Ameliorative Effects of Intranasal AntagomiR-125b in AD
Abstract
Background
Alzheimer's disease (AD) is a global public health crisis. Currently, there are no treatments to prevent or halt the disease. Given the precedence of chronic neuroinflammation in triggering AD-like neurodegeneration and an observed upregulation of NFkB-driven miRs i.e. mir-125b positively correlating with AD, silencing specific micro-RNA with antisense-microRNA (antagomir) to miR-125b was undertaken to evaluate its efficacy in ameliorating AD-like neurodegeneration in 5XFAD transgenic mice modeling AD.
Objective
This study evaluated therapeutic potential of intranasally (IN) delivered 2'-O-Methyl/locked nucleic acid (LNA)-modified antagomir 125b-5p (AM-125b) in correcting neurobehavioral outcomes in 5XFAD transgenic mice modeling AD.
Methods
5XFAD mice were intranasally administered with AM-125b (8 nMols/2μl/week) for 8 weeks. Controls received equal volume of nasally delivered saline-vehicle containing equal length 2'-O-Methyl/LNA modified scrambled nucleotides for the same duration. After confirming miR-125b-5p blocking ability of AM-125b using QRTPCR, ameliorative efficacy of intranasal AM-125b in improving spatial reference working memory and in reducing cerebral levels of total and oligomeric Aß, total and phospho-tau, and key inflammatory markers were evaluated using Y-maze and ELISA.
Results
Results confirmed direct brain targeting of AM-125b after intranasal delivery. Intranasally delivered AM-125b significantly improved reference working memory (52-55% increased alterations between three arms, p < 0.0001) along with reduced cerebral levels of total and oligomeric Aß (2.1-2.5-fold reduction, all values, p < 0.0001), reduced total and phospho-tau (2.4-2.5-fold reduction, all values, p < 0.0001) and reduced inflammatory markers (2.2-2,3-fold reduction, all values, p < 0.0001).
Conclusions
Intranasally delivered AM-125b significantly improved neurobehavioral deficits in 5XFAD mice modeling AD.
Keywords
Alzheimer's disease, AntagomiRs, Phospho-tau, Oligomeric Aß, Spatial Working Reference Memory
Abbreviations
Aß: ß-amyloid; AD: Alzheimer's disease; AM: AntagomiR; APP: ß-amyloid precursor protein; BBB: Blood brain barrier; BCSFB: Blood cerebrospinal fluid barrier; IL-1ß: Interleukin-1ß; IN: Intranasal; LNA: Locked nucleic acid; Lts: Littermates; mRNA: Messenger ribonucleic acid; MCI: Mild cognitive impairment; miR/miRs: Micro RNA(s); NFkB: Nuclear factor kappa B; Nucleotide: nt; oAß: oligomeric ß-amyloid; 2'-O-ME: 2'-O-Methyl; PKR: Protein kinase ribonucleic acid activated protein; Phospho-tau: Phosphorylated tau protein; PSEN1: Presenilin1; QPCR: Quantitative polymerase chain reaction; ROS: Reactive oxygen species; RNA: Ribonucleic acid; TNFα: Tumor necrosis factor alpha; Tgs: Transgenic mice; 3'-Untranslated region: 3'UTR; YM: Y maze
Introduction
Alzheimer's disease (AD) is a global public health crisis currently afflicting ~6 million Americans (and ~ 40 million people worldwide). By the middle of the century, these numbers will escalate to ~16 million Americans (and ~150 million people worldwide) suffering from AD, if breakthrough disease-modifying treatments are not discovered [1,2]. Currently, there are no treatments to prevent or halt the disease. There is a growing consensus that Alzheimer's is a multifactorial disease involving an interplay of many deregulated "aging" factors occurring much earlier than the actual onset of the disease [3], among which oxidative damage [4-6] constitutes one of the prime factors resulting from high energy requirement of brain with its modest anti-oxidant defense, and hence vulnerable to oxidative damage caused by reactive oxygen species (ROS) [7], along with chronic inflammation [8-10], cholinergic dysfunction [11-13], insulin resistance [14-16] and other factors. Recently, it has been implicated that increase in cerebral ß-amyloid (Aß) in the aging brain either due to reduced Aß clearance, influx of peripheral Aß due to blood brain barrier (BBB)/blood cerebrospinal fluid barrier (BCSFB) breach caused by age-related oxidative damage, inflammation-Protein kinase RNA activated protein (PKR) induced Aß formation [17], or Aß overproduction due to familial mutations, all result in cerebral Aß accumulation and tend to destroy synaptic integrity fundamental to cognitive decline observed in prodromal AD and/or mild cognitive impairment (MCI) [3]. Oxidative stress and chronic neuroinflammation constitute the earliest biochemical changes in triggering AD [7]. Emerging evidence indicates that each of these biochemical changes taking place early on in AD, are regulated by small non-coding microRNAs (miR/miRs) [18].
MicroRNAs (miR/miRs) are highly conserved ~22-nucleotide (nt) long non-coding RNAs that function as post-transcriptional regulators of gene expression shaping the transcriptome of a cell [19,20]. MicroRNAs regulate gene expression by interfering with translation of their target messenger RNAs (mRNAs) via binding to the 3'-untranslated region (3'-UTR) of mRNAs to induce repression or degradation of target mRNA [21], thus blocking translation of mRNA into proteins [22,23]. The activity of any given miR can be experimentally inhibited by antisense oligonucleotides. In order to attain the in vivo stability, antisense oligonucleotides are chemically modified [24]. Among all, the "Locked nucleic acid (LNA) conformation, also known as 2'-O-Methyl nucleic acid modification [25] results in enhanced hybridization to target mRNA with increased resistance to degradation, sensitivity and selectivity [26,27].
