different port models from a single manufacturer. Using positron emission tomography with 15 ports for FDG contrast and 15 more for NaF contrast, this study has proven that AngioDynamics™ port lines including SmartPort™ CT, BioFlo™ Port, and BioFlo™ Dual Port demonstrate low uptake when injected directly into the port septum. All ports were measured to have a mere 1% retention on average following an immediate flush of 10 mL saline. Examination of the results for each port type with respect to the remaining activity following multiple saline priming injections with PET imaging at each injection reveals each is ideally suited for potential use in clinical Nuclear Medicine PET contrast studies.
Acknowledgements
We would like to thank the AngioDynamics™ for the donation of devices for this study.
Conflicts Statement
The authors have no relevant financial interests in the manuscript and no other potential conflicts of interest to disclose.
References
- Johnson Kathleen A (2009) Power injectable portal systems. J Radiol Nurs 28: 27-31.
- Graham ML, Mutch LA, Rieke EF, et al. (2010) Refinement of vascular access port placement in nonhuman primates: Complication rates and outcomes. Comp Med 60: 479-485.
- Carroll PL (1998) Reducing the risk of needlestick injury associated with implanted ports. Home Healthc Nurse 16: 225-234.
- Plumb AA, Murphy G (2011) The use of central venous catheters for intravenous contrast injection for CT examinations. Br J Radiol 84: 197-203.
- Emilio Bombardieri, Cumali Aktolun, Richard P Baum, et al. (2003) FDG-PET Procedure guidelines for tumor imaging. Eur J Nucl Med Mol Imaging 30: 115-124.
- Surov A, Rusner C, Weigand K, et al. (2012) Radio-opacity and incidental identified mechanical complications of totally implantable venous access devices placed in the chest. Acta Radiol 53: 1035-1039.
- Long NM, Smith CS (2011) Causes and imaging features of false positives and false negatives on18F-PET/CT in oncologic imaging. Insights Imaging 2: 679-698.
- Guiffant G, Durussel, Flaud P, et al. (2013) Power port contrast medium flushing and trapping: Impact of temperature, an in vitro experimental study. Med Devices 6: 133-140.
- Bhargava P, Kumar R, Zhuang H, et al. (2004) Catheter-related focal FDG activity on whole body PET imaging. Clin Nucl Med 29: 238-242.
- Siosteen AK, Celsing F, Jacobsson H (2005) FDG uptake in catheter-related thrombus simulating relapse of lymphoma. Clin Nucl Med 30: 338-339.
- Soper DS (2016) P-value calculator for an analysis of variance (ANOVA) study.
- Vines DC, Green DE, Kudo G, et al. (2011) Evaluation of mouse tail-vein injections both qualitatively and quantitatively on small-animal PET tail scans. J Nucl Med Technol 39: 264-270.
- Berry-Pusey BN, Chang YC, Prince SW, et al. (2013) A semi-automated vascular access system for preclinical models. Phys Med Biol 58: 5351-5362.
- Fiebig Teresa, Figueiredo Giovanna, Boll Hanne, et al. (2013) A low cost metal-free vascular access mini-port for artifact free imaging and repeated injections in mice. PLoS One 8: e65939.
- Gossman MS, Seuntjens JP, Serban MF, et al. (2009) Dosimetric effects near implanted vascular access ports: An examination of external photon beam dose calculations. JACMP 10: 3-15.
Corresponding Author
Michael S Gossman, Regulation Directive Medical Physics, 104 Hildeen Court, Russell, KY 41169, USA, Tel: 606-329-0060.
Copyright
© 2017 Gossman MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.