Treatment of Post-Stroke Shoulder Pain of Multi-Factorial Etiology
Abstract
Chronic pain after stroke is a common, yet often overlooked sequela of cerebrovascular injury. Among the syndromes seen in post-stroke pain (PSP) are central post-stroke pain (CPSP) and complex regional pain syndrome (CRPS). Shoulder pain develops in a large subset of patients with residual upper-extremity weakness. Shoulder pain after stroke is multifactorial. Treatment of pain in these patients can be challenging when the exact etiology is unclear, and may necessitate multiple, successive therapeutic approaches before an effective remedy is found. Here we present a case of Post-Stroke Shoulder Pain of multifactorial etiology in which multiple treatment strategies were utilized, including successful trial of a cervical spinal cord stimulator (SCS) device.
Keywords
Chronic Pain, Shoulder Pain, Poststroke Pain, Poststroke Shoulder Pain, Central Poststroke Pain, Cervical Spinal Cord Stimulator
Introduction
Individuals who suffer a stroke are at risk for the development of chronic pain syndromes, with prevalence of up to 50% [1]. Post-stroke pain (PSP) can be divided into several subtypes, including central post-stroke pain (CPSP), complex regional pain syndrome (CRPS), pain stemming from spasticity, shoulder pain, and headache [2]. However, many PSP patients exhibit symptoms that are multi-factorial in etiology.
CPSP occurs in 8%-14% of patients following stroke [3]. The onset of pain is typically gradual, occurring within 6 months as somatosensory deficits are resolving. It is paroxysmal, accompanied by allodynia and hyperalgesia, and often constant, although there can be intermittent periods of relief. Pain is commonly described as aching, lacerating, freezing, burning or squeezing in character [4]. Although historically associated with thalamic insults, CPSP can be mediated by a vascular lesion anywhere along the spinothalamocortical pathway [3]. The pathophysiology is poorly understood; however, proposed mechanisms include central disinhibition, central sensitization, and imbalance of stimuli [3]. The severity of the pain is usually variable, increases with external stimulation, and is relieved by rest [5]. CPSP can co-exist with other post-stroke pain subtypes. Some common combinations are CPSP and Complex Regional Pain Syndrome (CRPS), and CPSP and shoulder pain [4].
Shoulder pain after stroke is common, with an estimated prevalence of 25-50% in stroke patients [4]. Its development is likely multifactorial, involving glenohumeral subluxation, impingement, rotator cuff tears, bicipital tendinitis, and CRPS [4]. Glenohumeral subluxation can occur as a result of weakness in the muscles that surround and provide stability to the shoulder joint. The joint is most vulnerable to subluxation in the period immediately after stroke, when muscle tone in the upper extremity is flaccid [6]. Subluxation itself can result in further complications, including CRPS and secondary brachial plexus injury.
The estimated incidence of CRPS after stroke is between 2 and 49% [7,8]. CRPS is characterized by pain, edema, vasomotor abnormalities, and patchy demineralization of bone in an extremity, and is divided into two types based on the absence (Type I) or presence (Type II) of a definable nerve lesion. The majority of stroke patients with CRPS are diagnosed as Type I, however micro-trauma to nerves may in fact explain their symptoms [9]. As subluxation is more common in stroke patients who develop CRPS, and the severity of weakness and immobility in the shoulder are related to the likelihood of developing CRPS, glenohumeral joint instability and biomechanical impairment are likely implicated in post-stroke CRPS [10-12].
These etiologies of post-stoke pain each have unique treatment strategies, and the integration of those strategies can make care of the patient with multi-factorial post-stroke shoulder pain quite challenging.
Case Presentation
We will describe the treatment course in a case of severe post-stroke shoulder pain, which was multi-factorial in etiology. Our patient is a 45-year-old female who suffered a left middle cerebral artery stroke of suspected embolic origin 3 years prior, and subsequently developed right hemibody pain most severe in the right upper extremity (RUE). This was initially managed by a neurologist as an outpatient before she was referred to the pain management service with the diagnosis of CPSP. She had shown little response to conservative therapy, and had been unable to tolerate topiramate and amitriptyline due to side effects. She underwent extensive physical and occupational therapy, which helped with the weakness she was experiencing in her right hemibody, but did not decrease her pain. She had also attempted desensitization therapy without success. The patient had moderate reduction in her pain with gabapentin, and was transitioned to pregabalin with some improvement, but still reported suboptimal pain control. Of particular concern to her was focal right shoulder pain that she described as sharp and stabbing in nature, and which escalated intermittently throughout the day.
