Neuroanesthesia in an Awake Patient in a University Hospital in Latin America: Case Report
Abstract
The anesthetic technique for tumors resection that affect extensive motor areas with an awakened patient, poses a challenge for surgical teams. The advancement in neuroimaging and the incursion of neuronavigation as a surgical tool, allow reducing motor sequelae and extending the safety margins in the resection of these types of tumors.
The case report of a 25-year-old patient with a 6 by 5 cm glioma, 5 cm successfully resected under anesthesia with awake patient and neuronavigation techniques is presented. The Global Deterioration Scale (GDS-FAST) after three years of the procedure indicates a very mild cognitive deficit.
Conclusion
The use of neuronavigation under anesthesia with fully awake patient becomes the best therapeutic alternative in the approach of tumors that compromise the eloquent areas allowing establishing safe margins for tumor resection and significantly reducing subsequent neurological sequelae.
Keywords
Anesthesia, Awake neurosurgery, Motor cortex, Neurosurgical procedures
Introduction
Intraoperative or neuronavigational brain mapping aims to help with surgical resection of brain tumors, reducing the risk of functional sequelae. Retrospective randomised studies on large populations have shown that this technique can optimize surgical approach while reducing postoperative morbidity [1]. The resection of tumors within or near eloquent motor areas, in particular the precentral convolution, always implies a compromise between the extension of the resection and the preservation of the motor function. Especially in gliomas, surgical reduction of the tumor significantly affects survival and therefore should be as wide as possible [2].
One of the dilemmas of brain tumor surgery in the eloquent cortex is the way to obtain benefits for the patient in terms of prolonged survival, maintenance or even improvement of function, quality of life and at the same time not to run the risk of new neurological deficits [3,4]. The craniotomy with the awake patient is a functional-focus neurosurgery where the anesthesiologist should keep the patient conscious and collaborator to allow his specific neurological evaluation, offering him a conscious sedation and analgesia for his comfort without altering neurological monitoring and maintaining control of hemodynamics, brain physiology, ventilation and airway [5]. As the debate continues on the advantages of regional anesthesia versus general anesthesia for many forms of surgery, there is an increasing number of indications in intracranial surgery for the patient to be awake during part or all of the operation [1,6,7]. The traditional indication for craniotomy with awake patient has been the surgery of the epilepsy since the seventeenth century [8] and in particular the temporal lobectomy where the excision can invade the eloquent cortical areas eloquent (motor areas and of the language). Arteriovenous tumors or malformations are surgeries where the lesion may compromise speech, motor function, sensory or visual cortex, which requires intraoperative functional tests or cortical mapping and therefore the need for the patient to be awake [9].
The most common anesthesiological approach has been local anesthesia [10]. This approach allows patients to be kept in an awake and cooperative state in order to decrease false negative results during stimulation of language areas. Anesthesia is then usually provided using a combination of local anesthesia (local infiltration and regional blockade) and Intravenously (IV) with medications to provide sedation, anxiolysis, and supplemental analgesia during long procedures [11]. Propofol allows a rapid induction and has little effect on the respiratory function of the patient with spontaneous respiration. Pain control can be achieved by scalp block or local infiltration for the implantation of deep brain electrodes. In addition, low doses of remifentanil are recommended for trepanation (i.e., tumor or epilepsy surgery). The nasopharyngeal airway can be. Adequate antiemetic prophylaxis is required to protect the patient from vomiting [12].
Clinical Case
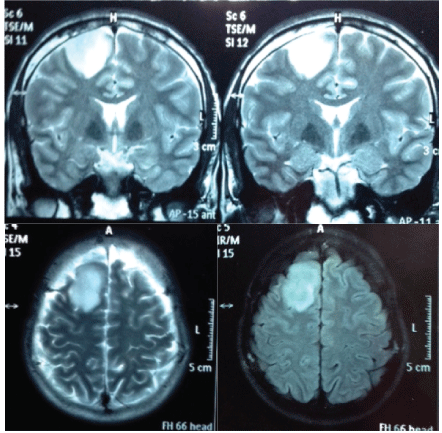
A 25-year-old male patient, who presented with sudden frontal pulsatile headache followed by generalized clonic tonic convulsion with postictal recovery. On physical examination patient was alert, conscious, with 15/15 in Glasgow scale, without motor or sensory deficit. He was brought to the ER, a month later presenting new convulsive episode of the same characteristics to the previous one. A simple brain tomography shows evidence of a lesion extending into motor area (Figure 1). The tumor size was 37 × 28 mm and slight local compressive effect. Such patient benefits from surgery by neuronavigation while awake due to the risk of postoperative motor sequelae.
