A Rare Case of Jejunal Perforation due to Post-transplant Lymphoproliferative Disease 27 Years after Liver Transplant
Abstract
Post-transplant lymphoproliferative disease (PTLD) is a well-known post-transplant malignancy common in both solid organ and stem cell transplants. The incidence of PTLD in adult liver transplant recipients is approximately 2.8%. While most often presenting in the first year after transplant, PTLD can occur at any time. This case report narrates a 61-year-old female with a history of liver transplant in 1992 for autoimmune hepatitis that was found to have a jejunal perforation due to PTLD 27 years later. The patient presented to the hospital with a fifteen hour history of left sided abdominal pain that was progressively worsening. The patient reports taking Imuran, Cellcept and Prednisone daily for immunosuppression. Computed tomography revealed pneumoperitoneum and free fluid centered on a focal area of enteritis in the proximal jejunum, as well as wall thinning of several areas of the small intestine. The patient was taken to the operating room for an exploratory laparotomy where a perforation was identified on the antimesenteric portion of the distal jejunum. A small bowel resection was performed and the patient's postoperative course was unremarkable. On pathologic analysis the patient was found to have a monomorphic, diffuse large B-cell lymphoma with a high proliferative rate. This case demonstrates a rare presentation of post-transplant lymphoproliferative disease occurring 27 years after transplantation.
Keywords
Post-transplant lymphoproliferative disorder, Gastro intestinal perforation, Small bowel resection, Liver transplant, Case report
Introduction
Post-transplant lymphoproliferative disorder (PTLD) is a rare but serious group of heterogeneous lymphoproliferative disorders that may develop after hematopoietic stem cell and solid organ transplants [1]. It is the second most common malignancy in transplant patients, the most common being skin cancer [2]. It is estimated that up to 70-80% of cases are associated with the Epstein Barr Virus (EBV) [1,3]. Most cases of early PTLD are due to EBV while most cases of late PTLD are EBV negative. Apart from affecting the allograft, extranodal PTLD has a predilection for the gastrointestinal tract [4]. PTLD is often a silent disorder however patients may develop non-specific symptoms such as fever, weight loss and fatigue secondary to the viral infection or lymphadenopathy. Other symptomology occurs secondary to mass effect of surrounding organs or structures [5].
While rare, PTLD can present with perforation of hollow viscus. There is a lack of guidelines for the surgical management of abdominal catastrophes related to PTLD. Identification of and resection of the perforation should be performed with either diversion of fecal stream or primary anastomosis depending on the clinical situation.
Case Presentation
This is a 61-year Caucasian female who presented to the emergency department with a fifteen hour history of acute onset abdominal pain. The pain progressed from sharp, constant pain in her left abdomen to diffuse abdominal pain. The patient denied other symptoms including nausea, emesis, fevers, and chills. Past medical history was positive for squamous cell skin cancer, arthritis, hypertension, hypothyroidism, and autoimmune hepatitis. Past surgical history consisted of liver transplant in 1992 secondary to autoimmune hepatitis, appendectomy, tonsillectomy, thyroidectomy, breast lumpectomy, and excision of skin cancer. She has no significant family history. Her immunosuppressive regimen consisted of 175 mg Azathioprine every night, 3 mg Tacrolimus daily, and 13 mg of Prednisone daily.
Dedicated physical examination was performed revealing tachycardia with otherwise stable vitals. The patient's abdomen was firm with diffuse tenderness to palpation with increased sensitivity in the left, lower quadrant. There was a palpable mass in the left lower quadrant. Four quadrant percussion and rebound tenderness were present with involuntary guarding. Laboratory analysis was significant for a leukocytosis of 12.5 bil/L, neutrophilic left shift of 11.1 bil/L, and lactic acidosis of 2.5 mmol/L. The patient underwent computed tomography of the abdomen and pelvis with IV contrast as pictured in the below images.
