Modulation of Cadmium Induced Apoptotic, Cancer and Inflammation Related Cytokines by Diallyl Disulfide in Rat Liver Cells
Abstract
Cadmium (Cd), an environmental heavy metal pollutant, is one of the high risk factors for human diseases due to the exponential increase of its use in industrial processes and products during the last 50 years. It deposits mainly in the liver and leads to various diseases including cancer. Currently, there is a growing attention to explore natural compounds for the prevention of unfavorable effects of Cd in humans. Diallyl disulfide (DADS), an organosulfur compound in garlic is used as a prophylactic compound for various diseases in many countries. In this study, the modulatory effect of DADS against Cd toxicity was evaluated on the viability and cytokine proteins expression in cadmium chloride (CdCl2) treated normal rat liver CRL1439 cells. The liver cells were treated with CdCl2 for 24 h with or without DADS pre-treatment for 2 h and viability was measured using the crystal violet dye uptake assay. The cytokines protein expression was measured after 6 h of CdCl2 treatment using the RayBiotech human cytokine array 7 kit. The DADS pre-treatment dramatically increased the viability of CdCl2 treated cells to 52.0 ± 3.1% in comparison to CdCl2 alone treated cells (31.4 ± 2.2%). The CdCl2 treatment upregulated 8 inflammatory, 3 apoptosis, and 9 cancer pathway cytokines and downregulated 3 inflammatory pathway cytokines in liver cells. However, DADS pre-treatment attenuated all the cytokines except two cytokines affected by the CdCl2 treatment. The present study clearly demonstrated the protective effect of DADS against Cd toxicity through the modulation of cytokines expression in the CdCl2 treated liver cells.
Keywords
Cadmium chloride, Liver cells, Diallyl disulfide, Viability, Cytokines
Abbreviations
Cd: Cadmium; CdCl2: Cadmium chloride; DADS: Diallyl disulfide; IL: Interleukin
Introduction
Cadmium (Cd) is one of the economically valuable heavy metals utilized in a variety of products that are used in our daily life [1]. On the other hand, it causes a serious concern to both animal and human's health worldwide as an environmental pollutant [2,3]. Large quantities of Cd compounds are discharged into the environment from industrial activities and fossil fuels as industrial waste into the water, air, and soil leading to contamination of edible plants and seafood [4,5]. Studies have reported that two thirds of the Cd exposure through the diet is attributed to contaminated vegetables and one third to animal products [6]. Thus, ingestible food and water are the major sources of Cd exposure for nonsmokers with the Cd intake from food and water estimated between 50 to 100 µg per day. Previous report showed that the estimated weekly intake value for Cd via rice consumption even ranged from 20 to 82 µg Cd per kg bodyweight [7]. The foods with highest Cd concentration are shellfish, offal products, and certain seeds, but the most common sources of dietary Cd exposure are cereals, potatoes, root crops, and vegetables (around 80%).
Upon absorption, Cd forms complexes with biomolecules and produces toxicity in various organs. Cd accumulates mostly in the liver due to its role in metal homeostasis and detoxification via the first bypass. Analyses of mammalian organs have shown more Cd accumulation in liver and kidney than in other parts of the body [5,8]. It was reported that environmental Cd exposure was associated with hepatic necro-inflammation in both men and women [9]. In the same study, it was reported that individuals in the top quartile of creatinine-corrected urinary Cd had over a threefold increased risk of liver disease mortality. The liver injury is characterized by increased serum levels of hepatic transaminases and massive necrosis of hepatocytes [10]. In addition to the induction of necrosis, cadmium has been reported to induce apoptosis in liver cells [11]. Our previous reports showed that Cd induce cytokines which play an important role in Cd-induced toxicity in lung cells [12-14].