Her physical exam was significant for allodynia and hyperesthesia to the right hemibody, which were most severe on the right side of her face and in the RUE. Active and passive range of motion of the right shoulder was greatly limited due to pain, and there was significant tenderness to palpation of the right acromioclavicular (AC) and glenohumeral (GH) joints. No temperature or skin changes to the right upper or lower extremities were noted.
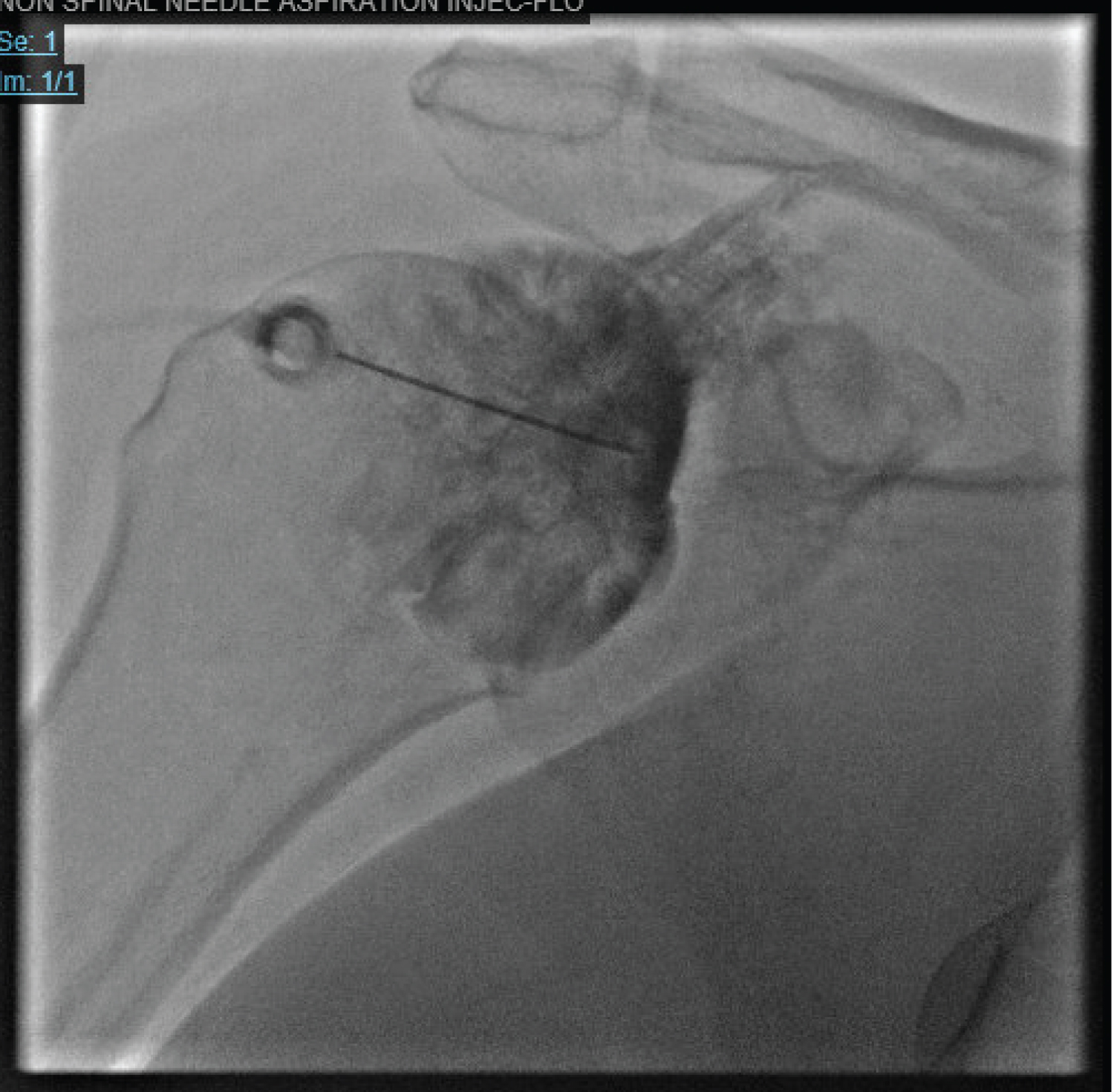
Due to concern for post-stroke shoulder subluxation, an X-ray of the right shoulder was ordered, which showed mild degenerative changes of the AC joint, but no acute fracture or dislocation. The patient subsequently underwent two AC joint corticosteroid injections with > 50% pain relief immediately post-procedure. She experienced 3 weeks of relief after each injection, however, the allodynia and hyperesthesia to the RUE persisted through this period. The patient stated that her shoulder pain was severely limiting her from participating in physical therapy. She was offered and elected to proceed with local anesthetic capsular distension at the right GH joint (Figure 1), along with right suprascapular and axillary nerve blocks.