Growing body of evidence indicates crucial role played by miRs in human health and diseases [28,29]. There are about > 2600 mature miRs currently characterized in human brain, of which only selected ~50 miRs have been found to be enriched within selective region(s) of the brain [19]. Increasing number of studies indicate that the dysregulation of miRs is fundamental to the etiology of neurodegenerative diseases including AD [3,30]. Multiple independent studies on brain gene expression patterns have indicated that in AD, about 1/3rd of the genes are upregulated while the rest 2/3rd of the genes are downregulated [31]. Interestingly, most of the upregulated pathogenic genes in AD are known to be under the transcriptional control of a pro-inflammatory mediator-nuclear factor kappa B (NFkB) [31] which are significantly upregulated in AD-specific anatomic brain regions [32]. Given the precedence of chronic neuroinflammation in triggering AD-like neurodegeneration and an observed significant upregulation of NFkB-driven miRs i.e. mir-125b positively correlating with AD progression [32-34], both in early and late onset AD [35-37], silencing miR-125b micro-RNA with anti-microRNA (antagomiR) is expected to shut-off AD-like neurodegeneration.
This study evaluated therapeutic potential of intranasally (IN) delivered 2'-O-Methyl locked nucleic acid (LNA)-stabilized antagomiR-125b in ameliorating neurobehavioral outcomes in 5XFAD transgenic mice modeling AD.
Materials and Methods
Animals
5XFAD mice harboring ß-amyloid precursor protein (APP) and presenilin 1 (PSEN1) transgenes, originally obtained from Dr. Vassar (Northwestern University, Chicago, IL) were used for generating 5XFAD transgenic colony [38,39]. One set of 5XFAD transgenic mice (Tgs) were used for studying brain uptake of radiolabeled antagomir 125b (AM-125b) up to 24 h after a single bolus intranasal (IN) administration. Experimental set of 5XFAD Tgs and non-transgenic littermates (Lts) were first used for assessing Y maze exploratory reference working memory, and then euthanized to collect brain tissues, left hemisphere was used to isolate total proteins for ELISA measurements of total and oligomeric Aß, total and phospho-tau and key inflammatory markers i.e. tumor necrosis factor alpha (TNFα) and interleukin-1beta (IL-1ß). While the right hemisphere was used to extract nucleic acids for quantitative polymerase chain reaction (QRTPCR) measurements of miR-125b-5p to confirm AM-125b-5p mediated inhibition of miR-125b-5p. All animal procedures and behavioral studies were performed in accordance with the institutionally approved protocol for the care and use of animals.
Anti-MicroRNA (AM) chemical modification
The single-stranded 2'-O-ME/LNA modified antisense RNA mixmiR-125b-5p (AM-125b-5p) as well as scrambled sequence-control with equal number of 2'-O-ME/LNA modified nucleotides and similar GC content were designed based on mmu-miR-125b-5p sequence (Accession # MIMAT0000136) (www.mirbase.org) as follows:
AM-125b-5p: (5'-A*G*G*G*A C T C t g g g a t t g a a*c*a*c*t-3')
Scrambled Control: (5'-A*A*C*A*G T G T g c g g c g a t t*a*c*g*a-3')
Capital bold letters represent LNA modifications, unbold lowercase letters represent 2"-O-ME modifications, and asterisks (*) represent phosphorothioate linkages (GenePharma, Shanghai, China).
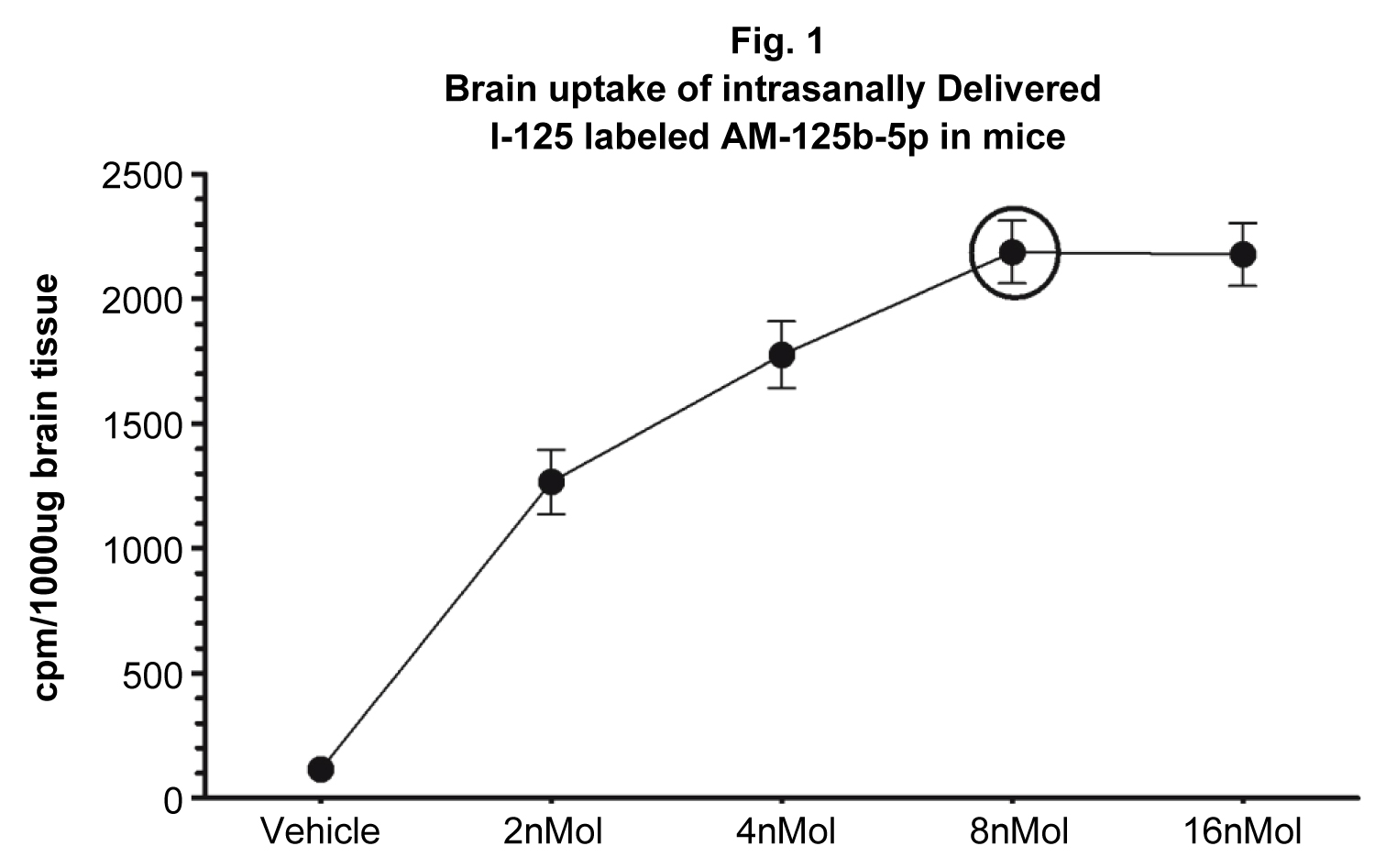
Intranasal administration and brain uptake of intranasally delivered AM125b-5p brain uptake studies: 5XFAD mice were nasally administered with a single bolus injection of I125-labeled AM-125b-5p (Iodobid, Pierce) [40] suspended in a saline vehicle 4 nMols/2 μl/naris ≡ 8 nMols/4 μl/animal/once (N = 10). Controls were IN-administered only with equal volume of saline vehicle 2 μl/naris/once (N =10). Mice were killed after 24 h, brains harvested, homogenized, centrifuged at 100,000 g (MTX Sorvall), and 100 μl of homogenate equating to 100 μg of brain tissue were recorded. The data were presented as cpm/100 ug (Figure 1).