Although the biological system protects itself by inducing the expression of antioxidant proteins to a certain extent, in the event of Cd accumulation in organs, the use of exogenous antioxidants or metal chelators provides additional protection against Cd induced oxidative stress [15,16]. The search for effective, nontoxic, natural compounds with antioxidant activity against Cd toxicity has been intensified in recent years [17,18]. Garlic (Allium sativum) has been used as a conventional food, in herbal therapy and in folk medicine in all parts of the world. It contains both water- and lipid-soluble organosulfur compounds (OSC) which have been reported to be responsible for its therapeutic properties. Diallyl disulfide (DADS) is one of the major organo-sulfur compounds in garlic and is highly stable and lipid-soluble [19]. However, studies related to cytokines expression in the liver during Cd-induced toxicity and the modulatory effects of natural compounds on Cd-induced inflammation are limited [20,21]. In this report, we evaluated the modulatory effect of DADS on Cd toxicity through viability studies and the study of the expression of a panel of 60 cytokine proteins which are involved in apoptosis, cancer and inflammation pathways in normal rat liver CRL 1439 epithelial cells cultured in vitro.
Materials and Methods
Reagents
F12K medium (1x), penicillin-streptomycin antibiotic solution (100x), amphotericin, fetal bovine serum (FBS), trypsin-EDTA solution (1x), phosphate buffered saline (PBS), cadmium chloride, 25% glutaraldehyde, diallyl disulfide (DADS) and crystal violet were purchased from Sigma-Aldrich company (St. Louis, MO, USA). The Human cytokine array 7 kit was purchased from RayBiotech, Inc. (Norcross, GA, USA).
Maintenance of cell line
A normal rat liver (catalog number CRL 1439) epithelial cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The supplied frozen cells were cultured according to ATCC instructions. In brief, the cells were grown in 10 ml of F12K medium containing 100 units of penicillin per ml, 100 µg of streptomycin per ml, 0.025 µg of amphotericin B per ml, and 10% FBS in T-75 cm2 tissue culture flasks at 37 ℃ in a 5% CO2 incubator (Nuaire Inc, Plymouth, MN, USA).
Treatment of cells
To investigate the modulatory effect of DADS against Cd toxicity in CdCl2 treated liver cells, 1 × 105 cells/well were seeded into 24 well tissue culture plate in 800 µl of complete medium and incubated overnight in a 5% CO2 incubator at 37 ℃ to attain stabilization. Following the stabilization, the cells were treated with CdCl2 (0 or 150 µM [27.45 ppm]) and DADS (150 µM) in a final volume of 1 ml in triplicate wells and incubated for 24 h at 37 ℃ in a 5% CO2 incubator. In the co-treatment group, cells were pre-treated with DADS for 2 h prior to the treatment with CdCl2. Cells incubated with only culture medium without CdCl2 or DADS served as the control cells.
Cell viability assay
The viability of the cells was evaluated using the crystal violet dye uptake assay as reported earlier [22]. In brief, at the end of the treatment period, glutaraldehyde (400 µl of 0.25% to make 0.07% final concentration in the well) was added to each well and incubated for 30 min at room temperature (RT) to fix the viable cells. Following this, the plates were rinsed with water to wash off the dead cells and dried under airflow inside the laminar hood for 10 min. The crystal violet solution (400 µl of 0.1%) was added to each well and incubated for 15 min, followed by several washes and dried for 10 min. The dye was solubilized in each well with the addition of 1 ml of 0.05 M sodium phosphate solution (monobasic) in 50% ethyl alcohol. The culture plates were read at 540 nm in a plate reader (Bio-Tek EL800 Plate Reader). The mean O.D. value of the control cells was taken as 100% and the other treated groups were calculated as a percent of the control.
Preparation of samples for cytokine array
To study the cytokines' protein expression of the CdCl2 treated liver cells and the modulatory effect of DADS, approximately 3.9 × 106 cells were plated in T-75 cm2 flasks in complete F12K medium and then were allowed to stabilize overnight. The cells were then treated with CdCl2 alone (0, 150 µM) or co-treated with 150 µM DADS and 150 µM CdCl2 in triplicate T-75 cm2 flasks. In co-treatment group, cells were pre-treated with 150 µM DADS for 2 h prior to treatment with CdCl2. The flasks were incubated for 6 h only at 37 ℃ in a 5% CO2 incubator to study the cytokines' expression at earlier time point. At the end of the incubation period, the cells were trypsinized, pooled together and pelleted by centrifuging at 2,500 RPM for 5 min. The cell pellet was suspended in 1 ml of 1x cell lysis buffer (from the Cytokine Array Kit) and lysed by homogenization in a vial under ice for 15 s (3x) using a Polytron homogenizer. The homogenate was transferred to an eppendorff tube and centrifuged at 10,000 RPM for 10 min at 4 ℃ to remove the lysed cell membrane debris. The supernatant was transferred to fresh tube and this cell lysate was used for cytokine array analysis or stored at -80 ℃ for future analysis.
