Effects of Non-Focused ELF-EMF Treatment on EEG: Preliminary Study
Abstract
Introduction
The central nervous system (CNS) is known to be sensitive to focused magnetic stimulation, which is now used routinely for the treatment of various disorders. Extremely Low Frequency Electro Magnetic Fields (ELF-EMF) have been used in various clinical settings and tests however, to date, little is known regarding their functional mechanisms in vivo and, in particular, as a total-body non-focused administration. The CNS acts as the first interactive organ, capable of modulating the effects of ELF-EMF treatment on living systems. This study analysed the effects of 5 different electromagnetic frequency-ranges on the CNS, by studying the endogenous response to exposure. Spectrometry was used to measure the strength of different cerebral wavebands in various brain topographies, together with a component of Z-ratio, as a comparative reference system.
Materials and methods
21 healthy subjects between 20 and 30 years of age were recruited and assessed on two different sessions, one sham day and one treatment day. The subjects were assessed during administration of 5 different ELF-EMF set-ups, using a SEQEX® device, all exposed to the same intensity of 20 μT (microtesla), with a protocol duration of 3 minutes, each using a Swipe method, with a progressive increase of 0.1 Hz. The setup range was devised as follows:
• 1-3 Hz (δ)
• 4-8 Hz (θ)
• 9-13 Hz (α)
• 15-29 Hz (β)
• 31-56 Hz (γ)
A 14 channel EEG device was used to measure the cortical responses of the 21 subjects during stimulation.
Results
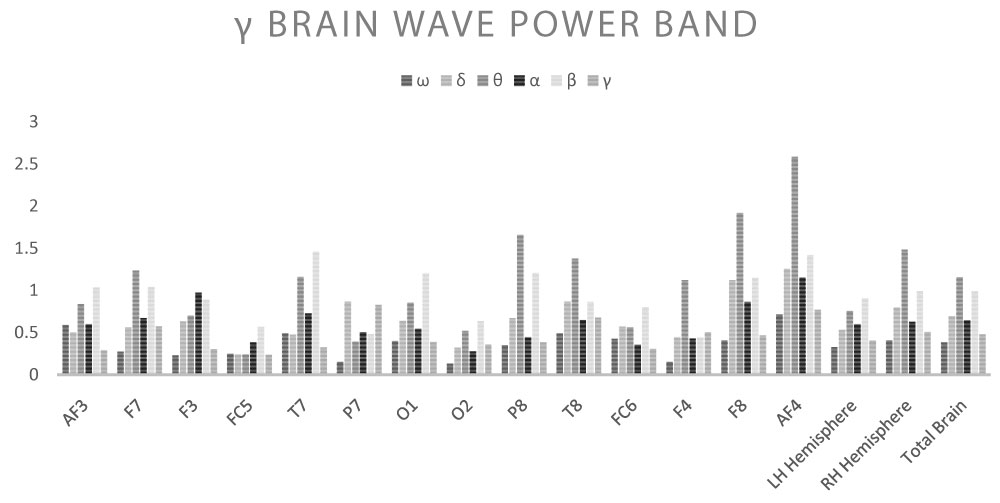
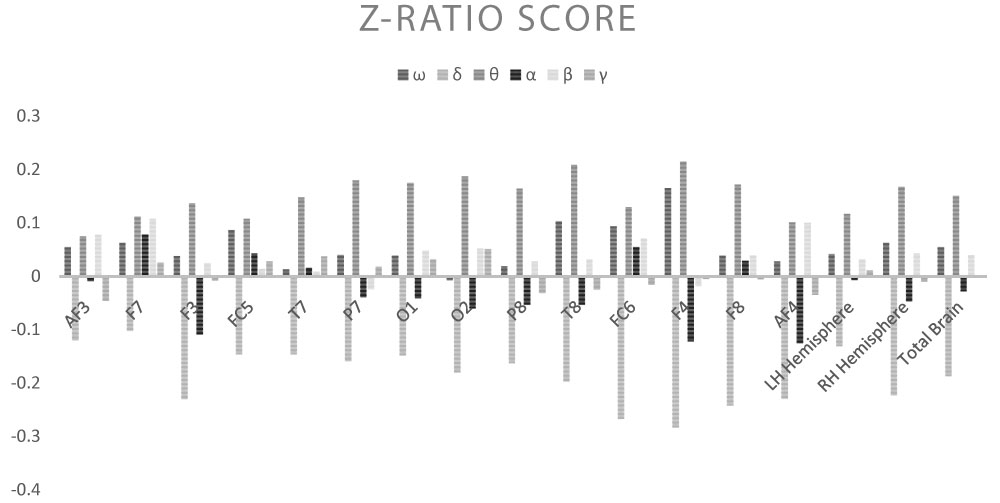
The response to the different electromagnetic stimulation set-ups varied, with activation of different brain derivations observed in EEG. In general, response was greater under stimulation within the δ range at 1-3 Hz, with a widespread increase in the band strength β (p < 0.05) and a ubiquitous decrease in the Z-Ratio (p < 0.05). Responses were minimal under stimulation in the γ range at 31-56 Hz, with a limited reaction in terms of activated areas and band strength, in any case coherent with the γ range, particularly in the right hemisphere (p < 0.01). Contrary to expectations, no significant increase was observed in the α band.