Preoperative Imaging
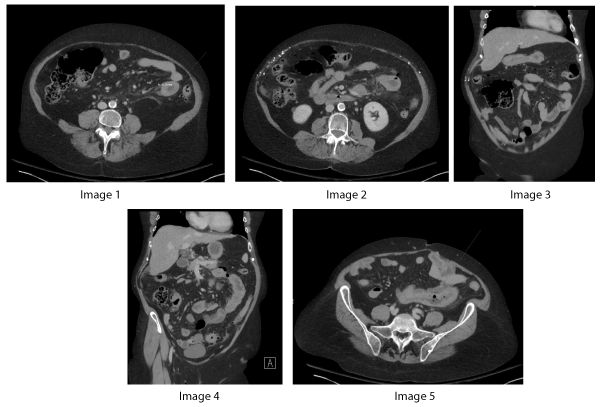
A CT of the abdomen and pelvis with intravenous contrast was performed that revealed jejunal bowel wall thickening indicated by the measurement caliper and arrow on axial Figure 1. The extent of wall thickening is also well demonstrated on coronal Figure 2 where nearly the entire segment of affected jejunum can be seen in the left abdomen, along with adjacent vascular engorgement. This image also demonstrates the presence of trace free fluid in the left lower quadrant and perihepatic space. There was also significant fat stranding surrounding the loop of jejunum, indicative of acute inflammation. Several images also demonstrate multiple foci of air throughout the mesenteric fat, consistent with pneumoperitoneum from jejunal perforation, as seen on Figure 3, Figure 4. On axial Figure 5, a segment of thickened small bowel is again demonstrated. The arrow indicates a focal air-containing defect in the bowel wall, suggesting a possible perforation site.
Pre-operative discussion was held between the surgical team and the patient with shared decision making. Given the imaging studies in the setting of an acute abdomen, the patient was taken emergently to the operating room for exploratory laparotomy. Extensive succus was encountered upon entry into the abdominal cavity. The bowel was examined proximally too distally in the standard fashion until a perforation was encountered on the antimesenteric side of a loop of bowel in the distal jejunum. Pathologically enlarged lymph nodes were confirmed near the site of perforation. Small bowel resection was performed with five centimeter margins proximally and distally. An extensive wash-out was performed with great yield and limited post-procedural contamination. A side-to-side stapled anastomosis was created without a diverting ostomy. The rest of the small intestine as well as the large intestine were inspected with no further areas of perforation noted. The patient was safely extubated and transferred to recovery in stable condition.
The patient had an uneventful postoperative recovery. Her diet was advanced and pain was well controlled. Preliminary pathology results came back with concern for lymphoma. Hematology and oncology was consulted and diagnosed the patient with post-transplant lymphoproliferative disorder. The patient was discharged home in stable condition. Several days later the final pathology report revealed monomorphic post-transplant lymphoproliferative disorder, diffuse large B-Cell lymphoma with high proliferative rate, double express or subtype. The patient followed up with the department of hematology and oncology after discharge and was prescribed DA-REPOCH regimen. In addition, the patient followed up with her transplant hepatology team who decreased the patient's immunosuppression regimen. The patient has had several hospital admissions since her surgery for inpatient chemotherapy sessions which were complicated by cytomegalovirus (CMV) infection, cellulitis and hospital acquired pneumonia and Pneumocystis Carinii Pneumonia (PCP). She is currently doing well and has completed chemotherapy.
Discussion
Post-transplant lymphoproliferative diseases are known complications following solid organ transplant and hematopoietic stem cell transplant. This pathology was first described in 1968 by Doak, et al. who highlighted two cases following renal transplantation [6]. It was formally described and categorized as a distinct clinical entity in the 2008 WHO classification of lymphomas. In 2016 the World Health Organization updated their classifications of PTLD with 6 categories: Plasmalytic hyperplasia PTLD, infectious mononucleosis PTLD, florid follicular hyperplasia PTLD, polymorphic PTLD, monomorphic PTLD, and classical Hodgkin lymphoma. The majority of cases are of B cell origin with diffuse large B cell lymphoma being the most common type. [1, 7,8] The incidence of PTLD varies depending on the type of transplant (with highest reported incidence in small bowel and multi-organ transplants) and is estimated to be 0.8-20%. A higher incidence has been attributed to the higher levels of immunosuppression required in these types of transplants. [2,9]
There is a known association between EBV infection and PTLD with an estimated 70-80% of cases being Epstin-Barr positive. While immunocompetent hosts infected with EBV can clear the primary infection, the virus may persist in B-lymphocytes in a latent state. There are four different types of latency associated with EBV which are differentiated by the EBV antigen expression pattern in infected B-cells. In EBV positive PTLD patients, the most common latency pattern is Type III [3]. Patients are at highest risk of developing PTLD one year after transplantation when immunosuppression is at its highest and these cases are most commonly driven by EBV. In EBV negative PTLD the pathogenesis is not as well defined. EBV negative PTLD occurs more commonly in older transplant patients, is a monomorphic type, and often has high risk features [10]. In this case report, final pathology demonstrated monoclonal, diffuse Large B-cell lymphoma with high proliferation rate, double expressor subtype (co-expression of BCL and c-myc). Analysis demonstrated rare Epstein Barr encoding region (EBER) presence in neoplastic B cells with the majority of neoplastic cells negative for EBER. While it is unclear the significance of the rare EBER presence in neoplastic B cells, most patients with EBV positive PTLD present within one year of transplant. With the majority of neoplastic cells negative for EBER we believe pathogenesis of this patient's PTLD is likely EBV negative which is consistent with the monoclonal lineage and higher grade of malignancy.