Protein estimation
The protein concentration was determined using the Pierce Bicinchoninic Acid (BCA) Assay (Thermo-Scientific Company, Rockford, IL, USA). The diluted albumin (BSA) standards and working reagent were prepared according to the kit instructions. Different concentrations of each standard and each unknown sample (25 µl) was pipetted in triplicate into appropriately labeled eppendorff tubes with 500 µl of working reagent and vortexed to mix. The tubes were incubated at 37 ℃ for 30 min and then read at 562 nm in a Beckman spectrophotometer. From the standard curve, the protein concentrations of cell lysates were determined.
Cytokine array analysis
The cytokines' protein expression was determined in cell lysate using Ray Biotech's Human Cytokine Antibody Array 7 kit (catalog # AAH-CYT-7). The array study was carried out according to manual instructions with minor changes. The membranes were blocked for 30 min and then hybridized with 400 µg of cell lysate protein (total volume 1 ml) for 2 h at RT. The membranes were washed with buffer I (3x) and buffer II (2x) for 5 min. Following this, the membranes were incubated with biotin-conjugated primary antibodies for 2 h at RT and then washed. Furthermore, the membranes were incubated with the HRP-conjugated streptavidin secondary antibodies at RT for 2 h, washed and finally incubated with detection buffer for 5 min for development. The chemiluminiscence of the arrays was then detected using Alpha Innotech's FluorChem FC2 machine and was analyzed by Alpha Ease FC software.
Statistical analysis
The viability data were presented as mean ± standard deviation (SD, n = 3). All treated cells data were presented as percentage values in comparison to the untreated control (100%). The data were analyzed for significance by one-way ANOVA, and then compared by Tukey's multiple comparison tests, using the GraphPad Prism Software, version 3.00 (GraphPad Software, Inc., San Diego, CA, USA). Differences with the respective untreated control were considered statistically significant when p < 0.05. For cytokine array, statistical analyses were performed using Minitab and Microsoft Excel. For pairwise comparisons, we performed both one-tailed and two-tailed two-sample hypothesis tests for the difference in the mean intensity values amongst corresponding cytokines exposed to cadmium (Cd) versus the control baseline cytokines and corresponding cytokines exposed to Cd and DADS (Cd + DADS) versus the control baseline cytokines. All Two-Sample Hypothesis tests were performed at the (α = 0.05) level of significance. Here, a statistical significance results in P-value < 0.05; thus, yielding a rejection of the null hypothesis in favor of the alternative hypothesis [23].
Results
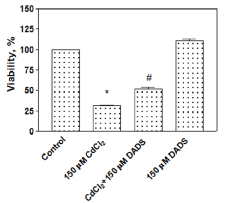
Modulatory effect of DADS on the viability of CdCl2 treated rat liver cells
The modulatory effect of DADS against Cd toxicity was examined through the viability assay and the results are shown in Figure 1. The cells treated with 150 µM CdCl2 alone showed a significant decrease (31.4 ± 2.2%) (P < 0.05) in cell viability, in comparison to untreated control cells (100%). However, the viability of the cells pre-treated with DADS (150 µM) for 2 h followed by 150 µM CdCl2 for 24 h was increased to 52.0 ± 3.1% (P < 0.05) in comparison to the cells treated with 150 µM CdCl2 alone for the same duration. Furthermore, cells treated with 150 µM DADS alone did not show toxicity (110.9 ± 3.9%) rather it slightly promoted cell proliferation (Figure 1). The viability data clearly showed a significant modulatory effect of DADS against Cd toxicity in rat liver cells.
Modulation of DADS on cytokines expression in CdCl2 treated rat liver cells
The cytokines' protein expression of cells treated with CdCl2 alone, DADS alone and a combination of CdCl2 and DADS in normal rat liver epithelial cells was examined and compared with the cytokines' expression of untreated control cells using Ray Biotech Cytokine Array 7. The upregulation is defined as the expression level that is 30% above the control cells expression, while downregulation is defined as the expression level 30% below the control cells expression (based on both one and two tailed tests). The regulated cytokines were grouped according to the pathway in which they function namely apoptosis, cancer or inflammation.