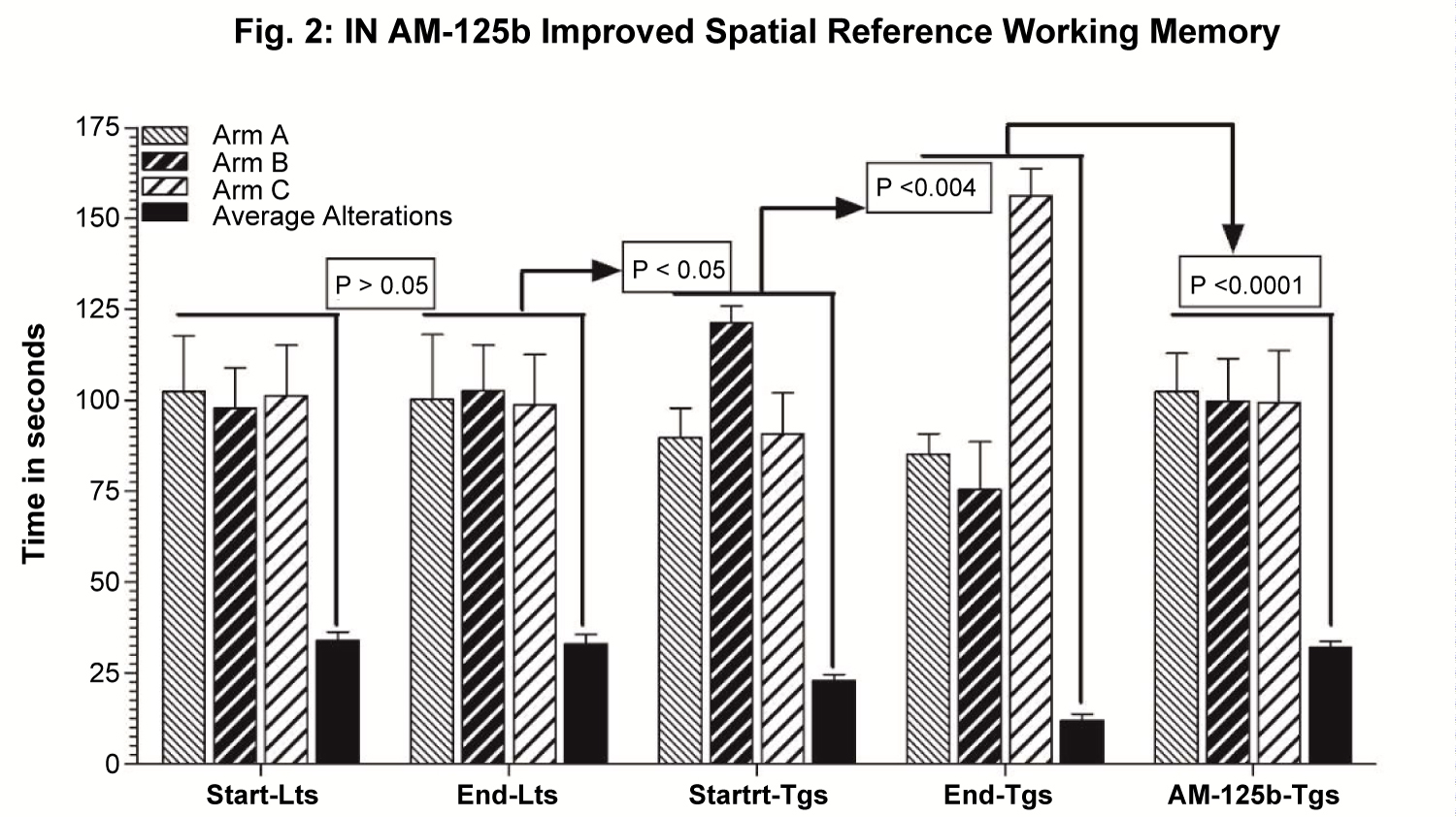
Behavioral studies: Experimental set of 5XFAD Tgs and non-transgenic littermate (Lts) controls were IN injected with AM-125b suspended in saline vehicle (4 nMols/2 μl/naris ≡ 8 nMols/4 μl/animal/week) for 8 weeks (Males/N = 5, Females/N = 5). Controls were IN injected with equal strength scrambled nucleotides suspended in saline vehicle (4 nMols/2 μl/naris ≡ 8 nMols/4 μl/animal/week) for 8 weeks Tgs (Males/N = 5, Females/N = 5) and Lts (Males/N = 5, Females/N = 5). Efficacy of IN administered AM-125b-5p in improving reference working memory in 5XFAD mice was evaluated using Y-maze (YM) performance [41,42].
