Postoperative Hypertension: When Blood Pressure Cuff and Arterial Line Disagree
Abstract
Hypertension is a commonly encountered condition by anesthesiologists yet there is no clearly defined approach to monitoring and managing blood pressure in the perioperative period. In this review, we focus on common dilemmas in perioperative management of hypertensive patients including preoperative evaluation and indications for postponement of elective procedures; appraise the commonly used modes of perioperative blood pressure monitoring; discuss acute hypertension in the postoperative period and the role of blood pressure in discharge criteria for patients in the post-anesthesia care unit; and we review the relevant pathophysiology of organ perfusion in chronic hypertension and the implication for postoperative blood pressure management in hypertensive patients. Finally, we propose a novel organized approach to perioperative blood pressure monitoring with emphasis on resolving conflict between invasive versus noninvasive modalities of measurement.
Introduction
Significant discrepancy in systemic blood pressure measurements between oscillometric cuff and arterial line is a commonly encountered scenario in the perioperative period. To our knowledge, there is no consensus strategy on how to best deal with such discrepancies and management is typically based on individual clinical judgement. In this review, we will explore some of the important clinical ramifications of this predicament and suggest rationale for how to proceed in this situation.
Materials and Methods
A literature search was conducted in PubMed and Ovid Medline electronic databases to gather published peer-reviewed articles related to perioperative blood pressure goals and modes of its monitoring. Key word searches included "perioperative hypertension", "intraoperative hemodynamic monitoring", "cerebral autoregulation", "invasive blood pressure monitoring", "noninvasive blood pressure monitoring", and "acute postoperative hypertension". The referenced literature includes eight guidelines and practice standards, two books, and thirty seven articles. After completing the literature review, we identified that a knowledge gap exists both in the approach to the management of perioperative hypertension as well as evidence-based selection of the blood pressure monitoring modality to guide this management. Based on the available studies, we proposed an algorithm for monitoring perioperative blood pressure using the appropriate invasive or noninvasive monitor when there is a discrepancy between the two methods.
Discussion
A gap of uncertainty
An aging population and the rising prevalence of obesity make it increasingly likely for anesthesiologists to encounter hypertension and related complications. Several guidelines have been published regarding managing perioperative BP (the American Society of Anesthesiologists Basic Standards for Pre-anesthesia Care, [1] Standards for Basic Anesthetic Monitoring, [2] and the Practice Advisory for Pre-anesthesia Evaluation), [3] but they neither include exact BP targets nor outline an algorithm for BP monitoring method. The American Society of Anesthesiologists Standards for Post-anesthesia Care lacks information on postoperative BP management, monitoring and its impact on discharge procedure. Monitoring of BP and heart rate is recommended but no target range is established. Absence of specific recommendations about BP targets and modality of BP monitoring leaves a gap of uncertainty in perioperative management of hypertensive patients, especially in a clinical setting in which NIBP and IBP monitors yield significantly differing results.
Preoperative evaluation and management of chronic hypertension
A thorough preoperative risk assessment should be performed prior to any elective, non-emergent surgical procedure. If a patient's preoperative BP is > 180/110 mmHg, consideration should be made to postpone the procedure to investigate for any underlying cause of severe hypertension and/or adjust outpatient medications until better baseline BP control is achieved [4]. There are no clear evidence-based recommendations on duration of postponement and overall it is not known if delaying surgery will have any effect on cardiovascular complication rate [5]. The minimum time needed for regression of some cardiac and vascular changes is usually considered to be 6 to 8 weeks, [6] but it may differ from patient to patient due to the magnitude of preexisting pathologic changes or comorbidities. Other conditions, such as diastolic dysfunction, may take many months to improve with medications [7]. For patients with other cardiovascular risk factors, the initiation of a beta-blocker 2 to 7 days before surgery decreases 30-day postoperative mortality and cardiac morbidity, although similar benefits have not been described when initiated for hypertension alone [8]. Decision regarding delaying surgery should be made after weighing all the risks and benefits for each patient.