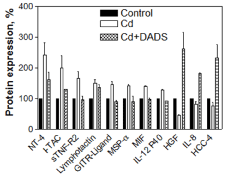
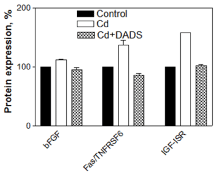
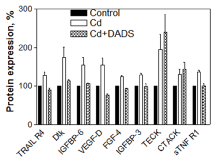
In the CdCl2 alone treated cells, 8 inflammatory, 3 apoptosis and 9 cancer pathway cytokines were upregulated, while 3 inflammatory pathway cytokines were downregulated. The upregulated cytokines involved in inflammation pathway were: NT-4, I-TAC, sTNF-R2, Lymphotactin, GITR-LIGAND, MSP-α, MIF, and IL-12 P40 (Figure 2); in apoptosis pathway: bFGF, IGF-1R, and Fas/TNFRSF6/APO (Figure 3); and in cancer pathway: TRAIL-R4, dtk, IGFBP6, VEGF-D, FGF-4, IGFBP-3, TECK, CTACK, and sTNF R1 (Figure 4). The downregulated cytokines involved in inflammatory pathway were: Hepatocyte growth factor (HGF), IL-8 and HCC-4 (Figure 2).
The pre-treatment of the cells with 150 µM DADS for 2 h prior to CdCl2 treatment resulted in the downregulation of 8 inflammatory, 3 apoptosis and 7 cancer cytokines that were involved in the pathways in comparison to cells treated with CdCl2 alone (Figure 2, Figure 3 and Figure 4). However, the TECK and CTACK cytokines involved in inflammation were more upregulated in the pre-treated cells than cells treated with CdCl2 alone (Figure 2). Furthermore, downregulated inflammatory cytokines (HGF, IL-8 and HCC-4) in CdCl2 alone treated cells were significantly up-regulated when pretreated with DADS (Figure 2). Thus, the results clearly showed a significant toxic effect of Cd on the cytokines expression involved in inflammation, apoptosis, and cancer pathways, while DADS pre-treatment modulated the Cd-induced cytokines in the liver epithelial cells.
Discussion
In this report, the modulatory effect of DADS was studied on the viability and cytokines protein expression in CdCl2 treated liver cells. We treated the cells with 150 µM CdCl2 based on our previous reports [15,16]. In our preliminary experiments, we used different concentrations of DADS for protection against Cd toxicity and 150 µM DADS was used in this study since higher concentrations showed toxicity (data not shown). The viability of the cells was measured using the cost-effective and reproducible crystal violet dye uptake assay [22]. The viability results clearly showed that CdCl2 significantly reduced the viability of liver cells (Figure 1, Lane 2). The pre-treatment of cells with DADS for 2 h protected the cells against cadmium toxicity (Figure 1, Lane 3) and, DADS alone did not show any toxicity in the liver cells (Figure 1, Lane 4).
Further, we studied the effect of Cd on the cytokines expression and modulatory effect of DADS on cytokines expression using cytokine array 7 analysis. The cytokines expression study was carried out at a shorter period (6 h) for clearer understanding of the cytokines expressed in cells exposed to CdCl2 within the period showing no cell death. Result from our previous study had shown no cell death at 8 h with the same CdCl2 concentration [22]. We studied the cytokine proteins expression in the cell lysate rather than the medium supernatant. Even though cytokines are secreted into the medium, they are diluted into the medium and hard to detect them in the cultured medium. The expressed cytokines from the cells were grouped according to their function in the cell pathological pathways namely; inflammation, apoptosis or cancer. The result from the cytokine array analysis of CdCl2 alone treated cells for 6 h resulted in upregulation (NT-4, I-TAC, sTNF-R2, Lymphotactin, GITR-LIGAND, MSP-α, MIF, and IL-12 P40) and downregulation (hepatocyte growth factor (HGF), IL-8 and HCC-4 cytokines) of certain cytokines involved in inflammatory pathway (Figure 2). The other previous studies also have ascertain the involvement of the observed cytokines in our study such as NT-4 [24], I-TAC [25], sTNF-R2 [26], Lymphotactin [27], GITR-LIGAND [28], MSP-α [29], MIF [30], IL-12 P40 [31], HGF [32], IL-8 [33], HCC-4 [34] as cytokines involve in the inflammation process. Previous reports showed the Cd-induced inflammation and mitigation through anti-inflammatory compounds [35,36].