Y-Maze (YM) assessment for evaluating spatial working reference memory Y-Maze (YM) test for evaluating spontaneous alteration behavior and exploratory activity to assess the spatial working reference memory that is stored temporarily and elicited actively during the completion of the task, a task known to involve hippocampus, septum, basal forebrain and pre-frontal cortex [43], will be performed as established [38,41,44]. The animals typically tend to explore a new arm of the maze rather than returning to one that was previously visited. Y maze is made up of dark grey acrylic material with three 40 cms high, 21 cm long and 4 cm wide identical arms at a 120° angle from each other. Each animal is placed in the center zone. After introduction to the center of the maze, the animal is given free access to all three arms in a single trial of 6 min duration. If the animal chooses a different arm than the one it arrived from, this choice is called an alteration. Alterations and total number of entries in each arm and the sequence of entries are video-tracked and recorded (AnyMaze). The Y maze activity index as the number of entries in each arm and percent alterations calculated as:
% Alterations = Total Number of Alterations/Total Number of Arms Entered × 100
The data were presented as Mean ± standard deviation (SD) and plotted (Figure 2).
Quantitative reverse transcriptase polymerase chain reaction (QRTPCR)
The mRNA expression of miR-125b-5p was quantitated by QRT-PCR as follows. Brain tissues RNA was isolated using TRIzol reagent (Invitrogen) and additionally treated with DNase I (Thermo Scientific) to destroy possible DNA contamination. The quality and quantity of RNA was measured by Nanodrop Lite (Thermo Scientific) and RNA agarose gels. High Capacity cDNA Reverse Transcription Kit (Applied Biosystems™) was used to convert 2 ug of isolated RNA into single-stranded cDNA which was used for QRT-PCR using QuantStudio-3 (Applied Bio-systems™). The expression of miR-125b-5p mRNA was quantitated using Taqman mRNA-specific assays using FastStart universal SYBR green master-mix (Rox) (Life Technologies). U6 mRNA was used as endogenous control to normalize the expression data of miR-125b. Specific primer pairs used were:
U6-RT: 5'ctcaactggtgtcgtggagtcggcaattcagttgagaaaaatatggaacgct-3'
U6-F: 5'-ctggtagggtgctcgcttcggcag-3'; U6- R: 5'-caactggtgtcgtggagtcggc-3'
miR-125b- RT: 5'-ctcaactggtgtcgtggagtcggcaattcagttgagtacaa-3'
miR-125b -F:5'-cgcgctccctgagaccctaac-3'; miR-125b- R: 5'-tggtgtcgtggagtcg-3'
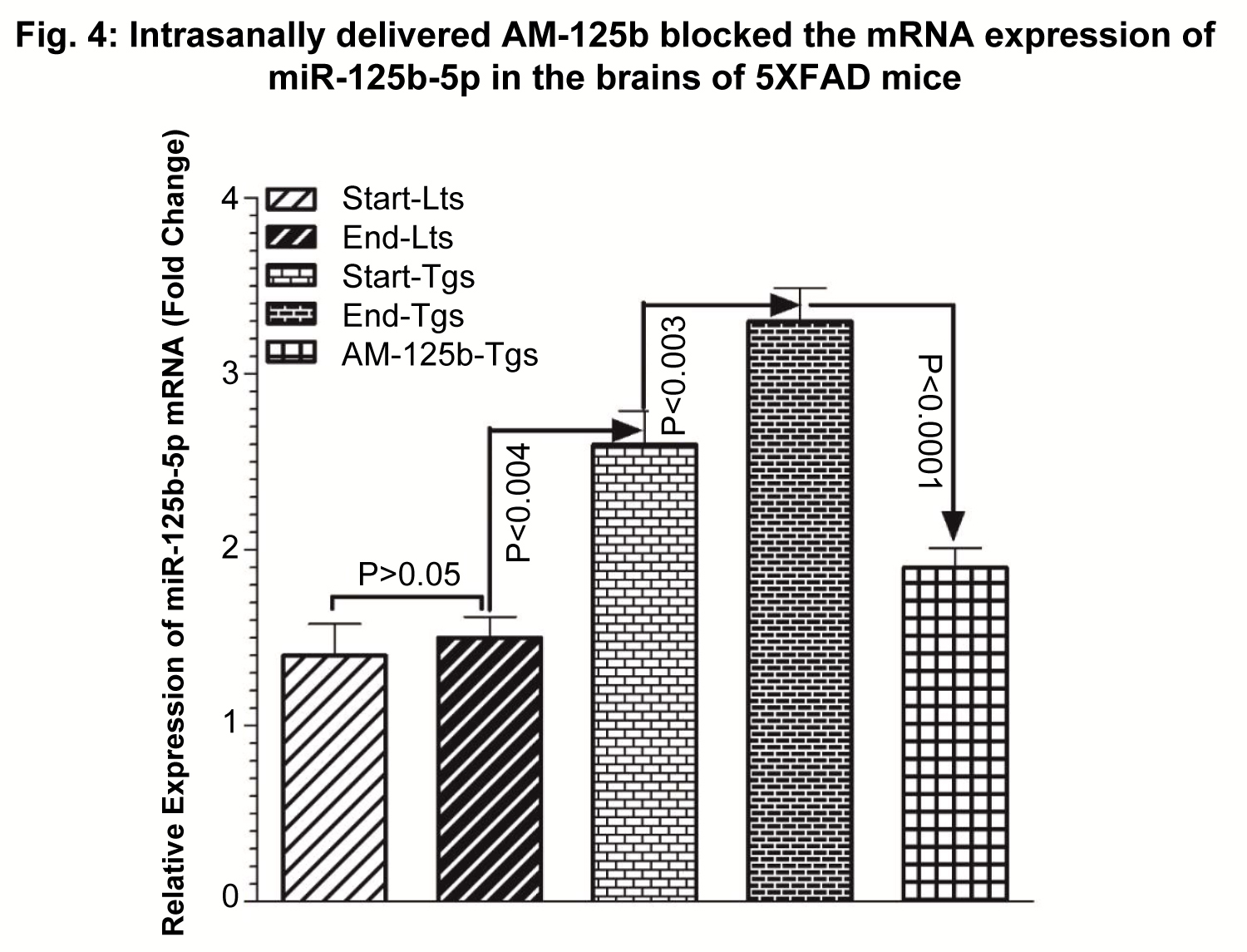
The expression of miR-125b-5p target mRNA was calculated relative to the endogenous U6 mRNA control. Comparative CT (ΔΔCT) method was used to quantitate differential mRNA expression and fold change, analyzed and plotted (Figure 3).