After these procedures, she was able to better participate in physical therapy. She reported 40-50% improvement in her right shoulder pain and increased range of motion 1 month after the interventions. However, she continued to experience intermittent episodes of severe pain in her right shoulder, and persistent allodynia/hyperesthesia of the right upper extremity. Two months after the GH injection, the patient reported that the pain relief had subsided. She was unable to lift her right arm above 90 degrees without significant pain, and stated that her ability to perform her daily activities was greatly impaired. Her medication regimen at that time consisted of pregabalin 150 mg TID, cyclobenzaprine 10 mg nightly, memantine 5 mg BID, and oxycodone 5-10 mg Q4H PRN.
Stellate Ganglion Block Procedure & Results
Given that the patient had failed all other therapies and was amenable to any intervention that would potentially alleviate her pain, she was offered and elected to proceed with a stellate ganglion block for treatment of refractory RUE pain. We felt that this intervention was reasonable to offer in this scenario as the stellate ganglion block has been successfully utilized to treat a wide range of conditions including CRPS, upper extremity pain, and even CPSP in one case report [13].
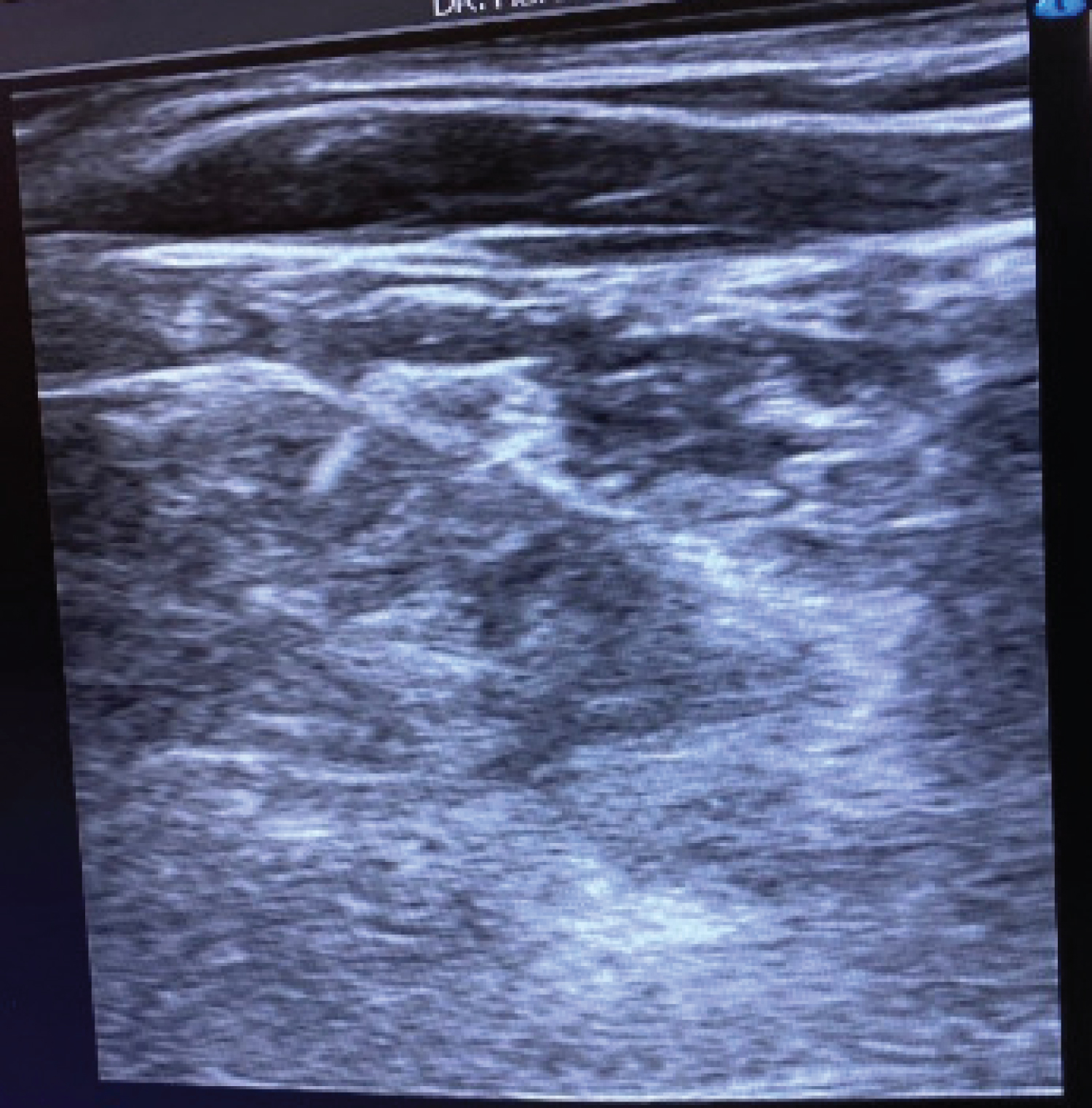
Informed consent was obtained. The patient was placed supine in a stretcher with a pillow underneath the lower cervical region to elevate the neck. She was directed to turn her head slightly to the left (contralateral) side. After the neck was prepped and draped, an ultrasound probe was used to visualize the cervical spine and count cervical articular pillars