Histological Evaluation of Pathology
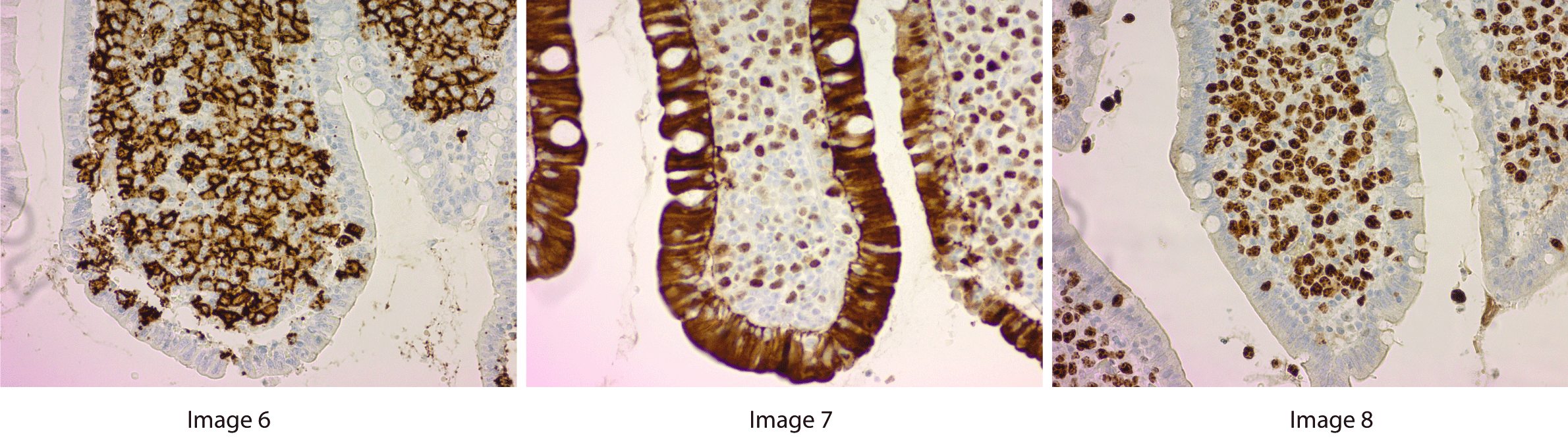
Figure 6, Figure 7, Figure 8 demonstrate preserved small intestine with ulcer bed and abnormal expansion of the lamina propria by atypical large cells. Figure 6 is a high power replicated view with stains indicating CD20 positive cells, a B-cell lineage marker. Figure 7 demonstrates the majority of cells are expressing C-myc protein, consistent with a c-myc translocation that is seen in Burkitt lymphoma and so called "double hit" lymphomas. In the clinical setting of transplant, the diagnosis is that of a post transplant lymphoproliferative disorder, likely Burkitt subtype. Figure 8 is a high power view with staining for Ki-67, indicating a high proliferation rate. The high Ki-67 stains and CD20 staining reveals that this is an aggressive B cell neoplasm.