Enzyme-linked immunosorbent assay (ELISA)
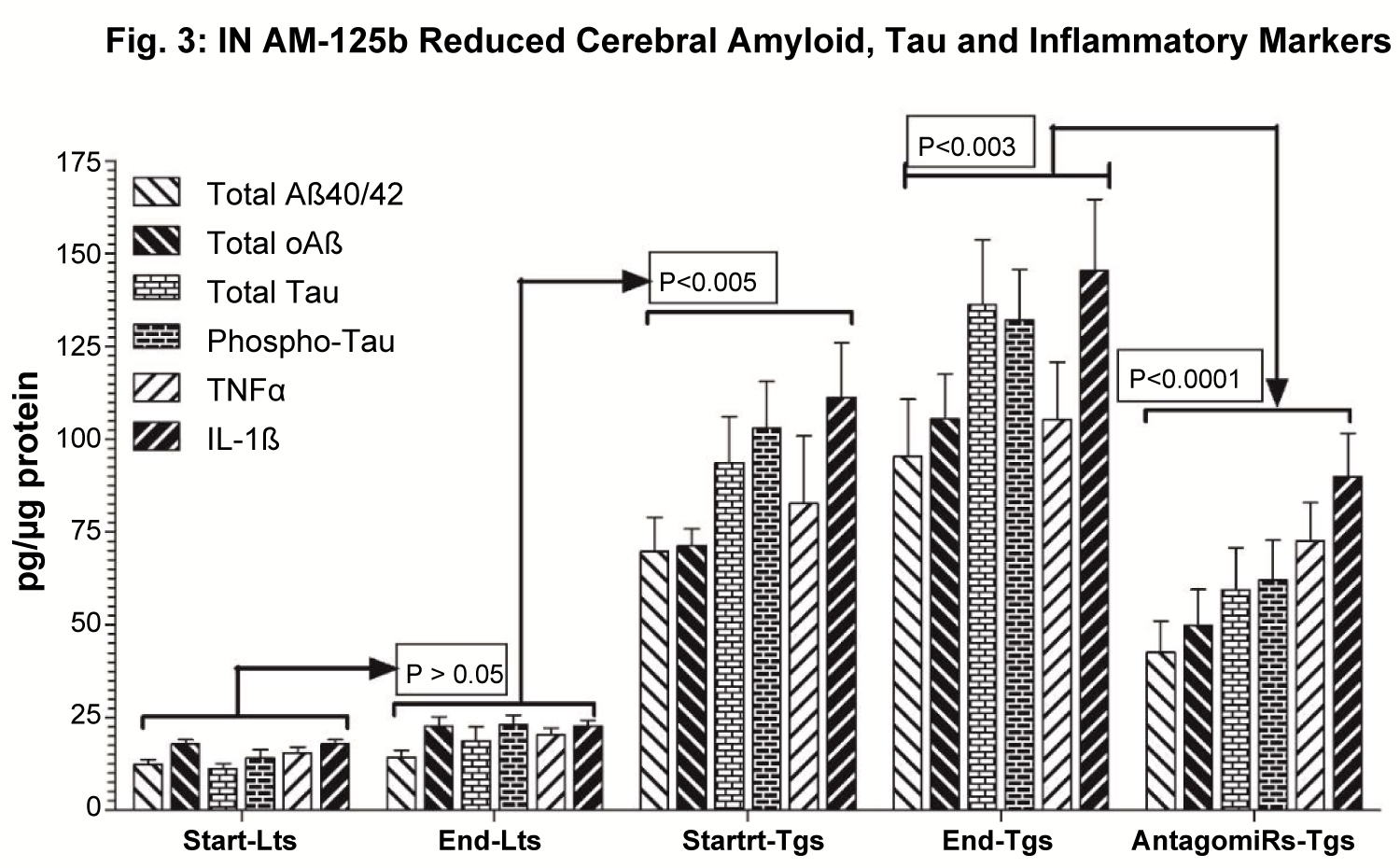
After behavioral studies, mice were euthanized, brains collected, brain tissue lysates prepared and subjected to ELISA measurement of cerebral levels of total and oligomeric Aß (BioSource) (Figure 3A), total and phospho-tau (BioSource) (Figure 3B), and inflammatory markers i.e. tumor necrosis factor alpha (TNFα) and interleukin-1-beta (IL-1ß) (R&D Systems) (Figure 3C), using commercial kits as established [44-46].
The data were presented as Mean ± standard deviation (SD) and plotted (Figure 4).
Statistical analysis
Data was initially subjected to column statistics in order to obtain respective group means with standard deviation (SD). The data was further subjected to analysis of variance (ANOVA), followed by Tukey post hoc test. A value of p < 0.05 was considered statistically significant.
Results
Intranasally administered AM-125b exhibited efficient brain targeting
Consistent with our previously reported findings [40], current study showed efficient brain uptake of radiolabeled AM-125b after a single bolus intranasal (IN) delivery in a dose-dependent manner. The results showed linear increase in the brain uptake of IN delivered AM-125b from 2nMol up to 8nMol, however, the next level increased concentration of 16 nMol did not exhibit significantly increased brain uptake (Figure 1). These results indicate 8 nMols to be the optimum concentration beyond which there may not be any further linear increase in the brain uptake, Therefore, the concentration of 8 nMols/single bolus IN administration was concluded to be optimum, and this optimum concentration was used in the follow up neurobehavioral studies.