For BP < 180/110 mmHg, elevated BP by itself has not been associated with increased postoperative morbidity or mortality and it is therefore likely safe to proceed with elective surgery [9-11]. Due to the lack of an independent link to major adverse cardiac events, elevated BP is not included as a risk factor in the American Heart Association and American College of Cardiology recommended Revised Cardiac Risk Index (RCRI) that is currently used to assess perioperative risk [8]. However, chronic hypertension is a known risk factor for ischemic heart disease, congestive heart failure, cerebrovascular disease and renal insufficiency, which are all included in the RCRI. Nevertheless, chronic hypertension remains an important factor to consider in patients undergoing surgery because it is associated with labile hemodynamics during general anesthesia and higher perioperative risk during noncardiac surgery if left ventricular hypertrophy is also present [12,13]. Considering the possibility of undiagnosed cardiovascular pathology associated with hypertension, preoperative evaluation with electrocardiogram and serum creatinine level is appropriate for chronically hypertensive patients. This would provide information for surgical risk stratification and help guide further evaluation [6,8,14].
Sometimes patients have adequately controlled hypertension during preoperative clinic visits, but their BP is unusually high on the day of surgery. This discrepancy can have a number of causes including missed medication dose(s) or sympathetic nervous system overstimulation due to preoperative anxiety and should be managed accordingly. Taken together, in the absence of any obvious treatable causes of preoperative hypertension and assuming the patient is asymptomatic, it is likely safe to proceed with surgery for hypertensive patients with BP < 180/110 mmHg [9].
Intraoperative hemodynamic monitoring
There are invasive (IBP) and noninvasive (NIBP) methods for perioperative BP monitoring with noninvasive being the most commonly used due to its convenience and low incidence of associated adverse events. The most widely used methods of NIBP measurements are oscillometry and auscultation. Although both rely on identifying a pressure range over which pulsatility is detected, the interchangeability of these two methods has been challenged. A review of ten studies suggested that oscillometry was less accurate as compared to auscultation; however these differences were not clinically significant [15]. The need for more accurate BP measurement technique is highlighted in situations such as arrhythmia or trauma where it directly affects management [15]. Moreover, the accuracy of oscillometry for individual patients can be unpredictable, and thus it is recommended the initial evaluation should include both methods for comparison [16]. Despite these drawbacks, oscillometric NIBP remains accurate in estimating MAP in stable, low-risk patients and has notable advantages over manual BP measurement: it doesn't require trained staff to take measurements, eliminates observer error, allows periodic automated BP measurements, and reduces the phenomenon of "white coat hypertension" in conscious patients [17,18]. Both methods are vulnerable to inaccuracy in certain conditions such as arterial stiffness, which is seen with high prevalence in patients older than 50 years of age [19], and in obese patients, regardless of whether or not the extremity used is conically-shaped [20]. Other possible causes of inaccuracy of oscillometric NIBP are given in Table 1 [21,22].
Many consider IBP monitoring to be the gold standard, as it directly measures intra-arterial pressures. An arterial line is used in patients who are anticipated to experience extremes of BP, are unstable, would be unable to be monitored accurately by NIBP means, require continuous monitoring, or are expected to have frequent intraoperative blood sampling. Despite that, there are no evidence-based indications for arterial line placement and the decision regarding its use are usually made on a case-by-case basis [23]. Use of IBP requires an invasive procedure that may produce rare but serious complications and requires trained personal for its placement, removal and care. Complications may be site nonspecific, affecting all arterial line sites, or specific to the particular anatomic location. For example, thrombosis, embolism or infections can complicate all arterial lines while direct injury or compression of adjacent nerves by hematoma is specific to certain sites-e.g. median nerve or brachial plexus injury from brachial artery or axillary artery puncture sites, respectively. A comparison of oscillometric NIBP and IBP methods is shown in Table 1.