Description of Anesthetic and Surgical Technique
The patient is 25 years of age and without comorbidity so it is classified as ASA 1. Anesthetic technique is performed with a fully awake patient with the help of local anesthetics and continuous infusion by means of a remifentanil and dexmedetomidine pump. At his entrance he was supplied with ringer lactate. He is monitored with electrocardiographic shunt DII, left radial arterial line, bladder probe, thermometer in the sternal region and is administered oxygen by cannula at 3 liters/minute.
Prophylaxis of Ranitidine 50 mgs, ondansetron 8 mgs, dexamethasone 8 mgs, diclofenac 75 mgs, phenytoin 750 mgs in infusion for 30 minutes, desmopressin 15 mgs, 30 minutes before incision and prophylactic antibiotic is given intravenously. A continuous infusion of dexmedetomidine at the rate of 0.5 mcg/kg for 15 minutes followed by 0.1 to 0.2 mcg/kg/hour accompanied by remifentanil to 0.05 mcg/kg/min by adjusting the infusion by pump to as needed. Once the proper sedation has been achieved, scalp block is performed by blocking the supraorbital, supratrochlear, zygomatic-temporal and auricular-temporal (Figure 2). In the posterior region the major occipital nerves, minor occipital and retroauricular nerve were blocked.
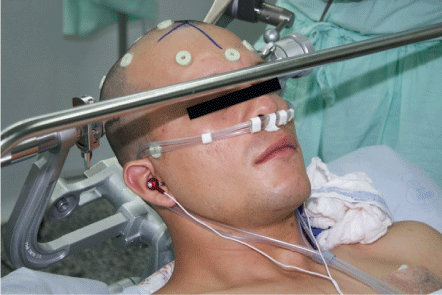
Scalp block is performed using a mixture of 2% lidocaine with epinephrine 15 ml plus 0.5% bupivacaine with epinephrine 25 milliliters (ml) plus normal saline 5 ml plus bicarbonate 5 ml for a total mixture of 50 ml. Once the scalp anesthesia has been checked, the Mayfield head is placed. For patient comfort, thermal blanket, intravenous fluid heater is placed and it's allowed the patient listen to the music of his choice using a custom audio system (Figure 3).
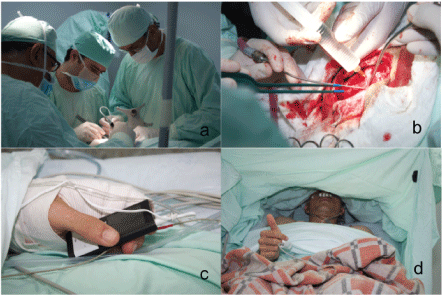
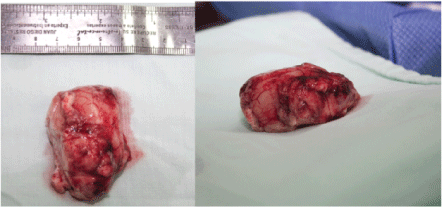
By using the neuronavigator, margins of intra-axial tumor lesion are delineated (Figure 3). Bipolar cortical stimulation of the posterior margin of the lesion is practiced without obtaining motor response that indicates immediate relationship with primary motor cortex. Under microsurgical technique, dissection and resection of the tumor lesion is practiced (Figure 4). During the procedure, the patient is asked to perform upper and lower limb movements, as well as reading, speaking, and language tests without finding alterations in the stimulation of the tumoural edges (Figure 4) 6 × 3.5 cm tumor lesion is excised (Figure 5). The procedure is completed without complications. The surgical procedure lasts approximately 6 hours during which the patient does not have an anesthetic and/or surgical complication, so at the end of the surgery, 3 mg of morphine is given, continuous infusion of medication is suspended and patient is transferred to the intensive care unit fully awake for postoperative surveillance. Patient is completely satisfied with the anesthesia technique employed and there is no evidence of postoperative pain. Patient is stable with normal vital signs and is admitted in ICU.