until a dropout was appreciated, effectively identifying C7. The ultrasound probe was transitioned up to the last appreciated cervical pillar to isolate C6. The probe was then transitioned anterior to visualize vital anatomy such as the internal jugular vein, carotid artery, thyroid tissue, thyroid artery, and longus colli muscle. Using an in-plane approach, a 22-gauge, 3.5 inch spinal needle was advanced just adjacent to the longus colli muscle (Figure 2). After repeated negative aspirations for blood, fluid, or air, a 5 mL mixture of bupivacaine 0.25% with dexamethasone 10 mg was intermittently injected in the visualized area. There was no evidence of intravascular or CSF placement. No paresthesias were noted. The needle was removed, the patient's neck was washed and dried, and a dressing was applied. The patient tolerated the procedure well. She was able to sit up after the procedure, and endorsed good pain relief prior to discharge home.
The patient had > 60% pain relief from baseline at post-procedure days 2 and 3 with steady return to her baseline pain level from day 4 onward. In contrast to previous interventions, the patient stated that the allodynia and hyperesthesia of her right upper extremity improved after the stellate ganglion block. At 12 days post-procedure the patient was at her baseline level of pain. She elected to proceed with a second stellate ganglion block. The second block was performed with the same technique as previously described. The patient once again tolerated the procedure well and endorsed 60% pain relief for 3 days post-procedure with gradual return of her pain. Her allodynia and hyperesthesia were once again improved after the intervention.
