Diagnostic Value of 123-I-MIBG Cardiac Scintigraphy for the Prediction of Conversion from Idiopathic REM Sleep Behavior Disorder to Dementia with Lewy Bodies, and the Differential Diagnosis of Neurodegenerative Diseases
Abstract
Objectives
The purpose of this study was to investigate the diagnostic availability of cMIBG in differentiating Parkinson's Disease (PD) and Dementia with Lewy Bodies (DLB) from related Neurodegenerative Diseases (NDDs) and to clarify the utility of cMIBG as a predicative biomarker for the conversion from Idiopathic REM Sleep Abnormal Behavior Disorder (iRBD) to α-synucleinopathies.
Materials and methods
A total 779 patients who underwent cMIBG were enrolled. Detailed analyses were performed in 27 patients with iRBD. The 568 patients with PD were classified as having DLB or PD1 (Hoehn & Yahr stage 1), PD2, PD3, or PD4, and 184 patients had other NDDs.
Results
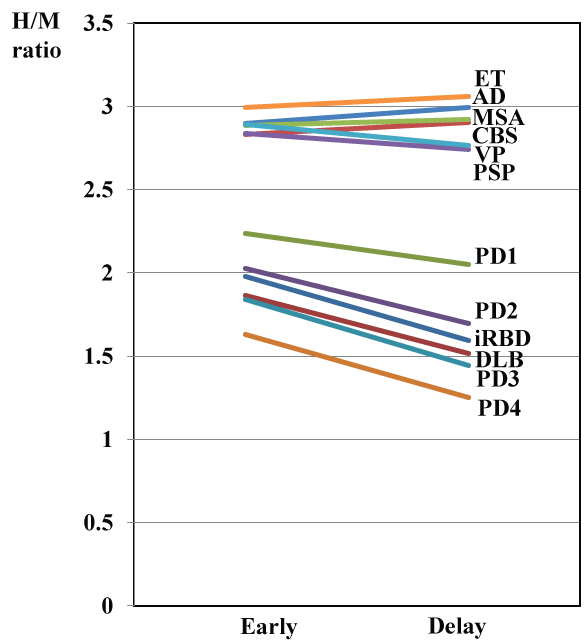
The Heart-to-Mediastinum uptake (H/M) ratios of the images from the patients with iRBD, PD, and DLB were significantly lower than those in other diseases. The sensitivity and specificity of differentiating DLB from AD were 88.9% and 95.2% in the early images, respectively, and 92.1% and 96.8% in the delayed images, respectively. In iRBD, PD and DLB patients, the H/M ratio decreased in the order of PD1, PD2, iRBD, DLB, PD3 and PD4. The cut-off values differentiating PD patients from those with NDDs were as follows: Early H/M ratio, 2.36 and delayed H/M ratio, 2.19. The H/M ratio in 55.6% of iRBD patients for the early images, and 74.0% of iRBD patients for the delayed images dropped to the level of the 95% confidence interval values of the DLB and PD3 patients. Four patients showed normal values for both the early and delayed H/M ratios, and three showed levels corresponding to PD1 or PD2.
Conclusions
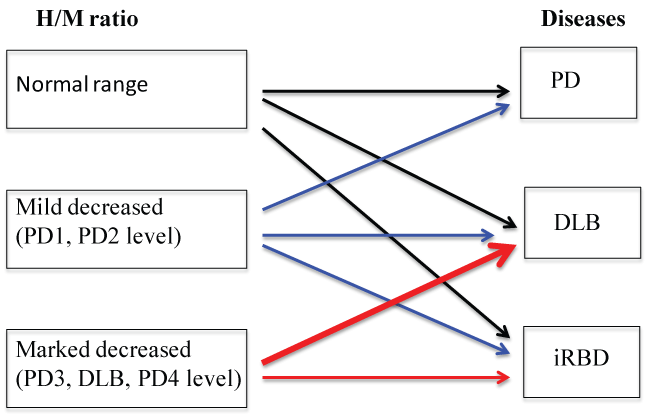
A marked decrease in the H/M ratio among iRBD patients might be a risk factor for the conversion to DLB. A normal or a mildly decreased H/M ratio among iRBD patients might be a risk factor for the conversion to PD or DLB, or continuing to have iRBD.
Keywords
MIBG cardiac scintigraphy, iRBD, DLB, Parkinson, Synucleinopathy
Introduction
Meta-iodobenzylguanidine (MIBG) is a physiologic analogue of noradrenaline, used to determine the location, integrity, and function of postganglionic noradrenergic neurons. 123I-MIBG Cardiac Scintigraphy (cMIBG) is a noninvasive diagnostic technique that is used to detect and evaluate sympathetic denervation [1]. In recent years, cMIBG has been reported as a useful tool for diagnosing PD [2] and differentiating PD from other parkinsonism's, such as Multiple System Atrophy (MSA) [3-5], Progressive Supranuclear Palsy (PSP) [4,5], Corticobasal Syndrome (CBS) [5], vascular parkinsonism [6] and drug-induced parkinsonism [7] and even from essential tremor [8].