In addition to the inflammatory cytokines, another set of upregulated cytokines (bFGF, IGF-1R and Fas/TNFRSF6/APO-1 proteins) observed in CdCl2 alone treated cells were involved in apoptotic pathway. Involvement of these cytokines in apoptosis has been investigated and confirmed in these studies: bFGF [37], IGF-1R [38], Fas/TNFRSF6/APO-1 [39]. Previous reports revealed that apoptosis plays an important role in Cd induced cytotoxicity and compounds which can prevent apoptosis can protect the cells against Cd toxicity [40,41].
The last set of upregulated cytokines in CdCl2 alone treated cells indicated involvement in cancer pathway and they were TRAIL-R4, dtk, IGFBP6, VEGF-D, FGF-4, IGFBP-3, TECK (also known as CCL25), CTACK (also known as CCL27), and sTNF R1. In support of our findings, earlier studies have also shown the involvement of cytokines such as TRAIL-R4 [42], Dtk [43], IGFBP6 [44], VEGF-D [45], FGF-4 [46], IGFBP-3 [47], TECK [48], CTACK [49], and sTNF R1 [50] in cancer development or progression. Several reports showed that Cd exposure causes cancer in the liver [9,51,52].
The analysis of the cytokines' protein expression revealed reduction in expression of cytokines in the cells pre-treated with DADS for 2 h prior to Cd treatment except for HGF, IL-8 and HCC-4 in inflammatory pathway and TECK and CTACK in cancer pathway in comparison to the level of cytokine protein expression observed in cells treated with CdCl2 alone (Figure 2, Figure 3 and Figure 4). The modulation of cytokines by DADS pre-treatment protects the cells from Cd toxicity which is reflected in the increase of cell viability in DADS pre-treated cells (Figure 1). The result observed in this study was supported by an earlier report which demonstrated that DADS reduce cancer [53], inflammation through regulation of pro-inflammatory cytokines production by inhibiting NF-κB and MAPKs expressions in cyclophosphamide treated Rats [54] and attenuate radiation-induced apoptosis [55]. Furthermore, other studies have also reported that sulfhydryl groups in garlic are very potent in reducing the Cd-induced hepatotoxicity in rats. In addition to diallyl disulfide, another sulfhydryl compound in garlic that has been shown to confer protection against Cd toxicity in rat liver is diallyl tetrasulfide. These species of sulfhydryl groups have been unequivocally demonstrated to have protective effect(s) against Cd toxicity through increase in anti-oxidant potential [59]. Our study clearly demonstrates the modulatory effect of the diallyl disulfide compound against Cd toxicity in rat liver epithelial cells through the regulation of cytokines expression which are responsible for inflammation, apoptosis and cancer pathways.
In conclusion, this report showed that pre-treatment of cells with DADS compound exhibited protective effect against Cd toxicity through modulation of cytokine proteins expression that resulted in increased viability in CdCl2 treated rat liver epithelial cells. This study suggests that use of a natural garlic compound can prevent the toxic effects of Cd in liver cells by modulating the cytokines' expression which in turn prevents cells from entering the inflammatory pathway. In addition, DADS, a major organosulfur compound in garlic has sulfhydryl groups that makes DADS a chelating agent of Cd.
Acknowledgements
We sincerely acknowledge the financial support of FAMU Title III, Dept. of Education (DOEHBGIPO31B4010808), RCMI, National Institute of Health (G12RR03020, G12D007582) for this research work.
Conflict of Interest
The authors have no competing interests.
References
- Faroon O, Ashizawa A, Wright S, et al. (2012) Toxicological profile for cadmium. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US).
- Rashid K, Sinha K, Sil PC (2013) An update on oxidative stress-mediated organ pathophysiology. Food Chem Toxicol 62: 584-600.
- Pan J, Plant JA, Voulvoulis N, et al. (2010) Cadmium levels in Europe: Implications for human health. Environ Geochem Health 32: 1-12.