Unfortunately, the symptoms of PTLD are quite vague with many patients being asymptomatic. Patients may present with viral symptoms, unexplained fever, or lymphadenopathy. They may also present with signs related to allograft dysfunction, with the allograft and the gastrointestinal tract being some of the most common extranodal sites [7]. Gastrointestinal symptoms can vary widely from vague abdominal pain, diarrhea, and lack of appetite to gastrointestinal bleeding, obstruction, and perforation [11-15]. In a retrospective study published by Cruz, et al. at the University of Pittsburgh, a total of 5,677 adult patients were studied with liver transplants performed from January 1983-December 2019. They identified thirty six patients who developed gastrointestinal PTLD, sixteen of which required emergency surgery. Of those sixteen patients, seven required surgery due to obstruction, six due to perforation and three due to gastrointestinal bleeding. Of the six perforations, two occurred following the initiation of chemotherapy [15].
Gastrointestinal perforation is an incredibly rare presentation of PTLD with only limited case reports and case series. In a literature review performed using Google Scholar and PubMed with keywords "post-transplant lymphoproliferative disorder" and "perforation", approximately seventeen cases of spontaneous gastrointestinal perforation as the initial presentation of PTLD have been reported [13,15-18]. To the best of our knowledge this case is the first reported case of PTLD diagnosed in a patient so late after transplant with the patient's perforation occurring 27 years following liver transplant.
Radiology plays an important role in the early diagnosis of PTLD lesions, in guiding biopsy, and in surveillance of treatment response. Contrast-enhanced computed tomography (CT) is the most widely used modality due to its ease of use and wide availability. Abdominal disease is seen in 50%-75% of patients with PTLD following renal, liver, or heart transplant and the abdominal cavity is the most frequently involved compartment in PTLD [19]. Bowel wall thickening is a nonspecific finding that, in the emergent setting, can be indicative of underlying inflammation. Wall thickening and dilation are the most common imaging findings in small bowel involvement of PTLD, followed in order of decreasing frequency by eccentric mass, luminal ulceration, and short-segment intussusception [19]. Extra nodal involvement is more common than nodal involvement in intra-abdominal disease [20], and the gastrointestinal tract and liver are the most commonly involved sites, although the prevalence of organ involvement varies with the type of transplant [19]. The imaging features of gastrointestinal involvement by PTLD are similar to those of non-Hodgkin lymphoma in immunocompetent patients, although ulceration is more common [21]. The distal small bowel is the most common site of involvement of hollow viscera, followed by the proximal large bowel, stomach, duodenum, and esophagus. The distal jejunum and ileum are the most common sites of small bowel disease [19].
The current standard for treatment of solid organ transplant PTLD, regardless of EBV status, is reduction of immunosuppression (RIS) with the addition of rituximab and chemotherapy [22]. Rituximab is a monoclonal anti-CD20 antibody which when combined with RIS has demonstrated improved overall response rates and complete response rates. While rituximab certainly changed the management of lymphoma and PTLD, it has a rare side effect of gastrointestinal perforation. A warning was issued by the manufacturer in 2006 warning of the very rare occurrence of perforation with their database recording 37 gastrointestinal perforations in a total of 730,000 patients. In this case report, the patient was treated with reduction in immunosuppression, chemotherapy, and rituximab (DA-REPOCH). Fortunately, she was able to complete treatment without complication of perforation. In one case report a patient presented with spontaneous intestinal perforation several months after bone marrow transplant successfully managed with emergent laparotomy. Postoperatively, they were treated with rituximab and developed a second intestinal perforation which was fatal [16]. While the complication of intestinal perforation with rituximab is exceedingly rare, it is important for the clinician managing the PTLD patients to be aware of this complication, especially if the presentation is with intestinal perforation.
Post-transplant lymphoproliferative disorder is a rare hematologic malignancy occurring after hematopoietic stem cell or solid organ transplant. It is very rare for PTLD to present with intestinal perforation. This case report describes the successful management of an abdominal catastrophe in a patient 27 years following liver transplantation on immunosuppressive therapy. The patient was successfully treated with laparotomy, small bowel resection and primary stapled side to side anastomosis. With a multi-modality approach to her surgical aftercare, the patient's pathology was properly managed and she continues to progress in her health. We describe one of few reported cases of gastrointestinal perforation as the initial presentation of PTLD after solid organ transplantation. To the authors' knowledge, we also present a patient with the longest latency from solid organ transplantation to diagnosis of PTLD following intestinal perforation that has been reported in the literature.
Conflict of Interest Statement
None Declared.