Cervical Stim Trial Procedure & Results
Although the stellate ganglion blocks did provide the patient with significant analgesia, the duration of relief was less than desired. Given the wide range of indications for this block, its success in alleviating the patient's pain for a brief period does not indicate that CRPS is the sole diagnosis. However, the fact that the patient meets Budapest criteria for diagnosis of CRPS and showed significant improvement in her allodynia and hyperesthesia after the block lead us to believe that there was a significant sympathetically-mediated component to her pain. As spinal cord stimulation (SCS) and dorsal root ganglion (DRG) stimulation have been shown to be effective in the treatment of sympathetically-mediated pain [14,15], we felt it was reasonable to consider cervical SCS placement in our patient. She elected to proceed with this intervention, and was scheduled for trial placement.
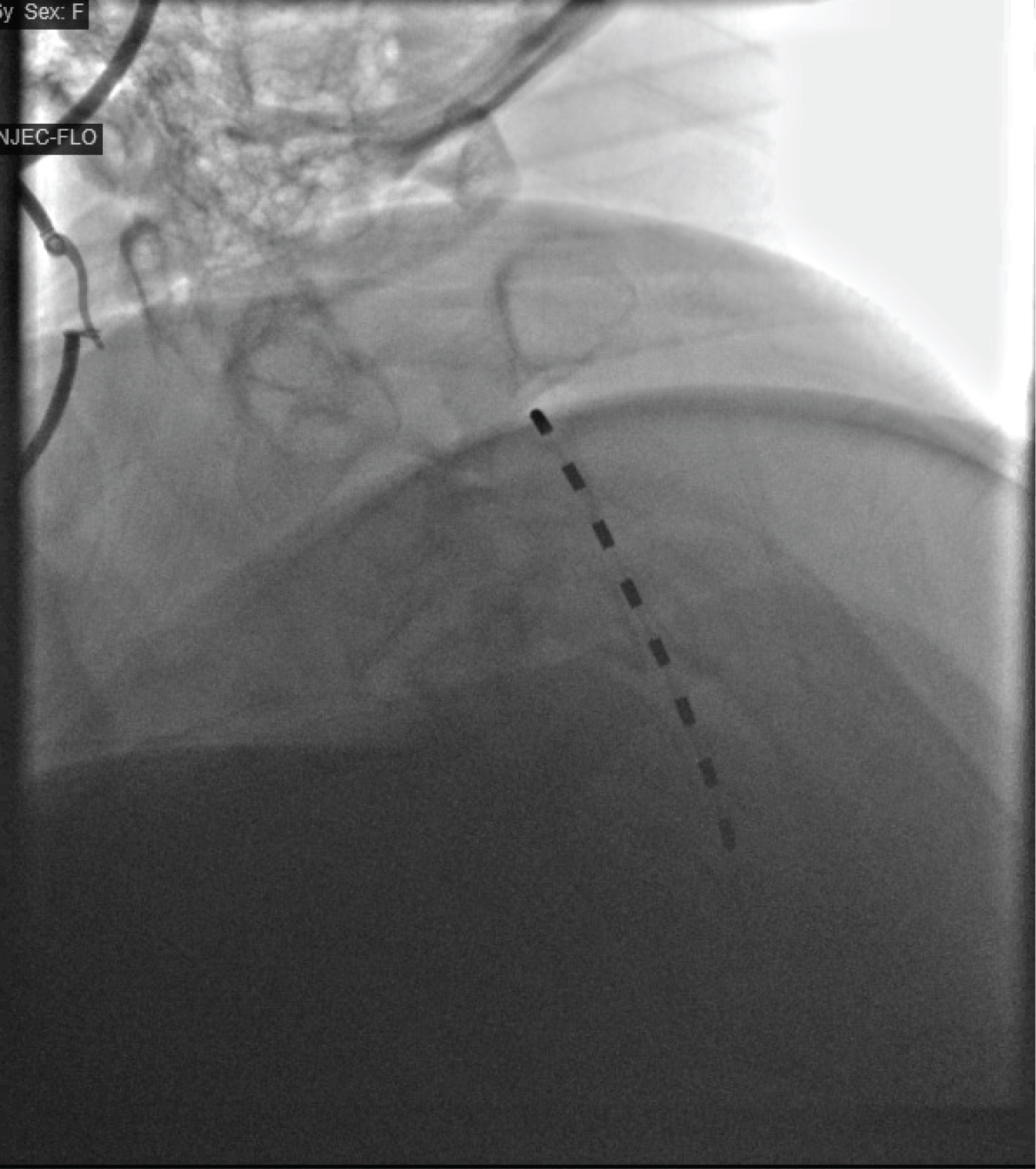
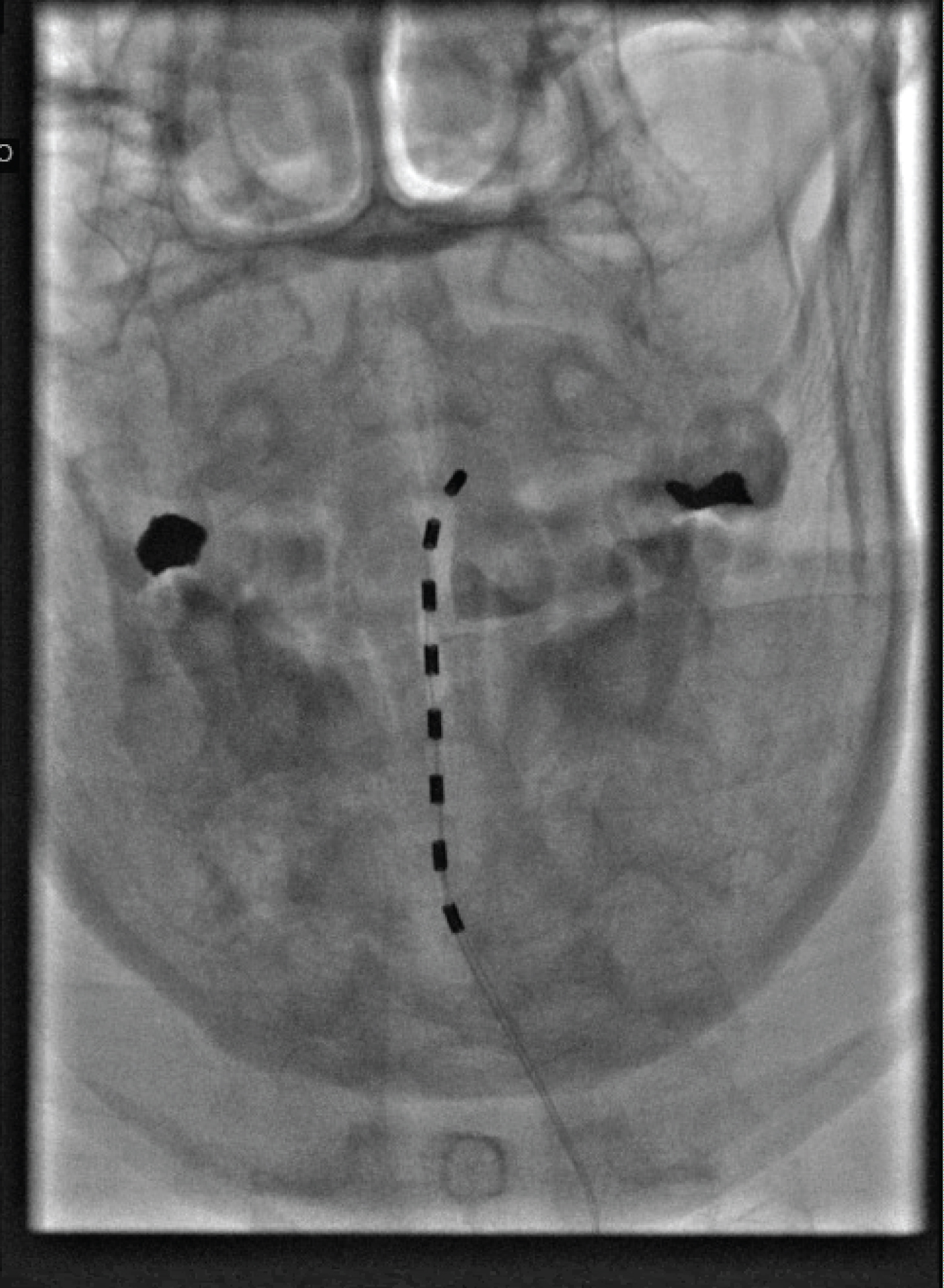
The patient was brought to the OR and placed on the fluoroscopy table in the prone position. The back was prepped and draped in sterile fashion. The T2/3 interspace was identified in the anteroposterior (AP) view. Skin was marked and infiltrated with 1% Lidocaine using a 25G needle. A 14G Touhy needle was incrementally advanced towards the epidural space using the loss of resistance technique. With the patient not under sedation and fully alert in order to give feedback for painful stimuli, the stimulator lead was partially advanced into the epidural space and a lateral image was taken to confirm posterior placement (Figure 3 and Figure 4). The stimulator lead was then incrementally advanced towards the top of C2. The SCS system was then tested and programmed with adequate coverage of the right upper extremity. The needle was then removed under fluoroscopic guidance, and lead placement was confirmed as unmoved with repeat imaging. The leads were then secured to the back with an occlusive dressing. The