- Kohrman H, Chamberlain CP (2014) Heavy metals in produce from urban farms in the San Francisco Bay Area. Food Addit Contam Part B Surveill 7: 127-134.
- Järup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238: 201-208.
- Nasreddine L, Nashalian O, Naja F, et al. (2010) Dietary exposure to essential and toxic trace elements from a Total diet study in an adult Lebanese urban population. Food Chem Toxicol 48: 1262-1269.
- Simmons RW, Pongsakul P, Saiyasitpanich D, et al. (2005) Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: Implications for public health. Environ Geochem Health 27: 501-511.
- Guirlet E, Das K (2012) Cadmium toxicokinetics and bioaccumulation in turtles: Trophic exposure of Trachemys scripta elegans. Ecotoxicology 21: 18-26.
- Hyder O, Chung M, Cosgrove D, et al. (2013) Cadmium exposure and liver disease among US adults. J Gastrointest Surg 17: 1265-1273.
- Ramamurthy CH, Subastri A, Suyavaran A, et al. (2016) Solanum torvum Swartz. fruit attenuates cadmium-induced liver and kidney damage through modulation of oxidative stress and glycosylation. Environ Sci Pollut Res Int 23: 7919-7929.
- Zou H, Zhuo L, Han T, et al. (2015) Autophagy and gap junctional intercellular communication inhibition are involved in cadmium-induced apoptosis in rat liver cells. Biochem Biophys Res Commun 459: 713-719.
- Odewumi CO, Latinwo LM, Ruden ML, et al. (2016) Modulation of cytokines and chemokines expression by NAC in cadmium chloride treated human lung cells. Environ Toxicol 31: 1612-1619.
- Odewumi CO, Fils-Aime S, Badisa VL, et al. (2015) Chemoprotective effect of monoisoamyl 2,3-dimercaptosuccinate (MiADMS) on cytokines expression in cadmium chloride treated human lung cells. Environ Toxicol 30: 704-711.
- Odewumi C, Latinwo LM, Sinclair A, et al. (2015) Effect of cadmium on the expression levels of interleukin-1α and interleukin-10 cytokines in human lung cells. Mol Med Rep 12: 6422-6426.
- Odewumi CO, Badisa VL, Le UT, et al. (2011) Protective effects of N-acetylcysteine against cadmium-induced damage in cultured rat normal liver cells. Int J Mol Med 27: 243-248.
- Odewumi CO, Buggs R, Badisa VL, et al. (2011) Mitigative action of monoisoamyl-2,3-dimercaptosuccinate (MiADMS) against cadmium-induced damage in cultured rat normal liver cells. Toxicol In Vitro 25: 1733-1739.
- White PA, Oliveira RC, Oliveira AP, et al. (2014) Antioxidant activity and mechanisms of action of natural compounds isolated from lichens: A systematic review. Molecules 19: 14496-14527.
- Tapia E, Zatarain-Barrón ZL, Hernández-Pando R, et al. (2013) Curcumin reverses glomerular hemodynamic alterations and oxidant stress in 5/6 nephrectomized rats. Phytomedicine 20: 359-366.
- Kay HY, Yang JW, Kim TH, et al. (2010) Ajoene, a stable garlic by-product, has an antioxidant effect through Nrf2-Mediated glutamate-cysteine ligase induction in HepG2 cells and primary hepatocytes. J Nutr 140: 1211-1219.
- Okoko T, Ere D (2013) Some bioactive potentials of two biflavanols isolated from Garcinia kola on cadmium-induced alterations of raw U937 cells and U937-derived macrophages. Asian Pac J Trop Med 6: 43-48.
- Lee J, Lim KT (2011) Inhibitory effect of plant-originated glycoprotein (27 kDa) on expression of matrix metalloproteinase-9 in cadmium chloride-induced BNL CL.2 cells. J Trace Elem Med Biol 25: 239-246.
- Badisa VL, Latinwo LM, Odewumi CO, et al. (2008) Cytotoxicity and stress gene microarray analysis in cadmium-exposed CRL-1439 normal rat liver cells. Int J Mol Med 22: 213-219.
- Mann T, Sherman D, Updegraff J (2004) Dispositional motivations and message framing: A test of the congruency hypothesis. Health Psychol 23: 330-334.