Intraoperatively, selected patients undergo placement of an IBP monitor to help guide hemodynamic management and oftentimes NIBP is continued intermittently as a confirmatory measure of BP. With this approach, more than one monitor of the same physiologic variable might have utility in quality control. If measurements do not correlate, this should trigger further investigation, thus providing for earlier detection of a potential faulty measurement. However, this can cause confusion when they differ significantly despite troubleshooting possible sources of measurement error.
Discrepancies between NIBP and IBP monitoring in patients has been well documented in the literature. Wax, et al. showed that even with proper application, NIBP tends to yield higher BP measurements in hypotensive patients and lower BP measurements in hypertensive patients, as compared to IBP [24]. This has been attributed to stiffened vessels requiring greater cuff pressure to occlude blood flow at low BP and to peripheral amplification of pulse pressure for arterial pressures at high BP (most relevant when measured at the radial artery, as compared to more central measurements) [25]. Therefore, based on these theoretical concerns, it would seem NIBP measurements would tend to more closely reflect central blood pressures at BP extremes. Monitoring BP by a single method may delay discovery of true hypo/hypertension, and consequentially delay initiation of appropriate intervention. However, very little has been published regarding management strategies in the context of general anesthesia as well as in the postoperative period. There exists a need to highlight a rational management strategy in this setting.
Recently published data regarding intraoperative management of BP found that the use of NIBP measurement to supplement IBP measurements was associated with decreased use of blood transfusions, vasopressor or inotrope infusions and antihypertensive medications when compared to IBP measurements alone [24]. However, less therapeutic interventions intraoperatively did not correlate with improved overall outcomes such as in-hospital survival [26].
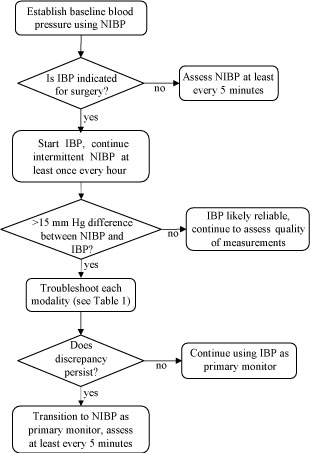
Figure 1 summarizes existing evidence into an algorithm that may facilitate selection of the appropriate monitoring mode if major discrepancy is found between IBP and NIBP measurements. To define discrepancy as "major" we chose a cutoff of ≥ 15 mmHg because such a difference in IBP and NIBP readings may indicate the presence of under damping/resonance phenomena which significantly distorts accuracy of IBP [27]. This artifact is found to occur in up to one third of intensive care unit (ICU) patients with mean deviation from coinciding systolic NIBP readings as high as ± 28.5 mmHg [27]. For reference, difference between invasive and noninvasive BP measurements ≥ 10 mmHg is perceived as clinically relevant by majority of anesthesia and critical care providers [28]. Inaccuracy of this size may affect clinical decisions on management with possible false-reassurance or unnecessary interventions. If "major" discrepancy in IBP and NIBP readings is noticed troubleshooting each modality for possible sources of error (as described in the Table 1) should be performed. If then a discrepancy persists, we suggest making clinical decisions relying more upon the NIBP monitor, as it was the monitor used to determine the patient's preoperative baseline. Although there has been some concern that such an approach may be suboptimal for critically ill patients, as NIBP has previously been believed to lack sufficient accuracy to guide therapy in that setting [29], this issue is currently under debate because no mortality benefit has been associated with the use of arterial line in ICU patients [30,31].