Recently, cMIBG has also been used to discriminate DLB from Alzheimer's Disease (AD) [9,10]. In 1995, we performed cMIBG in a 71-year-old male suffering from PD who showed almost complete loss of the MIBG uptake [11]. Thereafter, we reported that the myocardial MIBG uptake was reduced in cases of PD [2].
Idiopathic REM Sleep Behavior Disorder (iRBD) is characterized by a loss of REM sleep paralysis, allowing patients to "act out" vivid, often unpleasant dreams with vocal sounds and sudden, often violent arm and leg movements during REM sleep [12].
In recent years, iRBD has been reported to be an extremely powerful predictor or prodromal marker of neurodegenerative synucleinopathies, including PD, DLB, and MSA, and 80% of sufferers eventually develop neurodegenerative diseases [12-14].
It is often difficult to precisely diagnose and differentiate PD and DLB from other related NDDs using clinical consensus criteria alone. The purpose of this study was to investigate the diagnostic utility of cMIBG for differentiating PD and DLB from related NDDs and to clarify the utility of cMIBG as a predictive biomarker for the conversion from iRBD to neurodegenerative synucleinopathies.
Materials and Methods
Materials
A total 779 patients with neurodegenerative diseases who underwent cMIBG were recruited from the medical records and were enrolled in this study (Table 1). Twenty-seven patients with iRBD who met the clinical criteria [13] and underwent the cMIBG were enrolled in this study (Table 2). The 568 patients with PD were classified as having DLB (n = 126), Hoehn & Yahr [H&Y] stage 1 [PD1] (n = 58), stage 2 [PD2] (n = 106), stage 3 [PD3] (n = 230) and stage 4 [PD4] (n = 48). The remaining 184 patients included 62 patients with AD, 19 with CBS, 33 with MSA-P, 41 with PSP, 16 with essential tremor, and 13 with Vascular Parkinsonism and Dementia (VP).
Methods
Polysomnography (PSG), Dopamine Transporter (DaT) Single-Photon Emission Computed Tomography (SPECT), an olfactory test via an Odor Stick Identification Test for Japanese (OSIT-J), and brain SPECT were conducted in patients with iRBD.
The SPECT device used in this study was a two-head gamma camera (Infinia Hawkeye 4, General Electronics, U.S.A.) equipped with an Extended-Low-Energy-General-Purpose (ELEGP) collimator. The patient underwent 123I-MIBG cardiac scintigraphy during quiet respiration. SPECT images were obtained at 15 min (early image) and 4 h (delayed image) after the intravenous injection of 111 MBq of 123I-MIBG. Planar images were obtained in 128 × 128 matrices, with an acquisition time of 150 sec, and an energy window of 159 keV (± 10%). Only the planar anterior image was used in this study. Using the Region of Interest (ROI) method, the early and delayed 123I-MIBG Heart-to-Mediastinum uptake (H/M) ratio on the anterior view and the washout rate were calculated. After manually setting a circular ROI at the center of the heart, a mediastinum rectangle ROI was determined on the upper third of the mediastinum. The 123I-MIBG H/M ratios measured by an ELEGP collimator were then corrected to ME collimator-comparable values using a calibration phantom [15].
123I-FP-CIT was used for the DaT SPECT imaging. When "the number of Standard Deviations (SDs) from the mean" exceeded -1.64, the uptake in the striatum was judged to be abnormally decreased (DaTQUANT, GE Healthcare, U.S.A). The Regional Cerebral Blood Flow (rCBF) changes were acquired for 20 minutes, beginning 30 minutes after the administration of 111 MBq of 123I-IMP. The easy Z-score Imaging System (eZIS) [16], and Voxel Based Stereotactic Extraction Estimation (vbSEE) [17] were used in this study for the quantitative assessment of brain SPECT images. Among the parameters of the hypoperfusion assessment, the extent of an abnormal region in each segment (proportion of the coordinates with a Z-value that exceeds the threshold value, among all coordinates within a segment), and severity (average Z-value of the coordinates with a Z-value that exceeds the threshold value), we used the latter parameter to assess the hypoperfusion in the vbSEE analysis. Given that iRBD might be a factor predicting DLB, we determined the frequent hypoperfuion areas by analyzing the severity scores in 30 patients with DLB and selected the following 6 areas; superior occipital gyrus, angular gyrus, middle temporal gyrus, precuneus, cuneus and inferior temporal gyrus, depending on the grade of severity scores. The cut-off values using the total severity scores of these 6 areas in the control subjects were as follows; age 50 - 59 years, 18.28 (N = 27); 60 - 69 years, 21.91 (N = 21); 70 - 79 years, 17.60 (N = 23) and > 80 years, 14.83 (N = 18).