patient tolerated the procedure well. She endorsed 50% pain relief immediately post-procedure. She ambulated without difficulty and was discharged home with instructions to follow up in 5 days. She reported 50% pain relief with significantly improved ROM and function of the RUE at 5 days post-procedure. The improvement in function was primarily due to full resolution of the hyperesthesia, allodynia, and edema previously present in the RUE. Strength in the affected extremity improved to 4-5/5 with no pain. She had no discomfort with deep pressure throughout the RUE.
Discussion
In this case it is likely that there were multiple post-stroke pain syndromes superimposed on one another, highlighting the challenge in diagnosing and treating pain in this patient population. Given that CPSP and CRPS have many similar features, it can be difficult to distinguish between the two. This is further confounded by the fact that both syndromes can appear together, and are both associated with shoulder pain.
There is a notable lack of class I and II studies investigating treatment for CPSP. Review of the available literature indicates that amitriptyline is recommended as the first-line medication, and gabapentin and pregabalin are viable second-line medications [3,5]. This patient was unable to tolerate therapy with amitriptyline, and was currently taking pregabalin. In patients with CPSP refractory to pharmacological therapy, repetitive transcranial magnetic stimulation (rTMS) and deep brain stimulation (DBS) have shown positive results [3]. While DBS is an invasive surgical procedure, rTMS is considered safe and well tolerated and would be a reasonable option to consider in this patient. Spinal cord stimulation has not been systematically evaluated for treatment of CPSP, but could be a treatment option for patients refractory to conservative measures. Initial results in this patient were promising, and we will continue to follow-up to determine the long-term efficacy.