Recommendations for postoperative hypertension in patients recovering in the post-anesthesia care unit
Hypertension remains a common postoperative complication and affects 3 to 9% of all PACU patients with much higher rates after certain procedures. For instance, carotid endarterectomy, cardiac surgery, abdominal aortic surgery, and intracranial surgery may have postoperative hypertension rates as high as 90% [32]. Despite the widespread and longstanding recognition of acute postoperative hypertension (APH), there is no consensus on a more precise, quantitative definition [32]. Some authors define APH as systolic BP > 190 mmHg with or without diastolic BP ≥ 100 mmHg in at least two consecutive measurements postoperatively [33], while others recommend treatment threshold for APH as BP > 160/90 mmHg or greater than 20% elevation from preoperative baseline BP [32]. APH can begin to manifest in the first 20 minutes postoperatively and lasts on average 3-4 hours [33]. These patients often have a history of chronic hypertension, and the postoperative hypertension is usually transient in nature.
Poorly controlled postoperative hypertension can have its own secondary cardiovascular or bleeding complications. These effects are attributed to after load-associated increased myocardial workload and increased intravascular hydrostatic pressure, respectively. The first steps in management are to reverse any precipitating factors such as pain, bladder distension, hypervolemia, hypoxia, hypercarbia and hypothermia, with the goal of controlling hypertension in order to protect organ perfusion and function. Other causes of postoperative hypertension to consider include rebound hypertension after withdrawal of antihypertensive medications, hypertension associated with head trauma, and hypertension caused by acute catecholamine excess (e.g. pheochromocytoma). The approach to manage postoperative hypertension is not uniform, and there is no consensus regarding monitoring strategies, treatment thresholds/targets or pharmacologic agents [34].
Which monitor should be used in PACU for guiding APH management and subsequent determination of patient's fitness for discharge? We the authors submit that since the patient's preoperative determination of BP baseline and management was guided by NIBP, it would be reasonable to rely on NIBP in the event of discrepancy between monitoring modalities. This method should thus more accurately reflect overall trends in patients' BP.
Perioperative blood pressure guidelines
The American Society of Anesthesiologists Practice Guidelines for Post-anesthetic Care published in 2013provide recommendations that can assist providers in clinical decision making in the recovery room [35]. However, recommendations for post-anesthesia BP management are limited and only state: "Routine monitoring of pulse and blood pressure should be done during emergence and recovery, and electrocardiographic monitors should be immediately available" [35]. No clear strategy is provided in terms of diagnosis, management or discharge of post-anesthetic hypertension.
Although practice guidelines do not specifically recommend or discuss the use of scoring systems in pre-discharge patient assessment, the latter can help in defining acceptable margins for BP in postoperative patients. The most commonly used scoring systems are Post-anesthetic Discharge Scoring System (PADSS) [36] and Modified Aldrete system [37]. In relation to patients' preoperative baseline, PADSS defines optimal BP as within ± 20% while Modified Aldrete describes optimal BP as within ± 20 mmHg. Utilization of these scoring systems is recommended by the Joint Commission for Accreditation of Healthcare Organizations (JCAHO) and the Canadian Anesthetists Society (CAS), but the impact of their implementation on postoperative mortality and morbidity is unclear [38].