References
- Bishnoi R, Bajwa R, Franke AJ, et al. (2017) Post-transplant lymphoproliferative disorder (PTLD): Single institutional experience of 141 patients. Exp Hematol Oncol 6: 26.
- LaCasce AS (2006) Post-transplant lymphoproliferative disorders. Oncologist 11: 674-680.
- Swerdlow SH, Campo E, Pileri SA, et al. (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127: 2375-2390.
- Crombie J, LaCasce AS (2019) Epstein barr virus associated b-cell lymphomas and iatrogenic lymphoproliferative disorders. Front Oncol 9: 109.
- Paya C, Fung J, Nalesnik M, et al. (1999) Epstein-barr virus-induced posttransplant lymphoproliferative disorders. Transplantation 68: 1517-1525.
- Kamdar KY, Rooney CM, Heslop HE (2011) Posttransplant lymphoproliferative disease following liver transplantation. Curr Opin Organ Transplant 16: 274-280.
- Bakker N, van Imhoff G, Verschuuren E, et al. (2007) Presentation and early detection of post-transplant lymphoproliferative disorder after solid organ transplantation. Transplant Int 20: 207-218.
- Friedburg JW, Aster JC (2021) Epidemiology, clinical manifestations and diagnosis of post-transplant lymphoproliferative disorders.
- Doak PB, Montgomerie JZ, North JD, et al. (1968) Reticulum cell sarcoma after renal homotransplantation and azathioprine and prednisone therapy. Br Med J 4: 746-748.
- DeStefano C, Desai S, Shenoy A, et al. (2018) Management of post-transplant lymphoproliferative disorders. Br J Haematol 182: 330-343.
- Jayananda, Sriraksha, Gollol Raju, et al. (2019) Post transplant lymphoproliferative disorder: A Rare cause of gastrointestinal blood loss anemia. American Journal of Gastroenterology 114: S1123.
- Kruel CR, Ruiz RM, Shiller SM, et al. (2014) Posttransplant lymphoproliferative disorder presenting as a small bowel obstruction in a patient with pancreas transplantation alone. Proc (Bayl Univ Med Cent) 27: 346-348.
- Krishna KC, Sivaramakrishna G, Kumar PM, et al. (2009) Posttransplant lymphoproliferative disease presenting as jejunal perforation. Indian J Nephrol 19: 82.
- Osawa H, Uemura M, Nishimura J, et al. (2015) Surgical management of perforated gastrointestinal posttransplantation lymphoproliferative disorder after heart transplantation. Int Surg 100: 358-364.
- Cruz R, Ramachandra S, Sasatomi E, et al. (2012) Surgical management of gastrointestinal posttransplant lymphoproliferative disorders in liver transplant recipients. Transplantation 94: 417-423.
- Hsu Y, Liao W, Wang H, et al. (2009) Catastrophic gastrointestinal manifestations of post-transplant lymphoproliferative disorder. Dig Liver Dis 41: 238-241.
- Vaidya R, Habermann TM, Donohue JH, et al. (2013) Bowel perforation in intestinal lymphoma: Incidence and clinical features. Ann Oncol 24: 2439-2443.
- Aimoto M, Yamane T, Inoue A, et al. (2010) Epstein-Barr virus-associated post-transplant lymphoproliferative disorder diagnosed by the episode of intestinal perforation following allogeneic hematopoietic stem cell transplantation. Rinsho Ketsueki 51: 1775-1780.
- Borhani AA, Hosseinzadeh K, Almusa O, et al. (2009) Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics 29: 981-1000.
- Pickhardt PJ, Siegel MJ (1999) Posttransplantation lymphoproliferative disorder of the abdomen: CT evaluation in 51 patients. Radiology 213: 73-78.
- Dodd GD 3rd, Greenler DP, Confer SR (1992) Thoracic and abdominal manifestations of lymphoma occurring in the immunocompromised patient. Radiol Clin North Am 30: 597-610.
- Dierickx D, Vergote V (2019) Management of post-transplant lymphoproliferative disorders. Hemasphere 3: 74-77.
Corresponding Author
Marcos Rosado, DO Beaumont Hospital General Surgery, 28080 Grand River Avenue, Farmington Hills, MI 48336, USA.
Copyright
© 2021 Rosado M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.