Conclusions
The electrical activity of the CNS exhibits frequency dependent sensitivity to treatment with ELF-EMF. This observation, if confirmed in further studies and relevant for two main reasons: Firstly, it offers insight for an improved understanding of the mechanisms of action of ELF-EMF on complex biological systems from the perspective of preventing disorders caused by electromagnetic pollution. Secondly it creates several implications for possible integration into existing neurological and psychiatric therapeutic approaches [1].
Introduction
We dispose today of an ever-increasing evidence of biological interactions with electromagnetic fields and living organisms. Regarding neurological/psychiatric scopes, excellent results have been achieved using Transcranial Magnetic Stimulation (TMS) [2-5]. However, much remains unknown regarding the effects of low frequency, extremely low intensity electromagnetic fields (ELF-EMF) on the cerebral activity of subjects under non-focused, total-body treatment. This exposure simulates the lowest levels of natural environmental exposure of the human body to commonly occurring EMF. The evidence, to date, indicates that local ELF-EMF stimulation modifies cerebral electrical activity, with a significant increase in the alpha waves range, in the frontal regions [5]. Local cranial stimulation with electromagnetic fields in a frequency range of 1-60 Hz and an intensity scale of 20-100 µT, demonstrated the sensitivity of the central nervous system (CNS), expressed as modified cerebral electrical behaviour [6-9].
The authors decided to observe the effects of non-focused, total body treatment with ELF-EMF on the CNS, in order to clarify:
• Potential environmental exposure to spurious electromagnetic frequencies.
• Potential treatments that might assist diverse medical disciplines.
• Mechanisms of action that lead to measurable behavioural [10] and physiological variations, monitored by Heart Rate Variability (HRV) [11].
It was thus decided to analyse cerebral electrical activity by EEG, as in previous studies listed in the bibliography [6-9]. The aim of the present study was to achieve a qualitative and quantitative understanding regarding non-focused stimulation, identical to the one used to test responses of the autonomic nervous system (ANS) [11], as a specific encephalic electrical reaction.
Materials and Methods
The study was conducted at the Azienda Pubblica di Servizi alla Persona (APSP) [Public Agency for Personal Health Services] "Santa Maria" retirement home, located in Cles (Trentino, Italy).
For the study, the authors recruited 21 volunteers, healthy subjects between the age of 20 and 30 years: 15 women and 6 men. The subjects were assessed on two different days:
• On one day (sham day) the EEG of the subjects was recorded at rest, with eyes closed, lying on a physiotherapy couch, with a device for generating ELF-EMF switched on but not emitting a field.
• On the other day the EEG of the subjects was recorded at rest, with eyes closed, lying on a physiotherapy couch, with a device for generating ELF-EMF operating and emitting an electromagnetic field.
Throughout the data collection phase, none of the subjects were informed of the nature and aim of the study: The only information provided regarded the type of ELF-EMF therapy to be given (method of administration of treatment, duration) and that EEG measurements that would be taken. None of the volunteers on either of the days were informed whether or not there was an actual administration of ELF-EMF. All the measurements were taken between 3 p.m. and 6 p.m.
A SEQEX® device was used for the study, produced and distributed by the Italian company S.I.S.T.E.M.I. Srl. (Trento, Italy), certified CSQ ISO-13485. These devices produce complex electromagnetic fields, using an analogue mechanism in a range of frequencies from 1 to 80 Hz, and variable intensities from 1 to 20 µT. The field parameters were tested by the manufacturing company using specialized equipment: A GM08 Gaussmeter produced by the Hirst company. The electromagnetic field produced by the device control unit (on which the parameters of the electromagnetic field are set), is emitted from a mat containing a Helmholtz coil that generates the ELF-EMF. Individual patients were asked to lie on the mat to receive non-focused total-body treatment with weak electromagnetic fields.