Guidelines for managing patients with extreme high blood pressures in the perioperative period
Existing data suggests that higher BP goals may be more beneficial for chronically hypertensive patients than lower ones [39], but some worrisome signs should never be overlooked. Critically elevated BP threatens perfusion and can lead to end-organ damage. Systolic BP > 180 mmHg and diastolic BP > 120 mmHg is a hypertensive urgency and should be promptly treated if diagnosed peri-operatively, although individual patient's history of preexisting hypertension and preoperative BP measurements should also be considered [39]. However, there are no data showing any benefit of rapid BP correction in cases of hypertensive urgency; on the contrary, it may cause cerebral, cardiac or renal hypoperfusion. If evidence of end-organ damage is present in the context of elevated BP it is considered a hypertensive emergency and more aggressive measures should be taken. Manifestations of end-organ damage can be neurologic (hypertensive encephalopathy, stroke, subarachnoid hemorrhage, intracranial hemorrhage, eclampsia), cardiovascular (myocardial ischemia/infarction, acute left ventricular dysfunction, acute pulmonary edema, aortic dissection), renal (acute renal failure/injury), ophthalmologic (retinopathy with papilledema), or hematologic (microangiopathic hemolytic anemia). The most common clinical symptoms in hypertensive emergency are chest pain, dyspnea, and neurological deficits. The most common types of end-organ damage are cerebrovascular accident, acute pulmonary edema, and hypertensive encephalopathy [40]. In hypertensive emergency, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) recommends 25% decrease in MAP over the initial first hour of therapy with BP goal of 160/100-110 mmHg over next 2-6 hours [41]. In terms of pharmacotherapy, the ideal medication would have rapid onset, short duration, be easily titratable, allow for dose adjustments, have low incidence of toxicity, and few contraindications. A parenteral route of administration is preferred to oral due to rapid onset, ease of titration and tolerance in the context of concomitant nausea/vomiting [34]. The goal of therapy should be to stop and prevent further damage by reversing the pathological process. "Normalization" of BP should not necessarily be the goal in the initial management of hypertensive emergencies [41].
If concurrently monitored NIBP and IBP are vastly different with NIBP being much higher and suggestive of hypertensive urgency, we suggest antihypertensive medications be titrated with the aim of achieving NIBP ± 20% of the patient's baseline, because the baseline was determined using this BP method. On the contrary, if IBP is suggestive of hypertensive urgency despite a more normal NIBP measurement, we suggest efforts be made to carefully titrate antihypertensive medications with the aim of achieving NIBP at 20% below the patient's baseline while monitoring closely for clinical signs of inadequate systemic perfusion. These signs might include orthostatic lightheadedness or near-syncope, ST depressions on the electrocardiogram, or decreased urinary output. With this approach, the goal is to prevent both the damaging effects of both extreme hypertension as well as inadequate perfusion.
Maintaining blood pressure near baseline
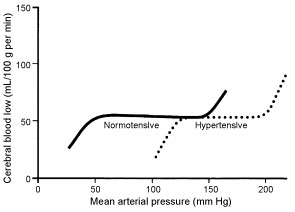
Vital organs require steady perfusion over a wide range of blood pressures in order to adequately meet metabolic demand. This is achieved by an autoregulatory mechanism which relies mainly on varying resistance arteriole diameter in order to maintain constant blood flow over a range of blood pressures (Figure 2). Although still not fully understood, studies of cerebral blood flow autoregulation have implicated the role of endothelium, hypoxic metabolites and neurogenic stimulation in this process. Normally, cerebral autoregulation occurs between MAP of 50 to 150 mmHg. Cerebral blood flow thus remains constant with varying pressure within this range. Outside this range, any increase or decrease in MAP results in proportional increase or decrease in cerebral blood flow, both of which may cause cerebral injury. Failure of autoregulation at lower MAPs results in hypoxic injury, and at higher MAPs it can causecerebral edema [42]. In chronic hypertension, autoregulation occurs across a range of higher MAPs and is thus said to be "right wardly shifted", as shown in Figure 2 [43]. Furthermore, endothelial damage and loss of vessel elasticity from chronic hypertension leads to unpredictable changes in BP [42]. Rapid reduction and normalization of BP in chronic hypertension can lower the MAP below the autoregulatory range, resulting in impaired cerebral perfusion and hypoxic injury.
Coronary blood flow autoregulation also exhibits "rightward-shift" to a higher MAP range in chronic hypertension. Increased after load in hypertension causes increased intracardiac pressure which can cause subendocardial ischemia or infarction. In chronically hypertensive patients, a higher MAP range of coronary autoregulation ensures adequate myocardial perfusion and protects the myocardium from ischemic injury. Accordingly, overaggressive BP reduction has been shown to be associated with cardiac ischemic events due to low diastolic BP [44]. Additionally, chronic hypertensive patients who have elevated pulse pressure with low diastolic BP are prone to subclinical myocardial damage and coronary events [45]. The recent VISION study linked intraoperative tachycardia and hypotension to myocardial injury after noncardiac surgery [46].