The clinical diagnosis of iRBD was made according to the criteria of Schenck CH, et al. [12]. Probable DLB was diagnosed according to the criteria of McKeith IG, et al. [18]. The clinical diagnosis of probable AD was made according to the National Institute of Neurological and Communicative Disorders and Stroke - Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria [19]. The clinical diagnosis of MSA-P was made according to the criteria of Gilman, et al. [20], while that of PSP was made according to the current criteria of the National Institute of Neurological Disorders and Stroke (NINDS) and the Society for Progressive Supranuclear Palsy [21], and that of CBS was made according to the criteria of Armstrong, et al. [22].
In clinical practice, there often is diagnostic doubt due to the overlap in the clinical symptoms and signs. Such patients were not included in this study, and patients with diabetes mellitus, heart failure, cardiac ischemic heart disease, or patients under pharmacological treatment (like haloperidol and selegiline) that could influence the MIBG uptake were also excluded from this study [23].
Statistical analyses
We used the IBM-SPSS v.22, (U.S.A) and StatMate v.5. (Atms, Tokyo, Japan) software programs for the data analyses. Descriptive data are reported as mean and Standard Deviation (SD) or as number and percentage. Differences between each disease were assessed with a one way ANOVA, followed by the Tukey's test for pair-wise comparison when ANOVA showed s signify difference. To estimate the predicative ability, the sensitivity and specificity of the different items were calculated.
This study was approved by the ethics committee of the Nagasaki Kita Hospital.
Results
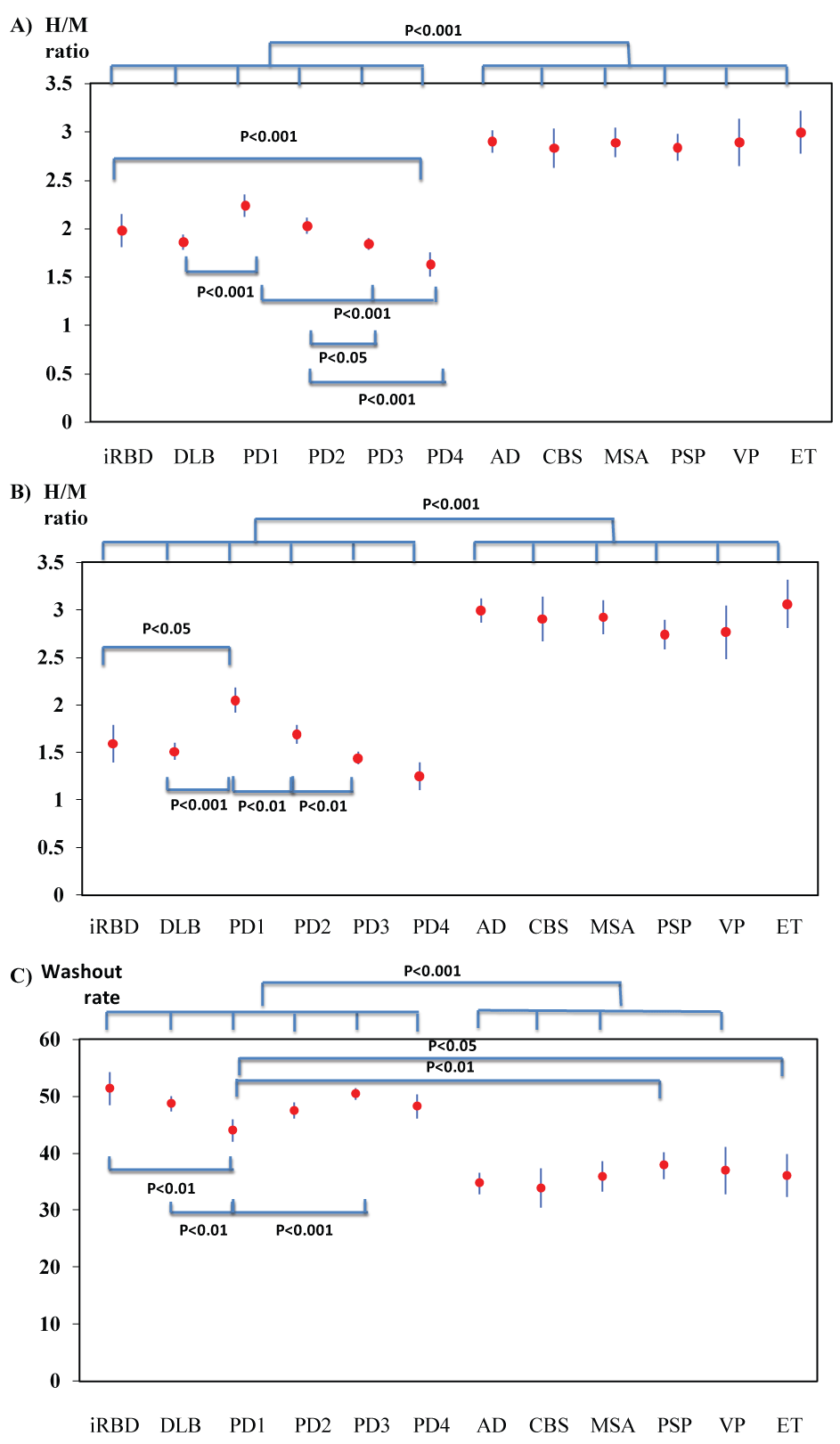
The H/M ratios for the early and delayed images for each disease are listed Table 3. All of the data were compared using a one-way analysis of variance (Tukey's test). The upper 95% confidence interval of the H/M ratio in PD1 was 2.353 for the early images and 2.182 for the delayed images. The upper 95% confidence interval of the washout rate was 40.22% for PSP which was the highest value among non-PD NDDs. Based on these results, the diagnostic criteria (cut-off values) differentiating PD group from other NDDs were defined as follows: Early H/M ratio of 2.36, delayed H/M ratio of 2.19, and washout rate of 40.3% (Table 3).
The H/M ratios of the images from the patients with iRBD, PD and DLB were significantly lower than those from the patients with other diseases (P < 0.001). Among the patients with iRBD, PD or DLB, the H/M ratio decreased in the order of PD1, PD2, iRBD, DLB, PD3, and PD4 (in the early images, PD1:DLB, P < 0.001, PD1: PD3 and PD4, P < 0.001, PD2: PD3, P < 0.05, PD2: PD4, P < 0.001, iRBD: PD4, P < 0.001, no significant differences were noted between PD1 and PD2, and in the delayed images, in addition to the same statistical results in the early images, PD1: PD2 showed a significant difference, P < 0.01) (Figure 1 and Figure 2).