Intranasal AM-125b improved spatial working reference memory
The results showed that Y maze spontaneous alteration in littermate controls (Lts) IN administered only with saline vehicle did not change significantly from 8 weeks of age (Start-Lts) up to 16 weeks of age (End-Lts) (p > 0.05) (Figure 2). Compared to End-Lts, start-Tgs at 8 weeks of age administered with saline vehicle only, exhibited unequal and restricted arm entries, more time spent in arm B, and 47% reduced alterations between A/B/C arms (p < 0.0001) (Figure 2). Compared to 8-week-old start-Tgs, Y maze spontaneous exploration in 16-week-old Tgs IN administered only with saline vehicle was further deteriorated by 29% (p < 0.0001), showing restricted exploration in A/B/C arms and more time spent in arm C (Figure 2). Intranasal administration of AM-125b significantly improved Y maze spontaneous exploratory behavior, as evidenced by almost equal number of entries in all arms A/B/C and 52% increased alterations between 3 arms in AM-125b-treated Tgs (p < 0.0001), which was observed to be normalized very much similar to Lts. Given the fact that Y Maze spontaneous alternation is a behavioral test that assesses damage to limbic and non-limbic brain regions, quantifying the spatial working reference memory that is stored temporarily and elicited actively during the completion of the task [43], equal exploration in all arms with ample number of entries in each arm suggests normal spontaneous exploratory behavior observed in Lts. By contrast, unequal time spent in all arms with relatively more time spent in randomly selected arm showing restricted arm exploration and reduced number of entries between these arms are indicative of impaired working memory in untreated Tgs. Antagomir-mediated blockade of miR-125b significantly improved Y maze exploration in AM-125b treated Tgs, suggesting therapeutic efficacy of intranasally delivered antagomir-125b in improving AD-like memory deficits.
Intranasal AM-125b successfully blocked cerebral expression of miR-125b
The results showed efficient blockade of miR-125b-5p after intranasal administration of AM-125b in 5XFAD mice. There was non-significant (p > 0.05) difference in the basal expression levels of miR-125b-5p mRNA between start-Lts vs. end-Lts (p > 0.05). This basal expression of miR-125b-5p mRNA was significantly increased in 8-week-old Tgs at the treatment start-point by 1.9-fold (p < 0.004) (Start/End-Lts vs. 8-week-old Tgs). In 16-week-old Tgs, there was observed further increase in the expression of miR-125b-5p mRNA by 2.4-fold (p < 0.003) (Start-Lts vs. 16-week-old Tgs). Intranasal administration of AM-125b successfully blocked the expression of miR-125b-5p mRNA, as evidenced by significantly decreased the levels of miR-125-5p mRNA by 1.8-fold (p < 0.0001) (16-week-old untreated Tgs vs. 16-week-old AM-125b treated Tgs) in Tgs intranasally injected with AM-125b.
Intranasal AM-125b reduced cerebral inflammation, Amyloid and Tau
It was interesting to see that AM-125b-mediated silencing of miR-125b resulted in reducing cerebral levels of total Aß, oAß, Tau, Phospho-Tau, TNFα and IL-1ß in AM-125b treated 5XFAD mice. In all these parameters studied, there was no significant difference start-Lts vs. end-Lts (p > 0.05). In general, basal cerebral levels of total Aß, oAß,Tau, Phospho-Tau, TNFα and IL-1ß in start/end Lts were increased age-dependently in 8-week-old (p < 0.0001) (Start/End-Lts vs. 8-week-old Tgs) and 16-week-old (p < 0.0001) (Start/End-Lts vs. 16-week-old Tgs) untreated Tgs. Intranasal administration of AM-125b resulted in significant reductions of cerebral levels of total Aß, oAß, Tau, Phospho-Tau, TNFα and IL-1ß (p < 0.0001) (16-week-old untreated Tgs vs. 16-week-old AM-125b treated Tgs) in AM-125b treated 5XFAD mice (Table 1).
Discussion
Early upregulation of neuroinflammation in AD and its persistence during the disease process in AD is characterized with the upregulation of a dimeric DNA binding protein NFkB as p50/p65 complex that has emerged as a ubiquitous transcription factor controlling diverse biological functions predominantly including inflammatory and immune functions [47]. NFkB activation and binding to the promoters of NFkB-sensitive genes via microRNAs, facilitates transcriptions of many pathogenic genes altered in many neurodegenerative conditions including AD [32]. MicroRNAs (miRs) bind to the complementary RNA sequences in the 3'-UTR on mRNA and thereby repress the expression of target mRNA [32]. Upregulated miRs are generally accepted to predominantly decrease the expression/levels of target mRNA, thus down-regulating the protein translated by that target mRNA, and vice versa [32].
NFkB regulated miRs have been shown to be significantly elevated in AD brain, among which common to aging brain and AD brain are significant upregulation of miR-125b [32]. Bioinformatics and multiple analytical techniques including RT-PCR, DNA-Array, Western blots, etc. have confirmed that miR-125b targets the 3"-UTR of several AD-related mRNAs [31,32]. Micro-RNA 125b was first shown to be upregulated in both stressed and differentiating mouse and human neurons, and has been implicated in neuronal development, cell-signaling and neurodegeneration [48]. NFkB-regulated miR-125b has been shown to be induced by neurotoxic aluminum sulfate that generates oxidative stress and ROS in human brain cells [49]. Consistent upregulation of miR-125b is associated with deregulated astroglial proliferation and linked to astrogliosis in various neurodegenerative conditions including AD [50].