This study tested 5 different electromagnetic set-ups, each of which included 3 frequency/intensity pairs named "steps", with a time-on administration and a pause or time-off cycle (expressed in seconds). A sinusoidal wave form was used to produce the emissions for all the steps. The 5 different set-ups were labelled with Greek letters corresponding to the brain waves of the same frequencies (δ, θ, α, β, γ). The characteristics of the individual set-ups are listed in the following Table 1.
All the set-ups had a treatment time of 3 minutes. The subjects were assessed without administration of any ELF-EMF, but with the device switched on (measurement labelled ω), and then subsequently a single time for each set-up listed in the Table 1. Each subject involved in the study was therefore assessed 6 times.
The volunteers were assessed lying down with eyes closed. The position of the wooden couch supporting the mat was arranged so that the subjects were unable to see the control unit. This expedient, combined with the absence of any perception when the field is generated, ensured that the subjects did not know whether or not the device was actually in operation. After each distinct assessment with the electromagnetic field, the subjects were asked to stand up and remain upright for 3 minutes, away from the device mat. All administrations were conducted using the same device and in the same environment, the integrated medicine clinic at the APSP Santa Maria (Cles), without distractions or stimulations from third parties (background music, air fresheners, etc.) or the medical staff.
EEG
Electroencephalography is the most widely used non-invasive method for the study of cerebral electrical activity [12]. An electroencephalogram trace (EEG) divides up cerebral electrical activity into different brain wave ranges [12] labelled with the Greek letters: δ, θ, α, β, γ.
In this study an Eopch+ [13,14] 14 channel wireless electroencephalography headset from the Emotiv® company, was used. Sample recording was set at 128 Hz. The following brain derivations were studied: AF3, F7, F3, FC5, T7, P7, O1, O2, P8, T8, FC6, F4, F8, AF4.
EEG: FFT and Power Band
The device operates with the EmotivPRO proprietary software, which is capable of recording a RAW trace and conducting real-time analysis of band strength by Fourier transform (FFT). In this way it is possible to measure the band strength for each cerebral wave except delta waves, which are not analysed by FFT because the strength of delta signal would possibly mask high frequency low amplitude oscillations. The Power Band reading was assessed for all the brain derivations studied. The emissions of the two individual hemispheres as the sum of same-side derivations, and overall brain emissions, were also assessed as more generic readings.
The software did not provide data for the endogenous response to the δ wave, while it subdivided the study of β waves into two subcategories: low and high. Consequently, the bands recorded were:
• θ (4-8 Hz),
• α (8-12 Hz),
• β-low (12-16 Hz),
• β-high (16-25 Hz),
• γ (25-45 Hz).
EEG: Z-Ratio
In order to resolve the information gap regarding any incidental modifications to the δ waves, the data obtained by the abovementioned software were downloaded in *.EDF format and analysed using the open source EDFBrowser software. This was used to analyse the Z-Ratio [15], taken here as a measurement to calculate cerebral activity at low and high frequencies within a period or "epoch" of 2 seconds. This period is expressed on a scale of -1 to +1, with negative values indicating a predominance of high frequency activity and positive values indicating a predominance of low frequency activity. The Z-Ratio is calculated as follows (the Greek letters represent the corresponding brain waves):
The data obtained for the Power Bands did not follow a Gaussian distribution and were consequently transformed using the Log in order to normalize the distribution and make it possible to conduct a parametric statistical analysis applying Student's t-distribution. The Z-ratio data again did not have a Gaussian distribution and they were analysed using the Mann-Whitney test. Transformation to a logarithmic scale and statistical analysis were conducted using the GraphPad® InStat software.