There is some evidence suggesting an advantage of individualized BP management of hypotension in the postoperative period. In one trial, individualized management of postoperative hypotension to within 10% of resting preoperative systolic BP reduced the risk of organ dysfunction when compared to the control group receiving a standard approach to hypotension management regardless of individual preoperative systolic BP [47]. It is unclear if a similar benefit exists for perioperative hypertension management of chronically hypertensive patients.
Conclusion
Hypertension is a commonly encountered perioperative problem and may pose hemodynamic management challenges and complications in the perioperative period. Patients with chronic hypertension should undergo a preoperative evaluation before any nonemergent elective surgery. Patients with BP > 180/110 mmHg should be further evaluated for signs and symptoms suggestive of end-organ dysfunction and thoughtfully managed, including consideration to delay elective cases for 6-8 weeks for medical optimization. Patients BP < 180/110 mmHg are likely safe to proceed to surgery unless other cardiovascular risk factors or concerning symptoms necessitate further work up. It is important to avoid aggressive treatment and maintain patients' BP near baseline in chronically hypertensive patients.
NIBP monitors are usually sufficient for hemodynamic monitoring in the perioperative period, however IBP may be needed. Advantages, limitations, and complications of both methods must be considered when utilizing them. Using both NIBP and IBP monitors concurrently during the intra- and postoperative periods may result in discordant BP values and pose a management dilemma. When NIBP and IBP measurements differ significantly in the postoperative period despite troubleshooting potential sources of error inherent to each modality, we suggest NIBP should generally be the preferred monitor for clinical decision making. This is our general recommendation because NIBP is the method used outside of the perioperative period to determine BP baseline and guide therapeutic management. It is reasonable to assume that factors affecting NIBP accuracy are unchanged between the preoperative and in the perioperative period, therefore accuracy of the monitor should be consistent in both settings. Although no specific BP targets are recommended for the postoperative period, commonly used pre-discharge scoring systems such as PADSS or Modified Aldrete do include BP criteria for discharge readiness from the PACU. The use of these scoring systems is recommended by JCAHO and the Canadian Anesthesia Society but their effect on outcomes is unclear. Further research is required to define perioperative BP targets for patients with chronic hypertension.
In postoperative hypertensive urgency or emergency, a more aggressive anti-hypertensive strategy has to be employed, preferably with rapidly titratable parenteral medications. We should be mindful that "normalization" of BP is not always the goal and may lead to more harm. In chronic hypertension the patient's preoperative BP should be considered as these patients' autoregulatory mechanisms occur at higher MAP values. When there is a discrepancy between IBP and NIBP, with NIBP suggestive of hypertensive urgency, we recommend titrating antihypertensive medications to ± 20% baseline MAP. When IBP is suggestive of hypertensive urgency despite a more normal NIBP, we recommend aiming for a MAP 20% below baseline using NIBP while closely monitoring for clinical symptoms of systemic hypoperfusion. Further research is required to elucidate the role for individualized BP management in the postoperative period in patients with chronic hypertension.
Disclaimer
Views expressed in the submitted article are our own and not an official position of the institution or funder.
Source of Support
None.
Conflict of Interest Declaration
None.
Declaration of Disclosures
None.
References
- (2015) Basic Standards for Preanesthesia Care.
- Lizasoain A, Tort LF, Garcia M, et al. (2015) Standards for basic anesthetic monitoring: Committee of Origin: Standards and practice parameters. Am Soc Anesthesiol 1: 1-4.
- Apfelbaum JL, Connis RT, Nickinovich DG, et al. (2012) Practice advisory for preanesthesia evaluation. Anesthesiology 116: 522-538.
