Investigation of MRI Contrast Release from Vascular Access Ports after Priming
Abstract
Vascular ports designed for venous access were injected with gadolinium contrast and imaged using MRI. Incrementally, the ports were flushed with saline and scanned to determine the amount of flush volume necessary to rid the injected gadolinium. A total of 15 ports were used in this study. Three different models were chosen based on their construction and design. Testing included 5 ports per model. The initial injection volume was 2 mL gadolinium, followed by saline flush volumes at 10 mL, 30 mL, 60 mL and 120 mL. After a flush of 10 mL saline, only residual gadolinium at 0.03 ± 0.01% on average was observable on MRI acquisition. It is apparent that a saline flush of minimally 10 mL is adequate to remove gadolinium from the port reservoir, relieving the potential for artifacts and false diagnoses.
Keywords
Artifacts, Contrast, Gadolinium, MRI, Ports
PACS Numbers
87.61.Jc, 87.19.uj, 87.61.-c
Introduction
Radiologists are now the most common physician specialist to implant vascular access ports. Many radiologists may not be fully acquainted with how imaging is affected by implantable devices such as vascular access ports, let alone contrast now being considered for use with them [1]. Outside of the clinic, researchers have been attempting to investigate some of these nuances. Although conventional totally implantable venous access ports are not approved for power contrast injections, in laboratory experiments one group examined temperature as a key parameter for the efficiency of specific port types to rid contrast after injection [2,3]. In the past, catheters were considered to potentially have limitations when contrast media is introduced. At the conclusion of a large clinical study where two hundred twenty-five patients were injected with contrast material through their indwelling catheter or through a peripherally placed intravenous catheter, no statistically significant difference was noted.
The medical community gained much ease once hearing of that power injection contrast media through central venous catheters for Computed Tomography (CT) examinations is feasible and safe when set hospital guidelines and injection protocols are followed [4]. Physicians such as medical oncologists are now comfortable enough to routinely offer injection of chemotherapeutic agents into totally implantable venous access ports. There still remains the skepticism as to whether they are appropriate to be considered for patients needing CT scans. This cynicism is often aggravated by published cases detailing how tumors are identified in CT to have spread along a catheter from the neck to the chest wall [5].
Knowing that rodent models are often applied to the development of clinical procedures like repeated medication administration, blood sampling, or intravenous contrast, we have chosen to consider the effects of contrast injection in vascular access ports using similar imaging techniques [6,7]. Given the considerable lack in research involving Magnetic Resonance Imaging (MRI) with vascular access ports, we have made significant effort here to focus attention to MRI imaging techniques only. In this study, we investigate retention rates in various vascular access ports, each having common designs and construction, when MRI contrast is injected.
Materials and Methods
Image acquisition was conducted using an Agilent Technologies® 9.4T MRI scanner (Santa Clara, CA). The scanner features a superconducting research magnet that has three times greater field strength than ordinarily used in hospitals. With the high field strength for scanning, an operating frequency of 400 MHz, and a highly stabled Agilent Technologies® cryo-shimming technology, the micro-MRI scanner produces imaging studies with unparalleled soft-tissue contrast resolution. Imaging RF coils and phase array coils were specifically engineered to allow the imaging of intricate small-scale structures, like neurologic networks in rodents with little aliasing artifact. The primary magnet encompasses a 31 cm bore, with a stepper couch for small specimen scanning. It will be here that vascular access ports are positioned for scanning.
T1-weighted images were obtained using a standard Spin Echo Multi-Slice (SEMS) imaging sequence with the following parameters: TR/TE = 500/11 msec; Matrix size = 192 × 192; Field of View (FOV) = 110 × 60 mm2; Orientation = axial; Number of slices = 27; Slice thickness = 1.0 mm, with no gap between slices.