Results
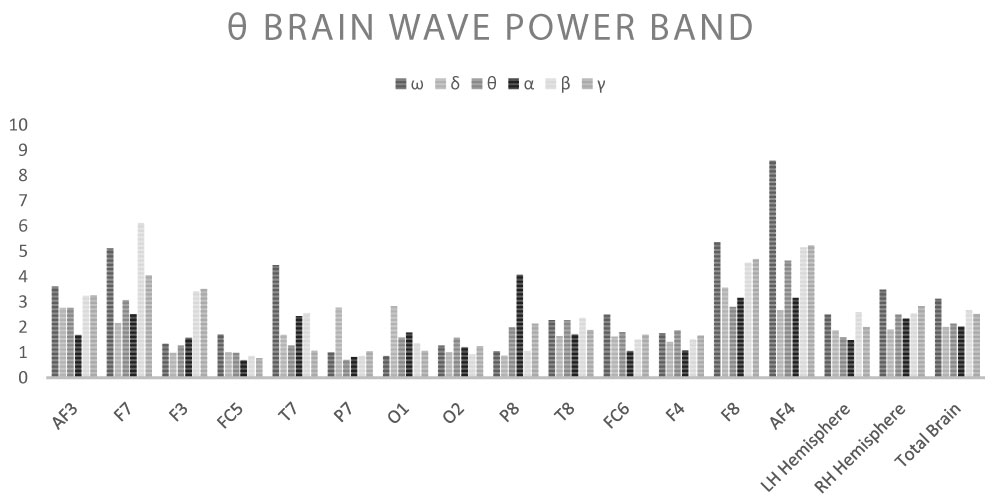
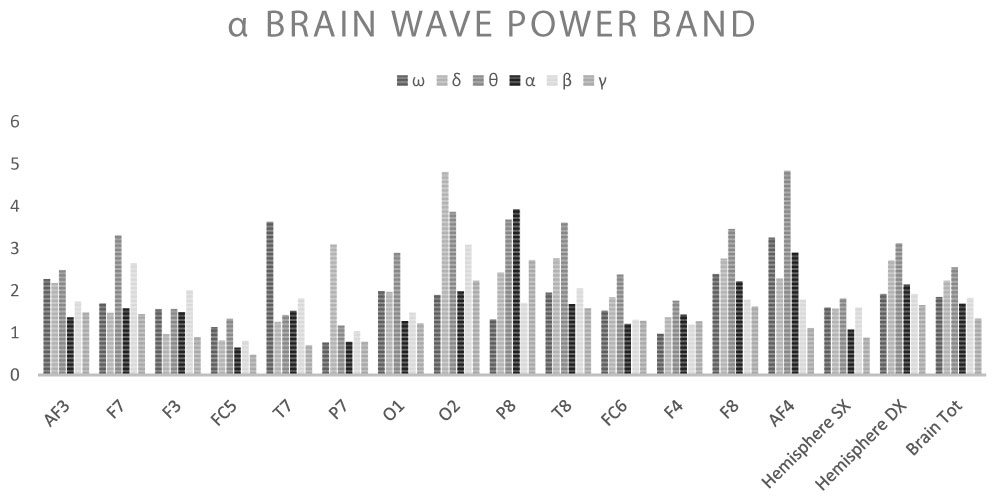
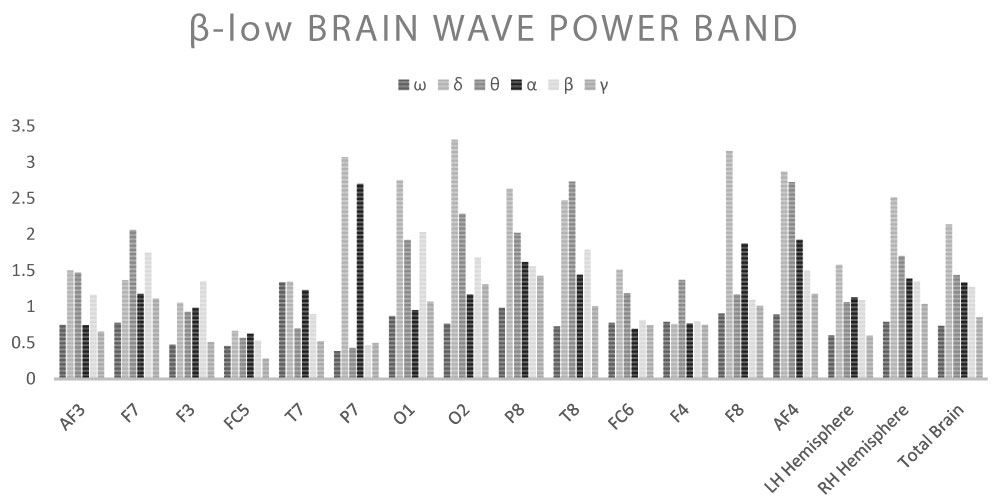
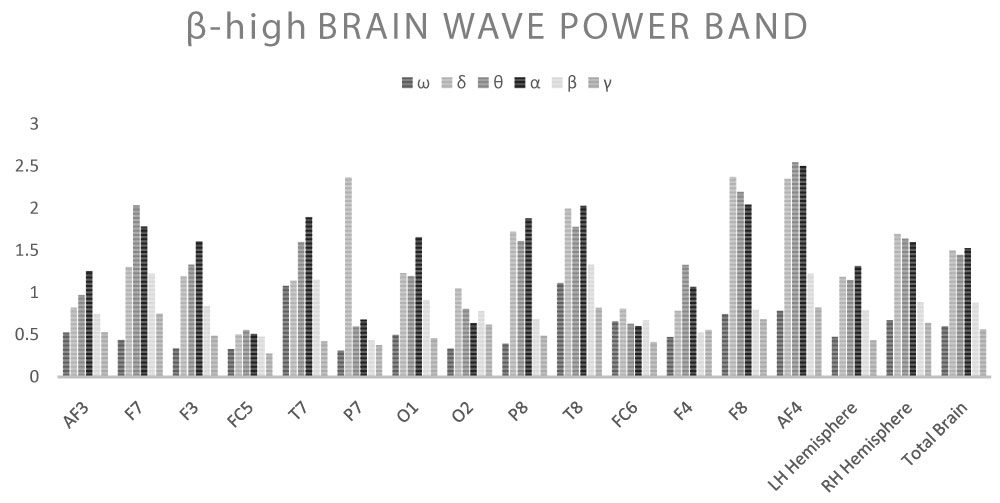
Shown below are the average power band values for the cerebral waves (θ, α, β-low, β-high, γ) for each derivation (Table 2, Table 3, Table 4, Table 5 and Table 6) (Graph 1, Graph 2, Graph 3, Graph 4 and Graph 5), and the Z-Ratio (Table 7 and Graph 6) obtained from the 6 measurements.
Effects of stimulation with ELF-EMF δ
Stimulation with frequencies in the δ range (1-2-3 Hz) produced an interesting increase in cerebral electrical activity in general, with an adaptive response. In detail:
• AF3: Decrease in Z-Ratio (average value ω: 0.055; average value under ELF-EMF: -0.12, p = 0.003);
• F7: Decrease in θ wave (average value ω: 5.117; average value under ELF-EMF: 2.168, p = 0.039), decrease in Z-Ratio (average value ω: 0.063; average value under ELF-EMF: -0.102, p = 0.0023);
• F3: Increase in β-low wave (average value ω: 0.476; average value under ELF-EMF: 1.058, p = 0.04), increase in β-high wave (average value ω: 0.338; average value under ELF-EMF: 1.195, p = 0.047), increase in γ wave (average value ω: 0.229; average value under ELF-EMF: 0.632, p = 0.028), decrease in Z-Ratio (average value ω: 0.038; average value under ELF-EMF: -0.23, p = 0.000);
• FC5: Decrease in Z-Ratio (average value ω: 0.087; average value under ELF-EMF: -0.146, p = 0.000);
• T7: Decrease in Z-Ratio (average value ω: 0.013; average value under ELF-EMF: -0.146, p = 0.016);
• P7: Increase in γ wave (average value ω: 0.152; average value under ELF-EMF: 0.867, p = 0.017), decrease in Z-Ratio (average value ω: 0.04; average value under ELF-EMF: -0.159, p = 0.000);
• O1: Decrease in Z-Ratio (average value ω: 0.039; average value under ELF-EMF: -0.148, p = 0.007);