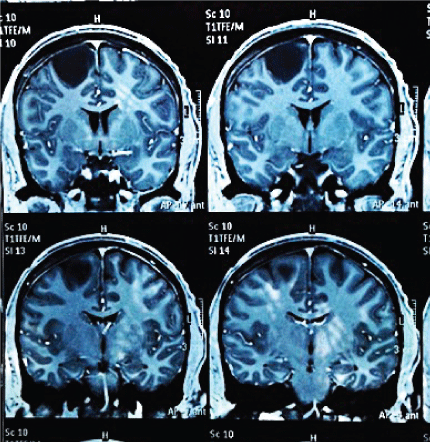
After three years of the procedure the patient present's normal intellect, postoperative magnetic resonance shows evidence of tumor resection without relapse (Figure 6), without neurological deficit, has an active life without restriction. However he presents with chronic convulsive symptoms that are controlled with valproic acid 250 mg 1/8 hours, and phenytoin 100 mg 1/8 hours. He shows isolated episodes of recent memory loss. In his postsurgical controls and after three years, the Global Deterioration Scale (GDS-FAST) is 2 with a very mild cognitive deficit. His superior executive functions are conserved with slight impairment of recent memory. No behavioral disturbances are presented. No motor deficits of any kind.
Discussion
Surgical resection of brain tumors by craniotomy in awake patients, with cortical stimulation and neuronavigation, are surgical alternatives that significantly improve the prognosis of the patient, because they allow the resection of tumors of great extension located in eloquent areas of the brain with a neurological valuation in real time [13]. According to the literature this technique favors a higher rate of total gross resection vs patients undergoing general anesthesia (37 vs. 14% respectively), fewer permanent neurological deficits (4.6% vs. 16%) and fewer new onset postoperative neurological deficits (3.3% vs. 58%); as Sacko or and collaborators evidence [14-16]. In our case, the patient, in the postsurgical, does not show any type of motor deficit, sensory, or language deficits, however he presents with chronic convulsive symptoms currently controlled. He has mild compromise of recent memory, despite this he is completely independent in the activities of the daily life. No recurrences are found in the tomographic controls.
This experience supports the importance of implementing advanced neurosurgery techniques that offer to the patient safe management alternatives, which are based on the improvement of their quality of life [17,18].
The advancement in anesthetic care has made an important contribution to the growing popularity of craniotomy with awake patient. However, there is not enough comparative evidence yet to make technical recommendations with an adequate degree of evidence and Recomendación [19].
In this context, it is of the utmost importance to have a multidisciplinary functional neurosurgery program that allows the identification of patients with adequate technological support which has a direct impact on the results [20,21]. Investment in these programs is feasible and cost-effective, even in developing countries like in Latin America, as they reduce hospital stay, stay in Intensive Care Unit, rehabilitation costs and allow the patient a faster recovery with a better prognosis [22,23].
Conclusion
The use of neuronavigation with anesthetic techniques with fully awake patient becomes the best therapeutic alternative in the approach of patients with tumors that compromise the eloquent areas. The evolution of these two medical disciplines have allowed the reduction of postsurgical sequelae as well as recurrences in this type of tumours.
In tribute to the memory of an excellent Neurosurgeon and especially a great Professor M.D. Jairo Sanchez R.I.P. University Clinic Rafael Uribe, Cali, Colombia.
References
- Conte V, Baratta P, Tomaselli P, et al. (2008) Awake neurosurgery: An update. Minerva Anestesiol 74: 289-292.
- Krieg SM, Shiban E, Buchmann N, et al. (2012) Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J Neurosurg 116: 994-1001.