Currently, no definitive treatment has been established for post-stroke CRPS; however, the optimal approach involves reduction in pain, maintenance of joint mobility, and restoration of function [4]. Pain control is absolutely essential, as it will allow patients to engage with therapy to restore and preserve function. This multi-prong approach necessitates an interdisciplinary team that incorporates occupational and physical therapists to provide mobilization and strengthening of the affected limb, edema control, and techniques for desensitization [4]. To target sympathetically-mediated effects, nerve blocks can be given at the level of the stellate ganglion, as was done in this patient. Gradual desensitization, which involves the application of an increasingly painful stimulus to the affected area that is thought to reset altered central sensory processing was not successful in this patient [16]. Drugs used for neuropathic pain have suggested benefit in CRPS, but their efficacy has not been studied in the stroke population [4].
Preventing the development of post-stroke shoulder pain through early intervention may be the most important component of treatment. After the acute phase of stroke, immediate joint stabilization and a physiotherapy regimen beginning with passive range of motion should be initiated [17]. The onset of severe upper extremity motor weakness and shoulder joint laxity renders it vulnerable to injury and GH subluxation, which in turn can lead to CRPS [4]. This sequence likely describes the etiology of our patient's condition, and would explain her combination of neuropathic and musculoskeletal symptoms. GH subluxation after stroke rarely resolves spontaneously [18]. Treatment options, including mechanical stabilization with shoulder slings, arm boards, and shoulder strapping are aimed at mechanical stabilization of the joint [4]. Simple analgesics and non-steroidal anti-inflammatory drugs are the first-line medical therapy [19]. Anti-spasmodic agents may be helpful if spasticity is contributing to the pain [20].