MicroRNA-125b is known to regulate neuronal synaptic functions, synaptic vesicle trafficking and neurotransmitter release, which when impaired in conditions such as AD, is reported to impair synaptic signaling and neurotransmitter release [51]. In addition, miR-125b is known to regulate cell cycle arrest and arachidonate 15-lipoxygenase (ALOX15) essential for conversion of docosahexaenoic acid (DHA) to neuroprotection D1 (NPD1), and therefore dysregulation of miR-125b leads to the down-regulation of cell cycle control and deficits in neurotrophic omega-3 fatty acids in the brain which in turn upregulates ß-secretase, prevents neurotrophic cleavage of ß-amyloid precursor protein (ßAPP) and increases Aß production [52]. In summary, upregulation of brain miR-125b is associated with glial cell proliferation (gliosis in AD), down-regulates synaptic vesicle-associated neurotransmitter release (synaptic degeneration in AD), conversion of omega fatty acids into neuroprotective DHA (DHA deficits in AD), stimulation of inflammatory response (neuroinflammation in AD), a shift from non-amyloidogenic to amyloidogenic processing of ßAPP leading to excessive Aß production (Aß toxicity at subtle levels in early AD and Aß pathology at advanced stages of AD). Observed age-dependent upregulation of miR-125b in untreated 5XFAD Tgs corroborates with previously reported role played by miR-125b in aggravating AD-like changes in 5XFAD mice. Furthermore, the role played by miR-125b in aggravating AD-like pathology is confirmed and reinforced by currently observed reduction of AD-like changes after AM-125b-mediated silencing of miR-125b mRNA. Thus, current finding on silencing miR-125b as a preventive and therapeutic strategy to treat AD, has a great potential of clinical translation.
Conclusion
This is one of the lead reports showing therapeutic efficacy of silencing microRNAs using a non-invasive nose-to-brain drug delivery method in ameliorating Alzheimer-like neurocognitive deficits.
Acknowledgements
Dr. Chun Xiao has contributed in carrying out all animal and experimental work. Dr. Neelima Chauhan has contributed in data analysis, interpretation of results and compiling the manuscript. All animal work has been conducted according to the institutional approval for care and use of animals (IACUC). This work has been supported in part by the facilities and resources at the Jesse Brown VA Medical Center Chicago and by the Westside Institute for Science and Education, Chicago, IL. The authors acknowledge the support provided by the Department of Pediatrics, University of Illinois at Chicago, Children's Hospital of the University of Illinois, Chicago, IL. Authors acknowledge the support provided by American University of Health Sciences, Signal Hill, CA. This work has been supported in part by National Institute of Health (AG039625, NS079614 NBC); and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, RR&D (I0880R, NBC).
References
- Alzheimer's Association Report (2020) 2020 Alzheimer's disease facts and figures. Alzheimers Dement 16: 391-460.
- Rampa A, Gobbi S, Belluti F, et al. (2013) Emerging targets in neurodegeneration: New opportunities for Alzheimer's disease treatment? Curr Top Med Chem 13: 1879-1904.
- Reddy PH, Tonk S, Kumar S, et al. (2016) A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer's disease. Biochem Biophys Res Commun 483: 1156-1165.
- Caracciolo B, Xu W, Collins S, et al. (2014) Cognitive decline, dietary factors and gut-brain interactions. Mech Ageing Dev 136-137: 59-69.
- Panickar KS, Jang S (2013) Dietary and plant polyphenols exert neuroprotective effects and improve cognitive function in cerebral ischemia. Recent Pat Food Nutr Agric 5: 128-143.
- Butterfield DA, Di Domenico F, Barone E (2014) Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim Biophys Acta 1842: 1693-1706.
- Prasad KN (2017) Oxidative stress and pro-inflammatory cytokines may act as one of the signals for regulating microRNAs expression in Alzheimer's disease. Mech Ageing Dev 162: 63-71.
- Norden DM, Muccigrosso MM, Godbout JP (2014) Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic cns injury, and neurodegenerative disease. Neuropharmacology 96: 29-41.
- Rosenberg GA, Bjerke M, Wallin A (2014) Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke 45: 1531-1538.
- Lim A, Krajina K, Marsland AL (2013) Peripheral inflammation and cognitive. Mod Trends Pharmacopsychiatri 28: 175-187.
- Anand P, Singh B (2013) A review on cholinesterase inhibitors for Alzheimer's disease. Arch Pharm Res 36: 375-399.
- Lendvai B, Kassai F, Szajli A, et al. (2013) a7 nicotinic acetylcholine receptors and their role in cognition. Brain Res Bull 93: 86-96.
- Hernandez CM, Dineley KT (2012) a7 nicotinic acetylcholine receptors in Alzheimer's disease: Neuroprotective, neurotrophic or both? Curr Drug Targets 13: 613-622.
- De La Monte SM (2012) Metabolic derangements mediate cognitive impairment and Alzheimer's disease: Role of peripheral insulin-resistance diseases. Panminerva Med 54: 171-178.
- Ho N, Sommers MS, Lucki I (2013) Effects of diabetes on hippocampal neurogenesis: Links to cognition and depression. Neurosci Biobehav Rev 37: 1346-1362.
- Blazquez E, Velazquez E, Hurtado-Carneiro V, et al. (2014) Insulin in the brain: Its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Front Endocrinol (Lausanne) 5: 161.
- Nillert N, Pannangrong W, Welbat JU, et al. (2017) Neuroprotective effects of aged garlic extract on cognitive dysfunction and neuroinflammation induced by beta-amyloid in rats. Nutrients 9.
- Hernandez-Rapp J, Rainone S, Hebert SS (2016) MicroRNAs underlying memory deficits in neurodegenerative disorders. Prog Neuropsychopharmacol Biol Psychiatry 73: 79-86.
- Hill JM, Lukiw WJ (2016) MicroRNA (miRNA)-mediated pathogenetic signaling in Alzheimer's Disease (AD). Neurochem Res 41: 96-100.
- Karnati HK, Panigrahi MK, Gutti RK, et al. (2015) miRNAs: Key players in neurodegenerative disorders and epilepsy. J Alzheimers Dis 48: 563-580.
- Keifer J, Zheng Z, Ambigapathy GA (2015) MicroRNA-BDNF negative feedback signaling loop in brain: Implications for Alzheimer's Disease. Microrna 4:101-108.