Both the early and delayed H/M ratios of DLB were significantly lower than those of PD1, but not significantly different from those of PD2, PD3, and PD4 (Figure 2A and Figure 2B). Both the early and delayed H/M ratios among the patients with non-PD diseases did not differ significantly by disease (Figure 2A and Figure 2B). The washout rates in the PD and DLB patients were significantly higher than those in the patients with other NDDs (P < 0.001) (Figure 2C).
The DLB patients were divided into two groups; DLB with Parkinsonism (78 patients) and DLB without parkinsonism (48 patients). The H/M ratio in the early images was 1.78 ± 0.38 for DLB with parkinsonism vs. 2.00 ± 0.49 for DLB without parkinsonism (P < 0.05), the H/M ratios in the delayed images was 1.40 ± 0.36 for DLB with parkinsonism vs. 1.68 ± 0.59 for DLB without parkinsonism (P < 0.01), and the washout rate was 49.9 ± 5.2% for DLB with parkinsonism vs. 46.6 ± 9.4% for DLB without parkinsonism (P < 0.05). Among DLB patients, the values in 14 of 126 (11.1%) on early images and 10 of 126 (7.9%) on delayed images exceeded the cut-off value. In contrast, among AD patients, the values in 3 of 62 (5.8%) on early images and 2 of 62 (3.2%) on delayed images fell below the cut-off value. The sensitivity and specificity for differentiating DLB from AD were 88.9% and 95.2% on early images, respectively, and 92.1% and 96.8% on delayed images, respectively. The washout rate in AD patients exceeded the cut-off value in 13 of 62 (21.0%). The sensitivity and specificity for differentiating DLB from AD were 92.1% and 79.0%, respectively, indicating that the specificity of the washout rate was lower than that of the H/M ratio.
The H/M ratio of the early and delayed images in iRBD patients fell below the cut-off value in 22 (81.5%) and 23 (85.2%) patients, respectively, and the washout rate increased in 24 (88.9%) patients. Among these iRBD patients, 15 of 27 (55.6%) on early images and 20 of 27 (74.0%) on delayed images dropped to the H/M ratio level as in the PD3 or PD4 patients (within the 95% Confidence Interval (CI) values of the PD3 and PD4 patients), and the washout rate exceeded the cut-off value of 40.3% in 24 of 27 (88.9%) patients (Table 2 and Table 3). Only 5 (18.5%) and 4 (14.8%) patients showed a normal value for the early and delayed H/M ratios, respectively. The H/M ratio in seven patients who were examined via early images and 3 who were examined via delayed images were within the H/M ratio ranges in PD1 or PD2 patients.