Currently post-stroke pain remains both under-recognized and under-treated. Improved recognition and treatment of pain syndromes such as the multi-factorial shoulder pain described in this case can have a significant impact on pain outcomes and overall quality of life in patients following stroke.
References
- Naess H, Lunde L, Brogger J (2012) The effects of fatigue, pain, and depression on quality of life in ischemic stroke patients: The bergen stroke study. Vasc Health Risk Manag 8: 407-413.
- (1979) Pain terms: A current list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain 6: S215-S221.
- Kumar B, Kalita J, Kumar G, et al. (2009) Central poststroke pain: A review of pathophysiology and treatment. Anesth Analg 108: 1645-1657.
- Harrison RA, Field TS (2015) Post stroke pain: Identification, assessment, and therapy. Cerebrovasc Dis 39: 190-201.
- Akyuz G, Kuru P (2016) Systematic review of central post stroke pain. What is happening in the central nervous system? Am J Phys Med Rehabil 95: 618-627.
- Faghri PD, Rodgers MM, Glaser RM, et al. (1994) The effects of functional electrical stimulation on shoulder subluxation, arm function recovery, and shoulder pain in hemiplegic stroke patients. Arch Phys Med Rehabil 75: 73-79.
- McLean DE (2004) Medical complications experienced by a cohort of stroke survivors during inpatient, tertiary-level stroke rehabilitation. Arch Phys Med Rehabil 85: 466-469.
- Kocabas H, Levendoglu F, Ozerbil OM, et al. (2007) Complex regional pain syndrome in stroke patients. Int J Rehabil Res 30: 33-38.
- Yu DT (2009) Shoulder pain and other musculoskeletal complications. In: Stein J, Harvey RL, Macko RF, et al. Stroke recovery and rehabilitation. (edn), DemosMedical, New York, 437-451.
- Dursun E, Dursun N, Ural CE, et al. (2000) Glenohumeral joint subluxation and reflex sympathetic dystrophy in hemiplegic patients. Arch Phys Med Rehabil 81: 944-946.
- Gokkaya NK, Aras M, Yesiltepe E, et al. (2006) Reflex sympathetic dystrophy in hemiplegia. Int J Rehabil Res 29: 275-279.
- Chae J (2010) Poststroke complex regional pain syndrome. Top Stroke Rehabil 17: 151-162.
- Liao C, Yang M, Liu P, et al. (2017) Thalamic pain alleviated by stellate ganglion block. Medicine (Baltimore) 96: e6058.
- Garg A, Danesh H (2015) Neuromodulation of the cervical dorsal root ganglion for upper extremity complex regional pain syndrome-case report. Neuromodulation 18: 765-768.
- Poree L, Krames E, Pope J, et al. (2013) Spinal cord stimulation as treatment for complex regional pain syndrome should be considered earlier than last resort therapy. Neuromodulation 16: 125-141.
- Harden RN (2001) Complex regional pain syndrome. Br J Anaesth 87: 99-106.
- Vasudevan JM, Browne BJ (2014) Hemiplegic shoulder pain: An approach to diagnosis and management. Phys Med Rehabil Clin N Am 25: 411-437.
- Zorowitz RD (2008) Recovery patterns of shoulder subluxation after stroke: A six-month follow-up study. Top Stroke Rehabil 8: 1-9.
- Dawson AS, Knox J, McClure A, et al. (2013) Stroke rehabilitation best practices writing group: Management of shoulder pain following stroke. In: Lindsay MP, Gubitz G, Bayley M, et al. Canadian best practice recommendations for stroke care. (edn), Heart and Stroke Foundation and the Canadian Stroke Network, Ottawa, Ontario, Canada, 47-50.
- Van Ouwenaller C, Laplace PM, Chantraine A (1986) Painful shoulder in hemiplegia. Arch Phys Med Rehabil 67: 23-26.
Corresponding Author
Stephen Hannaford, MD, Department of Anesthesiology, Rutgers New Jersey Medical School, 185 South Orange Ave, Newark, NJ 07103, USA
Copyright
© 2020 Hannaford S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.