• O2: Increase in β-low wave (average value ω: 0.765; average value under ELF-EMF: 3.312, p = 0.05), increase in β-high wave (average value ω: 0.334; average value under ELF-EMF: 1.053, p = 0.009), increase in γ wave (average value ω: 0.132; average value under ELF-EMF: 0.318, p = 0.012), decrease in Z-Ratio (average value ω: -0.007; average value under ELF-EMF: -0.18, p = 0.002);
• P8: Increase in β-high wave (average value ω: 0.391; average value under ELF-EMF: 1.724, p = 0.049), decrease in Z-Ratio (average value ω: 0.019; average value under ELF-EMF: -0.163, p = 0.007);
• T8: Increase in β-low wave (average value ω: 0.724; average value under ELF-EMF: 2.476, p = 0.043), decrease in Z-Ratio (average value ω: 0.103; average value under ELF-EMF: -0.197, p = 0.000);
• FC6: Decrease in Z-Ratio (average value ω: 0.094; average value under ELF-EMF: -0.267, p = 0.000);
• F4: Increase in β-low wave (average value ω: 0.788; average value under ELF-EMF: 0.762, p = 0.037), increase in β-high wave (average value ω: 0.471; average value under ELF-EMF: 0.781, p = 0.022), increase in γ wave (average value ω: 0.153; average value under ELF-EMF: 0.445, p = 0.000), decrease in Z-Ratio (average value ω: 0.165; average value under ELF-EMF: -0.283, p = 0.000);
• F8: Decrease in Z-Ratio (average value ω: 0.039; average value under ELF-EMF: -0.242, p = 0.000);
• AF4: Increase in γ wave (average value ω: 0.717; average value under ELF-EMF: 1.257, p = 0.025), decrease in Z-Ratio (average value ω: 0.028; average value under ELF-EMF: -0.229, p = 0.000);
• Average LH: Decrease in Z-Ratio (average value ω: 0.042; average value under ELF-EMF: -0.131, p = 0.000);
• Average RH: Increase in β-low wave (average value ω: 0.789; average value under ELF-EMF: 2.515, p = 0.05), decrease in Z-Ratio (average value ω: 0.063; average value under ELF-EMF: -0.223, p = 0.000);
• Total Brain: Decrease in Z-Ratio (average value ω: 0.055; average value under ELF-EMF: -0.187, p = 0.000).