A DaT scan revealed a decreased uptake in only 7 of 27 (25.9%) patients examined, all of whom showed a marked reduction in their H/M ratios on cMIBG (Table 2). PSG revealed REM Sleep without Atonia (RWA) in all patients except for two cases who were unable to sleep during the examination period. However, these patients showed the typical symptoms of iRBD in their history (Table 2). The olfactory test using the OSIT-J showed abnormal impairment in 20 of 26 tested patients (76.9%) (Table 2). An analysis of the SPECT images showed hypoperfusion in 14 of 26 (53.8%) iRBD patients (Table 2). There was no correlation between the H/M ratios in both early and delayed images and the severity scores of rCBF.
The duration of iRBD symptoms, j.e. vivid, often frightening dreams associated with simple or complex motor behavior during REM sleep, ranged from 2 to 30 years. Because of the relatively short observation periods in our hospital (6 months to 5 years), only 1 case (case 23) who showed a marked reduction in the cMIBG uptake and a deceased uptake on Dat SPECT progressed to DLB, but the other patients still have iRBD at present.
Discussion
Previous reports have shown promising results regarding the utility of cMIBG for differentiating PD from other neurodegenerative parkinsonism's, such as MSA [3-5], PSP [4,5], and CBS [5], and other neurological diseases, such as the essential tremor [8], vascular Parkinsonism [6] and AD [9,10]. The H/M ratio on cMIBG in patients with these non-PD diseases dose not markedly differ from that in normal control and diseased control subjects.
cMIBG results for PD, DLB and NDDs
In this study, because we could not examine normal subjects, and because no universal standard H/M ratio on cMIBG has been established. Since one of our objectives in this study was to differentiate PD and DLB from other NNDs, we set the cut-off value of the H/M ratio based on the upper limit of the 95% confidence interval in PD1 patients: An early H/M ratio of 2.36 and a delay ratio of 2.19. The cut-off value of the washout rate was determined using the upper limit of the 95% confidence interval in PSP patients which was the highest among those with NDDs. The findings in this study were essentially the same as those in the previous reports [3-7]. The H/M ratios of both the early and delayed images and the washout rate in the MSA, PSP, CBS, AD, essential tremor, and VP patients were clearly different from those of the PD and DLB patients.
Regarding the severity of PD and the H/M ratio on cMIBG, Rocchi C, et al. [24] reported that cMIBG was correlated with the Unified Parkinson's Disease Rating Scale (UPDRS) motor score and disease duration. In this study, we compared the severity of PD according to the H&Y stage and showed that both the early and delayed H/M ratios were clearly decreased in order of PD stages. However, these markers were useless in differentiation among the NDDs.
DLB was also clearly differentiated from AD, as shown in the previous papers [9,10,25,26]. Slaets S, et al. [9] reported that 95% of the patients were correctly classified as compared with a clinical or definite diagnosis at follow-up, with sensitivity and specificity values for diagnosing DLB of 100% and 75%, respectively.
Shimizu S, et al. [26] reported that the sensitivity and specificity of differentiating DLB from AD were 72.4% and 94.4% using the H/M ratio on cMIBG. The sensitivity and specificity in our results were 88.9% and 95.2% in the early images, respectively, and 92.1% and 96.8% in the delayed images, respectively, which were consistent with the values described in previous papers. The H/M ratio on cMIBG may aid in the conclusive diagnosis of cases suspected of being DLB or AD. Of note, a frequent concomitant finding in DLB is varying degrees of AD-type pathology, e.g. β-amyloid in neuritic plaques and hyperphosphorylated tau in neurofibrillary tangles. This overlap between the two most common, yet distinct , neurodegenerative dementias in terms of underlying pathology and clinical characteristics, often makes an antemortem diagnosis difficult. Nedelska Z, et al. [27] investigated the pattern and magnitude of the atrophy rates from two serial magnetic resonance imaging scans performed antemortem in autopsy-confirmed DLB and mixed DLB/AD patients, compared to AD and elderly non-demented controls. DLB patients without significant AD-type pathology were characterized by relatively low global and regional rates of atrophy, similar to those in controls. In contrast, the mixed DLB/AD patients displayed higher rates in the whole brain, temporo-parietal cortices, hippocampus and amygdala, along with ventricle expansion, similar to AD patients. It is actually impossible clinically to differentiate between AD patients with DLB pathology and DLB patients with AD pathology. In the present study, however, DLB was clearly differentiated from AD using the cMIBG. Further studies will be necessary to determine what the cMIBG findings look like in autopsy-confirmed patients with DLB/AD pathology.
cMIBG and other examinations for iRBD
The second objective of this study was to investigate whether iRBD may be a predictor of neurodegenerative synucleinopathies. In recent years, iRBD has been reported to be an extremely powerful predictor or prodromal marker of neurodegenerative synucleinopathies, including PD, DLB, and MSA, and the conversion rates from iRBD to full clinical synucleinopathies range from 17.7% to 90%, depending on the follow-up period [12,28-31]. Schenk CH, et al. [12] reported that over 80.8% (21/29) of patients with iRBD eventually developed synucleinopathies, including 13 PD, 3 DLB, and 2 MSA. However, a cMIBG examination was not performed in their studies. The most notable finding in the present study for iRBD patients was that the cMIBG H/M ratio had already decreased to within the 95% CI of DLB or PD3 in 55.6% of patients in the early images and 74% of patients in the delayed images.