- Fabian MR, Sonenberg N, Filipowicz W (2010) Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 79: 351-379.
- Jung HJ, Suh Y (2014) Circulating miRNAs in ageing and ageing-related diseases. J Genet Genomics 41: 465-472.
- Kempuraj D, Ahmed ME, Selvakumar GP, et al. (2020) Psychological stress-induced immune response and risk of alzheimer's disease in veterans from operation enduring freedom and operation Iraqi freedom. Clin Ther 42: 974-982.
- Flynt AS, Li N, Thatcher EJ, et al. (2007) Zebrafish miR-214 modulates Hedgehog signaling to specify muscle cell fate. Nat Genet 39: 259-263.
- Kaur H, Arora A, Wengel J, et al. (2006) Thermodynamic, counterion, and hydration effects for the incorporation of locked nucleic acid nucleotides into DNA duplexes. Biochemistry 45: 7347-7355.
- Stenvang J, Petri A, Lindow M, et al. (2012) Inhibition of microRNA function by antimiR oligonucleotides. Silence 3: 1.
- Codocedo JF, Rios JA, Godoy JA, et al. (2016) Are microRNAs the molecular link between metabolic syndrome and Alzheimer's Disease? Mol Neurobiol 53: 2320-2338.
- Basavaraju M, de Lencastre A (2016) Alzheimer's disease: Presence and role of microRNAs. Biomol Concepts 7: 241-252.
- Nadim WD, Simion V, Benedetti H, et al. (2017) MicroRNAs in neurocognitive dysfunctions: New molecular targets for pharmacological treatments? Curr Neuropharmacol 15: 260-275.
- Zhao Y, Alexandrov PN, Lukiw WJ (2016) Anti-microRNAs as novel therapeutic agents in the clinical management of Alzheimer's Disease. Front Neurosci 10: 59.
- Lukiw WJ (2012) NF-kappaB-regulated, proinflammatory miRNAs in Alzheimer's disease. Alzheimers Res Ther 4: 47.
- Pogue AI, Lukiw WJ (2018) Up-regulated pro-inflammatory MicroRNAs (miRNAs) in Alzheimer's disease (AD) and age-related macular degeneration (AMD). Cell Mol Neurobiol 38: 1021-1031.
- Maldonado-Lasuncion I, Atienza M, Sanchez-Espinosa MP, et al. (2019) Aging-related changes in cognition and cortical integrity are associated with serum expression of candidate MicroRNAs for Alzheimer Disease. Cereb Cortex 29: 4426-4437.
- Wu Y, Xu J, Xu J, et al. (2017) Lower serum levels of miR-29c-3p and miR-19b-3p as biomarkers for Alzheimer's Disease. Tohoku J Exp Med 242: 129-136.
- Herrera-Espejo S, Santos-Zorrozua B, Alvarez-Gonzalez P, et al. (2019) A systematic review of MicroRNA expression as biomarker of late-onset Alzheimer's Disease. Mol Neurobiol 56: 8376-8391.
- McKeever PM, Schneider R, Taghdiri F, et al. (2018) MicroRNA expression levels are altered in the cerebrospinal fluid of patients with young-onset Alzheimer's Disease. Mol Neurobiol 55: 8826-8841.
- Cheng B, Gong H, Xiao H, et al. (2013) Inhibiting toxic aggregation of amyloidogenic proteins: A therapeutic strategy for protein misfolding diseases. Biochim Biophys Acta 1830: 4860-4871.
- Chauhan NB, Davis F, Xiao C (2011) Wheat germ agglutinin enhanced cerebral uptake of anti-Abeta antibody after intranasal administration in 5XFAD mice. Vaccine 29: 7631-7637.
- Chauhan MB, Chauhan NB (2015) Brain uptake of neurotherapeutics after intranasal versus intraperitoneal delivery in mice. J Neurol Neurosurg 2: 1-9.
- Chauhan NB (2007) Intracerebroventricular passive immunization with anti-oligoAbeta antibody in TgCRND8. J Neurosci Res 85: 451-463.
- Chauhan NB, Gatto R (2010) Synergistic benefits of erythropoietin and simvastatin after traumatic brain injury. Brain Res 1360: 177-192.
- Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-Maze for assessment of spatial working and reference memory in mice. Methods Mol Biol 1916: 105-111.
- Mehla J, Chauhan BC, Chauhan NB (2014) Experimental induction of type 2 diabetes in aging-accelerated mice triggered Alzheimer-like pathology and memory deficits. J Alzheimers Dis 39: 145-162.
- Chauhan NB, Sandoval J (2007) Amelioration of early cognitive deficits by aged garlic extract in Alzheimer's transgenic mice. Phytother Res 21: 629-640.
- Ray B, Chauhan NB, Lahiri DK (2011) Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem 117: 388-402.
- Granic I, Dolga AM, Nijholt IM, et al. (2009) Inflammation and NF-kappaB in Alzheimer's disease and diabetes. J Alzheimers Dis 16: 809-821.
- Cui JG, Li YY, Zhao Y, et al. (2010) Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-kappaB in stressed human astroglial cells and in Alzheimer disease. J Biol Chem 285: 38951-38960.
- Kawahara M, Kato-Negishi M (2011) Link between aluminum and the pathogenesis of alzheimer's disease: The integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimers Dis 2011: 276-393.
- Pogue AI, Cui JG, Li YY, et al. (2010) Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci Lett 476: 18-22.
- Bykhovskaia M (2011) Synapsin regulation of vesicle organization and functional pools semin. Cell Dev Biol 22: 387-392.
- Zhao Y, Calon F, Julien C, et al. (2011) Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer's disease models. PLoS One 6: e15816.
Corresponding Author
Neelima Chauhan, VA Long Beach Healthcare System, Tibor Rubin VA Medical Center, Long Beach, CA, USA; Department of Pediatrics, University of Illinois at Chicago, Chicago, IL 60612, USA, Tel: 562-988-2278; Fax: 562-988-1791; E-mail: nchauhan51@gmail.com
Copyright
© 2020 Xiao C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.