Effects of stimulation with ELF-EMF θ
Stimulation with frequencies in the θ range (4-6-8 Hz) produced a very different response compared to the stimulation analysed above. In detail:
• AF3: No significant variation in brain waves and Z-Ratio;
• F7: No significant variation in brain waves and Z-Ratio;
• F3: Increase in γ wave (average value ω: 0.229; average value under ELF-EMF: 0.7, p = 0.033), increase in Z-Ratio (average value ω: 0.038; average value under ELF-EMF: 0.137, p = 0.039);
• FC5: No significant variation in brain waves and Z-Ratio;
• T7: Increase in Z-Ratio (average value ω: 0.013; average value under ELF-EMF: 0.148, p = 0.033);
• P7: Increase in γ wave (average value ω: 0.152; average value under ELF-EMF: 0.392, p = 0.000);
• O1: Increase in β-low wave (average value ω: 0.872; average value under ELF-EMF: 1.926, p = 0.042);
• O2: Increase in α wave (average value ω: 1.895; average value under ELF-EMF: 3.866, p = 0.016), increase in β-low wave (average value ω: 0.765; average value under ELF-EMF: 2.289, p = 0.024), increase in β-high wave (average value ω: 0.334; average value under ELF-EMF: 0.805, p = 0.02), increase in γ wave (average value ω: 0.132; average value under ELF-EMF: 0.518, p = 0.000), increase in Z-Ratio (average value ω: -0.007; average value under ELF-EMF: 0.187, p = 0.0137);
• P8: Increase in β-high wave (average value ω: 0.391; average value under ELF-EMF: 1.614, p = 0.049), increase in Z-Ratio (average value ω: 0.019; average value under ELF-EMF: 0.164, p = 0.03);
• T8: Increase in β-low wave (average value ω: 0.724; average value under ELF-EMF: 2.733, p = 0.028), increase in γ wave (average value ω: 0.491; average value under ELF-EMF: 1.379, p = 0.035), increase in Z-Ratio (average value ω: 0.103; average value under ELF-EMF: 0.209, p = 0.049);
• FC6: No significant variation in brain waves and Z-Ratio;
• F4: Increase in β-low wave (average value ω: 0.788; average value under ELF-EMF: 1.371, p = 0.034), increase in β-high wave (average value ω: 0.471; average value under ELF-EMF: 1.329, p = 0.006), increase in γ wave (average value ω: 0.153; average value under ELF-EMF: 1.121, p = 0.000), increase in Z-Ratio (average value ω: 0.165; average value under ELF-EMF: 0.215, p = 0.046);
• F8: No significant variation in brain waves and Z-Ratio;
• AF4: Increase in γ wave (average value ω: 0.717; average value under ELF-EMF: 2.583, p = 0.042), increase in Z-Ratio (average value ω: 0.028; average value under ELF-EMF: 0.101, p = 0.023);
• Average LH: No significant variation in brain waves and Z-Ratio;
• Average RH: Increase in α wave (average value ω: 1.921; average value under ELF-EMF: 3.12, p = 0.05), increase in β-low wave (average value ω: 0.789; average value under ELF-EMF: 1.702, p = 0.017), increase in β-high wave (average value ω: 0.672; average value under ELF-EMF: 1.639, p = 0.012), increase in Z-Ratio (average value ω: 0.063; average value under ELF-EMF: 0.168, p = 0.02);
• Total Brain: Increase in γ wave (average value ω: 0.382; average value under ELF-EMF: 1.158, p = 0.05), increase in Z-Ratio (average value ω: 0.055; average value under ELF-EMF: 0.151, p = 0.023).