DaT SPECT, an imaging technique that probes the integrity of the presynaptic nigrostriatal system, can be useful in the clinical evaluation of PD and other NDDs in the appropriate context. Kim YK, et al. [32] reported that the DaT densities in the putamen remained within the normal range in 11 of 14 iRBD patients. In the present study, only 7 of 27 (25.9%) iRBD patients showed a reduction in the DaT densities. Taken together, these findings indicate that DaT density changes are a minor phenomenon in iRBD and not useful as a predictive marker of neurodegenerative synucleinopathies. Recently Iranzo A, et al. [33] reported that 51of 87 (58.6%) iRBD patients showed a baseline DaT deficit, and 25 (28.7%) patients developed clinically defined synucleinopathy (PD in 11, DLB in 13, and MSA in 1) with a mean latency of 3.2 years from imaging. The differences in the abnormal frequency in the reported papers may depend on the diagnostic criteria and machine used for the measurement. Based on the findings from the DaT SPECT studies, the major diseases prone to convertion from iRBD to synucleinopathies are PD and DLB, and the MSA seems to be a minor disease because of its low frequency [12,33].
Regarding the findings from the cMIBG study, 5 to 10 years will be needed to draw any hard conclusions on whether or not the H/M ratios may get a position of predicting marker from iRBD to synucleinopathies. However, we believe that our finding that the H/M ratios showed a marked decrease to the level seen in PD3 or DLB has some clinical significance. PD is a progressive neurodegenerative disease and despite the diversity of its symptoms and signs, the severity of PD is classified according to the H&Y stage from I to V. The values of the H/M ratio decreased in the order of H&Y stages, and the H/M ratios in many iRBD patients decreased to the level seen in the PD3/PD4 and DLB patients during this study. Since PD does not start at the PD3 or PD4 level, we can assume that the iRBD patients with a marked decrease of the H/M ratios might be a risk factor of developing DLB from iRBD, but not PD. A normal H/M ratio or a mildly decreased H/M ratio in iRBD patients might be a risk factor of developing PD or DLB, or continuing to have iRBD (Figure 3). The results of our cMIBG study may be a useful indicator for selecting candidates for disease modification trials among iRBD patients.
Olfactory dysfunction is known to be a frequent and early feature of PD, Lewy Body Disease, and AD, often preceding the motor or cognitive symptoms by several years [34,37]. Recent data have indicated that over 90% of patients with PD have significant olfactory loss. Haehner, et al. [35] and Fantini, et al. [36] studied 54 consecutive PSG-confirmed iRBD patients and 54 age-and gender-matched control subjects with the Brief University of Pennsylvania Smell Identification Test (B-UPSIT). They found that 61.1% of iRBD patients, versus 16.6% of controls, had an abnormal olfactory function. The olfactory function as assessed using the OSIT-J was decreased in 76.9% of iRBD patients in the present study, as in previous reports. Two of six patients who showed normal scores also had normal cMIBG H/M ratios. While the number of cases was relatively small, this finding suggests that olfactory dysfunction may correlate with the cMIBG H/M ratio. Further studies will be needed in order to clarify this issue.
Some studies have investigated the Regional Cerebral Blood Flow (rCBF) changes using SPECT in patients with iRBD, showing conflicting results [38-40]. These inconsistent results may be attributed primarily to the differences in the assessment of cerebral perfusion (manual selection of regions of interest versus statistical imaging analyses), as well as patient characteristics. Shirakawa, et al. [38] showed decreased perfusion in the upper portion of the frontal lobe and the pons, whilst Mazza, et al. [39] found decreased perfusion in the frontal, temporal, and parietal lobes and increased perfusion in the hippocampus and putamen. In this study on the assumption of a factor predicting conversion to DLB, we used the data of DLB patients and a vbSEE analysis to determine the most likely sites of perfusion, as follows: Superior occipital gyrus, angular gyrus, middle temporal gyrus, precuneus, cuneus and inferior temporal gyrus, depending on the grade of severity scores. As a result, the total severity scores were increased in 53.8% of patients. There was no correlation between the H/M ratios in the delayed images and the severity scores of rCBF.