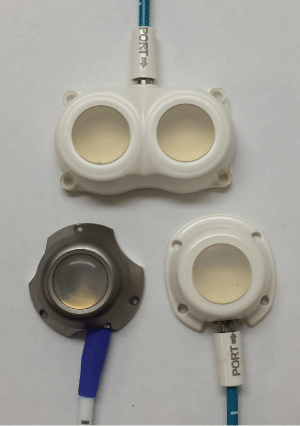
Ports chosen for the study include commercially available types manufactured by AngioDynamics™ (Latham, NY). Specific Models include the Smart Port™ CT, BioFlo™ Dual Port and BioFlo™ Port (Pl). These were chosen based on their typical construction and design. One port consisted of a dual septum for simultaneous injection if desired. The other two were merely representative of the common class of ports that were either mostly titanium or mostly plastic. A total of 15 ports were tested by the same protocol. For each port model, five (n = 5) ports were studied. Figure 1 illustrates the types used in this study.
The contrast agent used in MRI scans was Bracco Diagnostic, Inc.® (Milan, Italy) Model MultiHance® gadolinium-based contrast (gadobenate dimeglumine). Gadolinium alters the signals that are produced in the port and patient during the scan and provide better contrast and clearer images. As of October 26, 2009, it was estimated that approximately 3.2 million patients have received at least one dose of MultiHance® in the United States, and more than double this population received a dose internationally [8].
All fifteen ports were each primed with 2 mL normal saline prior to testing and kept for at least 24 hours at normal body temperature (37 ℃). The saline used for the study was Baxter® (Deerfield, IL) Model BSCl050124 isotonic irrigation fluid, composed of 0.9% sodium chloride. All injections were conducted using a non-coring 19 gauge needle. One additional port for each model was kept aside as a negative control. It was imaged without any contrast introduced in order to assess the background signal of the system.
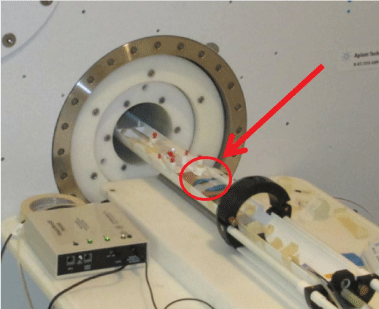
To initiate the contrast studies, a single port was chosen from the lot and taped to a cardboard mounting board. This mounting board was later affixed to the micro-MRI couch. A fluid catch device was arranged at the end of the couch to catch excess contrast or saline flow following injection. Figure 2 depicts the set-up for each exercise.
Positive control imaging began with a 2 mL injection of gadolinium into the rubber septum of the port being studied. An MRI acquisition then followed. The port was then flushed with 10 mL saline and rescanned. With 10 mL saline already introduced by this time, an additional 20 mL saline followed for the next scan, where a cumulative total of 30 mL saline was introduced. This progression was iteratively repeated to 60 mL and 120 mL of saline. The remaining ports were then consecutively mounted to the couch and scanned identically. Data analysis then followed using VnmrJ™ data acquisition software (Agilent Technologies®, Santa Clara, CA) for processing, visualization, spectroscopy and analysis.
Results
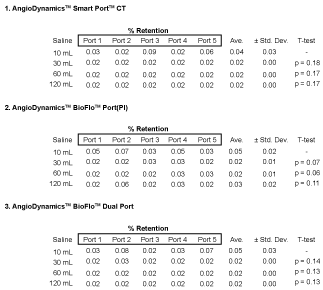
The average retention of all ports studied was a mere 0.03 ± 0.01% after the first saline injection. Apart from the consistency of all ports to rid the injected gadolinium contrast with only 10 mL saline, there were no patterns of inconsistency observed in analysis. Additional injections of contrast to 30 mL, 60 mL and 120 mL to follow did not alter the resulting retention. A successful outcome after the first saline injection was verified for five ports per model with 3 different models investigated. T-test p-values were hand-calculated to determine if any were statistical different, using the common threshold of p-value = 0.05 threshold for non-acceptance. T-test results showed no statistically difference amongst port retention. Tabulated retention rates and p-values for each port are presented in Figure 3.
Figure 4 illustrates the significance of saline flushing during MRI acquisition. The figure demonstrates contrast retention immediately after gadolinium injection, followed by retention removal when flushed with only 10 mL saline. It is apparent that a saline injection of 10 mL was adequate to eliminate nearly all of the remnant gadolinium from the port reservoir and connected catheter from MRI imaging.