Effects of stimulation with ELF-EMF α
Stimulation with frequencies in the α range (9-11-13 Hz) produced a minor response compared to the two cases described above. In detail:
References
- Vecchia P (2007) Exposure of humans to electromagnetic fields. Standards and regulations. Ann Ist Super Sanita 43: 260-267.
- Gorelick DA, Zangen A, George MS (2014) Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci 1327: 79-93.
- Nadine Dougall, Nicola Maayan, Karla Soares-Weiser, et al. (2015) Transcranial magnetic stimulation for schizophrenia. Schizophrenia Bulletin 41: 1220-1222.
- Narayana S, Papanicolaou AC, McGregor A, et al. (2015) Clinical applications of transcranial magnetic stimulation in pediatric neurology. J Child Neurol 30: 1111-1124.
- Farzan F, Vernet M, Shafi MM, et al. (2016) Characterizing and modulating brain circuitry through transcranial magnetic stimulation combined with electroencephalography. Front Neural Circuits 10: 73.
- Cvetkovic D, Jovanov E, Cosic I (2006) Alterations in human EEG activity caused by extremely low frequency electromagnetic fields. Conf Proc IEEE Eng Med Biol Soc 1: 3206-3209.
- Hausser K, Tellschaft D, Thoss F (1997) Influence of an alternating 3Hz magnetic field with an induction of 0.1 mT on chosen parameters of the human occipital EEG. Neurosci Lett 239: 57-60.
- Shafiei SA, Firoozabadi SM, Rasoulzadeh TK (2012) Study of the frequency parameters of EEG influenced by zone-dependent local ELF-MF exposure on the human head. Electromagn Biol Med 31: 112-121.
- Shafiei SA, Firoozabadi SM, Tabatabaie KR, et al. (2014) Investigation of EEG changes during exposure to extremely low-frequency magnetic field to conduct brain signals. Neurol Sci 35: 1715-1721.
- Betti, Marco PCP, Marco Saettoni, et al. (2019) Ion cyclotron resonance: Results and prospects for psychiatry. J Psychiatry Treat res 1: 16-24.
- Greco A, Destefani. A (2018) Effects of non-focused Elf-Emf treatment on Hrv: Preliminary study. International Journal of Depression and Anxiety 1.
- Pravdich NVV (1913) Ein Versuch der Registrierung der elektrischen Gehirnerscheinungen (In German). Zbl Physiol 27: 951-960.
- Badcock NA, Mousikou P, Mahajan Y, et al. (2013) Validation of the emotiv EPOC(®) EEG gaming system for measuring research quality auditory ERPs. PeerJ 1: e38.
- Badcock NA, Preece KA, de Wit B, et al. (2015) Validation of the emotiv EPOC EEG system for research quality auditory event-related potentials in children. PeerJ 3: e907.
- Albertario CL, Zendell SM, Hertz G, et al. (1995) Comparison of a frequency-based analysis of electroencephalograms (Z-ratio) and visual scoring on the multiple sleep latency test. Sleep 18: 836-843.
- Li JP, Chen S, Peng H, et al. (2014) Pulsed electromagnetic fields protect the balance between adipogenesis and osteogenesis on steroid-induced osteonecrosis of femoral head at the pre-collapse stage in rats. Bioelectromagnetics 35: 170-180.
- Morabito C, Rovetta F, Bizzarri M, et al. (2010) Modulation of redox status and calcium handling by extremely low frequency electromagnetic fields in C2C12 muscle cells: A real-time, single-cell approach. Free Radic Biol Med. 48: 579-589.
- Rossi E, Corsetti MT, Sukkar S, et al. (2007) Extremely low frequency electromagnetic fields prevent chemotherapy induced myelotoxicity. Electromagn Biol Med 26: 277-281.
- Raggi F, Vallesi G, Rufini S, et al. (2008) ELF magnetic therapy and oxidative balance. Electromagn Biol Med 27: 325-339.
- Vallesi G, Raggi F, Rufini S, et al. (2007) Effects of cyclotronic ion resonance on human metabolic processes: A clinical trial and one case report. Electromagn Biol Med 26: 283-288.