Takahashi M, et al. [41] reported that the strong quantitative correlation between the cMIBG uptake and the corresponding loss of sympathetic axons in patients with Lewy Body Disease, indicating cMIBG to be a powerful clinical tool for detecting the loss of these axons as a biomarker for the presence of Lewy bodies. However, the pathological findings of the cardiac sympathetic axons have not been described in iRBD patients. At present, none of the iRBD patients showed symptoms suggesting dysautonomia such as tachycardia, hypotension, constipation, or anhydrosis. When the loss of cardiac sympathetic axons develops in iRBD patients and its clinical and physiological significance remain unclear.
Regarding potential markers predicting the conversion from iRBD to synucleinopathies, particularly conversion to DLB, the accumulation of cases and long-term follow-up studies are now required to determine whether or not cMIBG findings may be a sensitive marker of conversion to DLB or PD, versus continuing to have iRBD.
Conclusions
The H/M ratios on the cMIBG were found to be the most reliable tool for differentiating PD and DLB from other NDDs such as MSA, PSP, CBS, AD, ET, and VP. The H/M ratio was correlated with the severity of PD, decreasing in the order of PD1, PD2, iRBD, DLB, PD3, and PD4. The H/M ratio in DLB patients was significantly lower than those in PD1 and PD2 patients, but did not significantly differ from those in PD3 and PD4 patients. A marked decrease in the H/M ratio among iRBD patients may be a risk factor of conversion to DLB. A normal or a mildly decreased H/M ratio among iRBD patients might be a risk factor of conversion to PD or DLB, or continuing to have iRBD.
References
- Spiegel J, Hellwig D, Farmakis G, et al. (2007) Myocardial sympathetic degeneration correlates with clinical phenotype of Parkinson's disease. Mov Disord 22: 1004-1008.
- Satoh A, Serita T, Seto M, et al. (1999) Loss of 123I-MIBG uptake by the heart in Parkinson's disease: assessment of cardiac sympathetic denervation and diagnostic value. J Nucl Med 40: 371-375.
- Braune S, Reinhardt M, Schnitzer R, et al. (1999) Cardiac uptake of [123I]MIBG separates Parkinson's disease from multiple system atrophy. Neurology 53: 1020-1025.
- Yoshita M (1998) Differentiation of idiopathic Parkinson's disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci 155: 60-67.
- Orimo S, Suzuki M, Inaba A, et al. (2012) 123I-MIBG myocardial scintigraphy for differentiating Parkinson's disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord 18: 494-500.
- Kim JS, Lee PH, Lee KS, et al. (2006) Cardiac [123I]metaiodobenzylguanidine scintigraphy for vascular Parkinsonism. Mov Disord 21: 1990-1994.
- Lee PH, Kim JS, Shin DH, et al. (2006) Cardiac 123I-MIBG scintigraphy in patients with drug induced parkinsonism. J Neurol Neurosurg Psychiatry 77: 372-374.
- Lee PH, Kim JW, Bang OY, et al. (2006) Cardiac 123I-MIBG scintigraphy in patients with essential tremor. Mov Disord 21: 1235-1238.
- Slaets S, Acker FA, Versijpt J, et al. (2015) Diagnostic value of MIBG cardiac scintigraphy for differential dementia diagnosis. Int J Geriatr Psychiatry 30: 864-869.
- Yoshita M, Arai H, Arai H, et al. (2015) Diagnostic Accuracy of 123I-Meta-Iodobenzylguanidine Myocardial Scintigraphy in Dementia with Lewy Bodies: A Multicenter Study. PLoS One 10: e0120540.
- Serita T, Irita A, Ueyama C, et al. (1995) Autonomic nervous system function of the heart in patient with Parkinson's disease. Therapeutic Research 16: 60-64.
- Schenck CH, Montplaisir JY, Frauscher B, et al. (2013) Rapid eye movement sleep behavior disorder: devising controlled active treatment studies for symptomatic and neuroprotective therapy-a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep Med 14: 795-806.
- Iranzo A, Tolosa E, Gelpi E, et al. (2013) Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: An observational cohort study. Lancet Neurol 12: 443-453.
- Schenck CH, Boeve BF, Mahowald MW (2013) Delayed emergence of a parkinsonian disorder or dementia in 81% of older males initially diagnosed with idiopathic REM sleep behavior disorder (RBD): a 16-year update on a previously reported series. Sleep Med 14: 744-748.
- Nakajima K, Okuda K, Matsuo S, et al. (2012) Standardization of metaiodobenzylguanidine heart to mediastinum ratio using a calibration phantom: effects of correction on normal databases and a multicentere study. Eur J Nucl Med Mol Imaging 39: 113-119.