- Randy L Jensen (2014) Navigated transcranial magnetic stimulation: Another tool for preoperative planning for patients with motor-eloquent brain tumors. Neuro Oncol 16: 1299-1300.
- Blanshard HJ, Chung F, Manninen PH, et al. (2001) Awake craniotomy for removal of intracranial tumor: Considerations for early discharge. Anesth Analg 92: 89-94.
- Carlos Ramírez-Paesano (2013) Anestesia para craneotomía con paciente despierto. Ramírez Paesano, Carlos. Revista Mexicana de Anestesiología 36: S1-S3.
- Meyer FB, Bates LM, Goerss SJ, et al. (2001) Awake craniotomy for aggressive resection of primary gliomas located in eloquent brain. Mayo Clin Proc 76: 677-687.
- Taylor M, Bernstein M (1999) Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: A prospective trial of 200 cases. J Neurosurg 90: 35-41.
- Marshall C (1967) Surgery of epilepsy and motor disorders. In: Walker AE, A history of neurological surgery. Hafner Publishing Co, New York, 288-305.
- John R Cormack, Timothy G Costello (2005) Awake craniotomy: Anaesthetic guidelines and recent advances. Australasian Anaesthesia 2005: 77-93.
- Penfield W (1958) Some mechanisms of consciousness discovered during electrical stimulation of the brain. Proc Natl Acad Sci U S A 44: 51-66.
- Duffau H (2005) Lessons from brain mapping in surgery for low grade glioma: Insights into associations between tumour and brain plasticity. Lancet Neurol 4: 476-486.
- Schulz U, Keh D, Fritz G, et al. (2006) "Asleep-awake-asleep"-anaesthetic technique for awake craniotomy. Anaesthesist 55: 585-598.
- González-Darder JM, González-López P, Talamantes-Escribá F, et al. (2011) Treatment of intrinsic brain tumors located in motor eloquent areas. Results of a protocol based in navigation, tractography and neurophysiological monitoring of cortical and subcortical structures. Neurocirugia (Astur) 22: 23-35.
- Sacko O, Lauwers-Cances V, Brauge D, et al. (2011) Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery 68: 1192-1198.
- Mao H, Berns S (2002) MRI in the study of brain functions: clinical perspectives. Medicamundi 46: 28-38.
- Khan OH, Mason W, Kongkham PN, et al. (2016) Neurosurgical management of adult diffuse low grade gliomas in Canada: A multi-center survey. J Neurooncol 126: 137-149.
- Vincent M, Rossel O, Poulin-Charronnat B, et al. (2016) Case report: Remote neuromodulation with direct electrical stimulation of the brain, as evidenced by intra-operative EEG recordings during wide-awake neurosurgery. Clin Neurophysiol 127: 1752-1754.
- Chan-Seng E, Moritz-Gasser S, Duffau H (2014) Awake mapping for low-grade gliomas involving the left sagittal stratum: Anatomofunctional and surgical considerations. J Neurosurg 120: 1069-1077.
- Meng L, McDonagh DL, Berger MS, et al. (2017) Anesthesia for awake craniotomy: A how-to guide for the occasional practitioner. Can J Anaesth 64: 517-529.
- Szelényi A, Bello L, Duffau H, et al. (2010) Workgroup for intraoperative management in low-grade glioma surgery within the european low-grade glioma network. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus 28: E7.
- Talacchi A, Santini B, Casagrande F, et al. (2013) Awake surgery between art and science. Part I: Clinical and operative settings. Funct Neurol 28: 205-221.
- Serletis D, Bernstein M (2007) Prospective study of awake craniotomy used routinely and nonselectively for supratentorial tumors. J Neurosurg 107: 1-6.
- Brown T, Shah AH, Bregy A, et al. (2013) Awake craniotomy for brain tumor resection: The rule rather than the exception? J Neurosurg Anesthesiol 25: 240-247.
Corresponding Author
Arenas Villamizar Angel-Ricardo, Medical Doctor Master Degree in Clinical Epidemiology, Chile.
Copyright
© 2017 Ana-María OP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.