Discussion
It is well known that undesirable isolated concentrations of contrast material can cause obscurity when physicians attempt diagnoses. Misinterpretation of artifacts and confusion between normal enhancing structures and tumors were found to be two of the most common reasons for errant false-positive findings [9,10]. Familiarity of artifacts and their sources is extremely important in order to learn how to eliminate them [11]. The usefulness of vascular access ports to assist imaging needs has been limited by the lack of thorough testing and evaluation as it relates to contrast injection. Here, we have demonstrated that there can be success in the administration of injectable gadolinium contrast directly in the port septum for MRI diagnostic imaging. The study was thorough in examining various port types by construction design and composition. Repeated succinct testing verified that retention of contract is a concern if there is no accompanying flush to remove it from the port afterward. Still, the investigation revealed no difficulty in any port model to eradicate 99% or more of the injected gadolinium concentration with only one 10 mL saline injection.
It should be noted that we have examined only a few types of vascular access ports. Although many ports used clinically today involve similar materials and similar shapes as the ones tested in this study, the reader is cautioned that there can be differences in retention for other makes and models when used with gadolinium injection. Additionally, it is not recommended that the user extrapolate or expect identical results when ports are injected with other contrast agents.
Conclusion
This study investigated retention rates for 15 ports. All ports were models currently marketed by AngioDynamics™, which have typical design features and composition used clinically. The experimental intent was to determine if there exists a significant amount of uptake in the vascular access port and catheter system when directly injected with gadolinium contrast prescribed by a radiologist to assist with MRI studies. Testing included five models per port type. The initial injection volume was 2 mL gadolinium, followed by saline flush volumes at 10 mL, 30 mL, 60 mL and 120 mL. Identical and consecutive testing revealed only residual gadolinium remained in the port when injected with saline at a volume of 10 mL. It is recommended that if these ports are to be used in radiological studies with gadolinium, saline should be used to flush the port prior to engaging in imaging. As provided in this study, removing MRI contrast from the port can rid nearly all imaging artifacts, which directly relate to the apprehension of potential false diagnoses.
Acknowledgements
We would like to thank the AngioDynamics™ for the donation of devices for this study.
Conflicts Statement
The authors have no relevant financial interests in the manuscript and no other potential conflicts of interest to disclose.
References
- Indrajit IK, Sivasankar R, D'Souza J, et al. (2015) Pressure injectors for radiologists: A review and what is new. Indian J Radiol Imaging 25: 2-10.
- Guiffant G, Durussel JJ, Flaud P, et al. (2013) Power port contrast medium flushing and trapping: Impact of temperature, and in vitro experimental study. Med Devices 6: 133-140.
- Goltz JP, Machann W, Noack C, et al. (2011) Feasibility of power contrast injections and bolus triggering during CT scans in oncologic patients with totally implantable venous access ports of the forearm. Acta Radiol 52: 41-47.
- Herts BR, O'Malley CM, Wirth SL, et al. (2001) Power injection of contrast media using central venous catheters: Feasibility, safety and efficacy. AJR Am J Roentgenol 176: 447-453.
- Lee CH, Day AS, Whang TZ (2013) Metastasis over implantable venous access ports. Head Neck 35: E314-E316.
- Fiebig T, Figueiredo G, Boll H, et al. (2013) A low cost metal-free vascular access mini-port for artifact free imaging and repeated injections in mice. PLos One 8: e65939.
- Hettinger PC, Li R, Yan JG, et al. (2011) Long-term vascular access ports as a means of sedative administration in a rodent fMRI survival model. J Neurosci Methods 200: 106-112.
- U.S. Food and Drug Administration (2009) Colloquia: Joint meeting of the cardiovascular and renal drugs and drug safety and risk management advisory committee, Gaithersburg.
- Millet I, Pages E, Hoa D, et al. (2012) Pearls and pitfalls in breast MRI. Br J Radiol 85: 197-207.
- Krupa K, Bekiesinska-Figatowska M (2015) Artifacts in magnetic resonance imaging. Pol J Radiol 80: 93-106.
- Kathiravan S, Kanakaraj J (2013) A review on potential issues and challenges in MR imaging. Scientific World Journal 2013: 783715.
Corresponding Author
Michael S Gossman, Regulation Directive Medical Physics, 104 Hildeen Court, Russell, Kentucky 41169, USA, Tel: 606-329-0060.
Copyright
© 2017 Gossman MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.