- Matsuda H (2007) Role of neuroimaging in Alzheimer's disease, with emphasis on brain perfusion SPECT. J Nucl Med 48: 1289-1300.
- Mizumura S, Kumita S, Cho K, et al. (2003) Development of quantitative analysis method for stereotactic brain image: assessment of reduced accumulation in extent and severity using anatomical segmentation. Ann Nucl Med 17: 289-295.
- McKeith IG, Dickson DW, Lowe J, et al. (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65: 1863-1872.
- McKhann G, Drachman D, Folstein M, et al. (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939-944.
- Gilman S, Wenning GK, Low PA, et al. (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71: 670-676.
- Litvan I, Agid Y, Calne D, et al. (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47: 1-9.
- Armstrong MJ, Litvan I, Lang AE, et al. (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80: 496-503.
- Solanki KK, Bomanji J, Moyes J, et al. (1992) A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta-iodobenzylguanidine (MIBG). Nucl Med Commun 13: 513-521.
- Rocchi C, Pierantozzi M, Galati S, et al. (2015) Autonomic Function Tests and MIBG in Parkinson's Disease: Correlation to Disease Duration and Motor Symptoms. CNS Neurosci Ther 21: 727-732.
- Kim JS, Park HE, Oh YS, et al. (2015) (123)I-MIBG myocardial scintigraphy and neurocirculatory abnormalities in patients with dementia with Lewy bodies and Alzheimer's disease. J Neurol Sci 357: 173-177.
- Shimizu S, Hirao K, Kanetaka H, et al. (2016) Utility of the combination of DAT SPECTand MIBG myocardial scintigraphy in differentiating dementia with Lewy bodies from Alzheimer's disease. Eur J Nucl Med Mol Imaging 43: 184-192.
- Nedelska Z, Tanis J, Ferman TJ, et al. (2007) Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain 130: 708-719.
- Iranzo A, Fernandez-Arcos A, Tolosa E, et al. (2014) Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 9: e89741.
- Ferini-Strambi L, Marelli S, Galbiati A, et al. (2014) REM Sleep Behavior Disorder (RBD) as a marker of neurodegenerative disorders. Arch ltal Biol 152: 129-146.
- Postuma RB, Gagnon JF, Bertrand JA, et al. (2015) Parkinson risk in idiopathic REM sleep behavior disorder preparing for neuroprotective trials. Neurology 84: 1104-1113.
- Postuma RB, Iranzo A, Hogl B, et al. (2015) Risk Factors for Neurodegeneration in Idiopathic Rapid Eye Movement Sleep Behavior Disorder: A Multicenter Study. Ann Neurol 77: 830-839.
- Kim YK, Yoon IY, Kim JM, et al. (2010) The implication of nigrostriatal dopaminergic degeneration in the pathogenesis of REM sleep behavior disorder. Eur J Neurol 17: 487-492.
- Iranzo A, Santamaria J, Valldeoriola F, et al. (2017) Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 82: 419-428.
- Miyamoto M, Iwanami M, Hirata K, et al. (2010) Olfactory dysfunction in idiopathic REM sleep behavior disorder. Sleep Med 11: 458-461.
- Haehner A, Hummel T, Reichmann H (2014) A clinical approach towards smell loss in Parkinson's disease. J Parkinsons Dis 4: 189-195.
- Fantini M, Postuma R, Montplaisir J, et al. (2006) Olfactory deficit in idiopathic rapid eye move-ments sleep behavior disorder. Brain Res Bull 70: 386-390.
- Yong-ming Zou, Da Lu, Li-ping Liu, et al. (2016) Olfactory dysfunction in Alzheimer's disease. Neuropsychiatr Dis Treat 12: 869-875.
- Shirakawa S, Takeuchi N, Uchimura U, et al. (2002) Study of image findings in rapid eye movement sleep behavioural disorder. Psychiatry Clin Neurosci 56: 291-292.
- Mazza S, Soucy JP, Gravel P, et al. (2006) Assessing whole brain perfusion changes in patients with REM sleep behavior disorder. Neurology 67: 1618-1622.
- Hanyu H, Inoue Y, Salcurai H, et al. (2011) Regional cerebral blood flow changes in patients with idiopathic REM sleep behavior disorder. Eur J Neurol 18: 784-788.
- Takahashi M, Ikemura M, Oka T, et al. (2015) Quantitative correlation between cardiac MIBG uptake and remaining axons in the cardiac sympathetic nerve in Lewy body disease. J Neurol Neurosurg Psychiatry 86: 939-944.
Corresponding Author
Mitsuhiro Tsujihata, Section of Neurology, Nagasaki Kita Hospital, 800, Motomuragou, Togitsu-chou, Nishisonogi-gun, Nagasaki, 851-2103, Japan, Tel: 095-886-8700.
Copyright
© 2017 Seto M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.