- (1997) The sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 157: 2413-2446.
- Casadei B, Abuzeid H (2005) Is there a strong rationale for deferring elective surgery in patients with poorly controlled hypertension? J Hypertens 23: 19-22.
- Fleisher LA (2002) Preoperative evaluation of the patient with hypertension. JAMA 287: 2043-2046.
- Sear JW (2008) Perioperative control of hypertension: When will it adversely affect perioperative outcome? Curr Hypertens Rep 10: 480-487.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. (2015) 2014 ACC / AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery : Executive Summary A Report of the American College of Cardiology / American Heart Associat. J Nucl Cardiol 22: 162-215.
- Crowther M, van der Spuy K, Roodt F, et al. (2018) The relationship between pre-operative hypertension and intra-operative haemodynamic changes known to be associated with postoperative morbidity. Anaesthesia 73: 812-818.
- Wolfsthal SD (1993) Is blood pressure control necessary before surgery? Med Clin North Am 77: 349-363.
- Goldman L, Caldera D (1979) Risks of General Anesthesia and Elective Operation in the Hypertensive Patient. Anesthesiology 50: 285-292.
- Prys-Roberts C, Meloche R, Foëx P, et al. (1971) Studies of anaesthesia in relation to hypertension. I. Cardiovascular responses of treated and untreated patients. Br J Anaesth 43: 122-137.
- Hollenberg M, Mangano DT, Browner WS, et al. (1992) Predictors of postoperative myocardial ischemia in patients undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 268: 205-209.
- Kristensen SD, Knuuti J, Saraste A, et al. (2014) 2014 ESC/ESA Guidelines on non-cardiac surgery : cardiovascular assessment and management The Joint Task Force on non-cardiac surgery : cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 35: 2383-2431.
- Skirton H, Chamberlain W, Lawson C, et al. (2011) A systematic review of variability and reliability of manual and automated blood pressure readings. J Clin Nurs 20: 602-614.
- Shahriari M, Rotenberg DK, Nielsen JK, et al. (2003) Measurement of Arm Blood Pressure Using Different Oscillometry Manometers Compared to Auscultatory Readings. Blood Press 12: 155-160.
- Myers MG, Godwin M, Dawes M, et al. (2011) Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension. Br Med J 342: d286.
- Pickering TG, Hall JE, Appel LJ, et al. (2005) Recommendations for blood pressure measurement in humans and experimental animals part 1 : blood pressure measurement in humans a statement for professionals from the subcommittee of professional and public education of the american heart association coun. Circulation 111: 697-716.
- Ochiai H, Miyazaki N, Miyata T, et al. (1997) Assessment of the Accuracy of Indirect Blood Pressure Measurements. Jpn Heart J 38: 393-407.
- Anast N, Olejniczak M, Ingrande J, et al. (2016) The impact of blood pressure cuff location on the accuracy of noninvasive blood pressure measurements in obese patients: An observational study. Can J Anesth 63: 298-306.
- Barash PG, Cullen BF, Stoelting RK, et al. (2009) Clinical Anesthesia. (6th edn), Wolters Kluwer/ Lippincott Williams and Wilkins.
- Longnecker DE, Sandberg WS, Mackey SC (2018) Anesthesiology. (3rd edn), McGraw Hill Education.
- Garland A (2014) Arterial lines in the ICU: A call for rigorous controlled trials. Chest 146: 1155-1158.
- Wax DB, Lin HM, Leibowitz AB (2011) Invasive and Concomitant Noninvasive Intraoperative Blood Pressure Monitoring: Observed Differences in Measurements and Associated Therapeutic Interventions. Anesthesiology 115: 973.
- Kuck K, Baker PD (2017) Perioperative noninvasive blood pressure monitoring. Anesth Analg 127: 408-411.
- Gologorsky E, Gologorsky A, Barron ME (2012) Intraoperative blood pressure measurement modalities are separate and not equal. Anesthesiology 116: 1394.