- Rauš Balind S, Selaković V, Radenović L, et al. (2014) Extremely low frequency magnetic field (50 Hz, 0.5 mT) reduces oxidative stress in the brain of gerbils submitted to global cerebral ischemia. PLoS One 9: e88921.
- Das S, Kumar S, Jain S, et al. (2012) Exposure to ELF- magnetic field promotes restoration of sensori-motor functions in adult rats with hemisection of thoracic spinal cord. Electromagn Biol Med 31: 180-194.
- Zhong C, Zhao TF, Xu ZJ, et al. (2012) Effects of electromagnetic fields on bone regeneration in experimental and clinical studies: A review of the literature. Chin Med J (Engl) 125: 367-372.
- Sieron A, Labus Ł, Nowak P, et al. (2004) Alternating extremely low frequency magnetic field increases turnover of dopamine and serotonin in rat frontal cortex. Bioelectromagnetics 25: 426-430.
- Bao X, Shi Y, Huo X, et al. (2006) A possible involvement of b-endorphin, substance p, and serotonin in rat analgesia induced by extremely low frequency magnetic field. Bioelectromagnetics 27: 467-472.
- Başar E, Düzgün A (2016) The CLAIR model: Extension of brodmann's areas based on brain oscillations and connectivity. Int J Psychophysiol 103: 185-198.
- Alhola P, Polo-Kantola P (2007) Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat 3: 553-567.
- M Tervaniemia, T Ilvonen, K karma, et al. (1997) The musical brain: Brain waves reveal the neurophysiological basis of musicality in human subjects. Neuroscience Letters 226: 1-4.
- Cook IA, O'Hara R, Uijtdehaage SH, et al. (1998) Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr Clin Neurophysiol 107: 408-414.
- Vidal F, Burle B, Spieser L, et al. (2015) Linking EEG signals, brain functions and mental operations: Advantages of the Laplacian transformation. Int J Psychophysiol 97: 221-232.
- Duffy FH (1989) Clinical value of topographic mapping and quantified neurophysiology. Arch Neurol 46: 1133-1134.
- Thatcher R (2010) Validity and reliability of quantitative electroencephalography. Journal of Neurotherapy 14: 122-152.
- Thatcher R, Lubar J (2008) History of the scientific standards of QEEG normative databases. In Thomas Budzinsky, H Budzinski, J Evans, et al. Introduction to QEEG and neurofeedback: Advanced theory and applications, (2nd edn), Academic Press, San Diego, 29-62.
- Thatcher R, RA Walker, CJ Biver, et al. (2003) Sensitivity and specificity of an EEG normative data base: Validation and clinical correlation. J Neurotherapy 7: 87-121.
- Tramontano G (2006) QEEG testing can discern reason for cognitive disorder: Digital EEG recordings of brainwaves can determine TBI etiology. Connecticut Lawyer, 14-16.
- Nuwer M (1997) Assessment of digital EEG, quantitative EEG, and EEG brain mapping: Report of the american academy of neurology and the american clinical neurophysiology society. Neurology 49: 277-292.
- Juri DK (2009) Quantitative EEG, event-related potentials and neurotherapy.
- O'Keefe J, Recce ML (1993) Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3: 317-330.
- Bland BH, Oddie SD (2001) Theta band oscillation and synchrony in the hippocampal formation and associated structures: The case for its role in sensorimotor integration. Behav Brain Res 127: 119-136.
- Felzer T, Freisleben B (2003) Analyzing EEG signals using the probability estimating guarded neural classifier. IEEE Trans Neural Syst Rehabil Eng 11: 361-371.
- Santini R, Santini P, Seigne M, et al. (2001) Symptômes exprimés par des riverains de stations relais de téléphonie mobile. Presse Med 30: 1594.
- Lenartowicz A, Loo SK (2014) Use of EEG to diagnose ADHD. Curr Psychiatry Rep 16: 498.
- Al Zoubi O, Ahmad M, Aki T, et al. (2019) EEG microstates temporal dynamics differentiate individuals with mood and anxiety disorders from healthy subjects. Front Hum Neurosci 13: 56.
- Mo HM, Ryan AO, Kristianna BW, et al. (2019) Strong correlation of novel sleep electroencephalography coherence markers with diagnosis and severity of posttraumatic stress disorder. Sci Rep.
Corresponding Author
Alessandro Greco, Medical Director, APSP (Public Agency for Personal Health Services) Cles, Italy
Copyright
© 2019 Greco A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.