- Romagnoli S, Ricci Z, Quattrone D, et al. (2014) Accuracy of invasive arterial pressure monitoring in cardiovascular patients: An observational study. Crit Care 18: 644.
- Gibbs NM, Larach DR, Derr JA (1991) The accuracy of Finapres (TM) noninvasive mean arterial pressure measurements in anesthetized patients. Anesthesiology 74: 647-652.
- Bur A, Hirschl MM, Herkner H, et al. (2000) Accuracy of oscillometric blood pressure measurement according to the relation between cuff size and upper-arm circumference in critically ill patients. Crit Care Med 28: 371-376.
- Lakhal K, Ehrmann S, Boulain T (2018) Non-invasive blood pressure monitoring in the critically ill: Time to abandon the intra-arterial catheter? Chest 153: 1023-1039.
- Gershengorn HB, Wunsch H, Scales DC, et al. (2014) Association between arterial catheter use and hospital mortality in intensive care units. JAMA Intern Med 174: 1746-1754.
- Haas C, LeBlanc J (2004) Acute postoperative hypertension: A review of therapeutic options. Am J Heal Pharm 61: 1661-1675.
- Sansone P, Caterina Pace M, Passavanti MB, et al. (2015) Postoperative Hypertension: Novel Opportunities in the Treatment of a Common Complication. J Hypertens Open Access 4: 8-11.
- Varon J, Marik PE (2008) Perioperative hypertension management. Vasc Health Risk Manag 4: 615-627.
- Apfelbaum JL, Silverstein JH, Chung FF, et al. (2013) Practice Guidelines for Postanesthetic Care. Anesthesiology 118: 291-307.
- Chung FF (1995) Recovery Pattern and Home-Readiness Ambulatory Surgery. Anesth Analg 80: 896-902.
- Aldrete JA (1995) The Post-Anesthesia Score Revisited. J Clin Anesth 7: 89-91.
- Ead H (2006) From Aldrete to PADSS: Reviewing Discharge Criteria After Ambulatory Surgery. J Perianesthesia Nurs 21: 259-267.
- Meng L, Yu W, Wang T, et al. (2018) Blood Pressure Targets in Perioperative Care. Hypertension 72: 806-817.
- Zampaglione B, Pascale C, Marchisio M, et al. (1996) Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension 27: 144-147.
- Lenfant C, Chobanian AV, Roccella EJ, et al. (2004) The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7).
- Shekhar S, Liu R, Travis OK, et al. (2017) Cerebral Autoregulation in Hypertension and Ischemic Stroke: A Mini Review. J Pharm Sci Exp Pharmacol 2017: 21-27.
- Kaplan NM (1994) Management of hypertensive emergencies. Lancet 344: 1335-1338.
- Selvaraj S, Steg PG, Elbez Y, et al. (2016) Pulse Pressure and Risk for Cardiovascular Events in Patients with Atherothrombosis from the REACH Registry. J Am Coll Cardiol 67: 392-403.
- Danzi GB, Cuspidi C (2017) Diastolic Blood Pressure and Myocardial Damage: What About Coronary Perfusion Time? J Am Coll Cardiol 69: 1645-1646.
- Abbott TEF, Pearse RM, Archbold RA, et al. (2018) A Prospective International Multicentre Cohort Study of Intraoperative Heart Rate and Systolic Blood Pressure and Myocardial Injury After Noncardiac Surgery. Anesth Analg 126: 1936-1945.
- Futier E, Lefrant JY, Guinot PG, et al. (2017) Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: A randomized clinical trial. JAMA 318: 1346-1357.
Corresponding Author
Alan M Smeltz, MD, Department of Anesthesiology, University of North Carolina at Chapel Hill, 101 Manning Drive, Chapel Hill 27514, USA.
Copyright
© 2019 Storozh OV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.