- Nassenstein C, Braun A, Erpenbeck VJ, et al. (2003) The neurotrophins nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 are survival and activation factors for eosinophils in patients with allergic bronchial asthma. J Exp Med 198: 455-467.
- Helbig KJ, Ruszkiewicz A, Semendric L, et al. (2004) Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology 39: 1220-1229.
- Hashikata A, Yamashita A, Suzuki S, et al. (2014) The inflammation-lipocalin 2 axis may contribute to the development of chronic kidney disease. Nephrol Dial Transplant 29: 611-618.
- Yeh PT, Lin FA, Lin CP, et al. (2010) Expressions of lymphotactin and its receptor, XCR, in Lewis rats with experimental autoimmune anterior uveitis. Graefes Arch Clin Exp Ophthalmol 248: 1737-1747.
- Kamimura Y, Iwai H, Piao J, et al. (2009) The glucocorticoid-induced TNF receptor-related protein (GITR)-GITR ligand pathway acts as a mediator of cutaneous dendritic cell migration and promotes T cell-mediated acquired immunity. J Immunol 182: 2708-2716.
- Li J, Chanda D, van Gorp PJ, et al. (2016) Macrophage stimulating protein enhances hepatic inflammation in a NASH model. PLoS One 11: e0163843.
- Oikonomidi A, Tautvydaitė D, Gholamrezaee MM, et al. (2017) Macrophage migration inhibitory factor is associated with biomarkers of alzheimer's disease pathology and predicts cognitive decline in mild cognitive impairment and mild dementia. J Alzheimers Dis 60: 273-281.
- Bedrossian N, Haidar M, Fares J, et al. (2016) Inflammation and elevation of Interleukin-12p40 in patients with schizophrenia. Front Mol Neurosci 9: 16.
- Cao XP, Han DM, Zhao L, et al. (2016) Hepatocyte growth factor enhances the inflammation-alleviating effect of umbilical cord-derived mesenchymal stromal cells in a bronchiolitis obliterans model. Cytotherapy 18: 402-412.
- Berger KI, Pradhan DR, Goldring RM, et al. (2016) Distal airway dysfunction identifies pulmonary inflammation in asymptomatic smokers. ERJ Open Res 2.
- Arakelyan A, Kriegova E, Kubistova Z, et al. (2009) Protein levels of CC chemokine ligand (CCL)15, CCL16 and macrophage stimulating protein in patients with sarcoidosis. Clin Exp Immunol 155: 457-465.
- Colacino JA, Arthur AE, Ferguson KK, et al. (2014) Dietary antioxidant and anti-inflammatory intake modifies the effect of cadmium exposure on markers of systemic inflammation and oxidative stress. Environ Res 131: 6-12.
- Zhang W, Zhi J, Cui Y, et al. (2014) Potentiated interaction between ineffective doses of budesonide and formoterol to control the inhaled cadmium-induced up-regulation of metalloproteinases and acute pulmonary inflammation in rats. PLoS One 9: e109136.
- Akasaka Y, Ono I, Kamiya T, et al. (2010) The mechanisms underlying fibroblast apoptosis regulated by growth factors during wound healing. J Pathol 221: 285-299.
- Xiong L, Kou F, Yang Y, et al. (2007) A novel role for IGF-1R in p53-mediated apoptosis through translational modulation of the p53-Mdm2 feedback loop. J Cell Biol 178: 995-1007
- Santourlidis S, Warskulat U, Florl AR, et al. (2001) Hypermethylation of the tumor necrosis factor receptor superfamily 6 (APT1, Fas, CD95/Apo-1) gene promoter at rel/nuclear factor kappaB sites in prostatic carcinoma. Mol Carcinog 32: 36-43.
- Ben P, Zhang Z, Xuan C, et al. (2015) Protective effect of L-Theanine on cadmium-induced apoptosis in PC12 cells by inhibiting the mitochondria-mediated pathway. Neurochem Res 40: 1661-1670.
- Panjehpour M, Alaie SH (2014) N-acetylcysteine prevents cadmium-induced apoptosis in human breast cancer MDA-MB468 cell line. Cell Mol Biol (Noisy-le-grand) 60: 33-38.
- Lalaoui N, MorlÉ A, MÉrino D, et al. (2011) TRAIL-R4 promotes tumor growth and resistance to apoptosis in cervical carcinoma HeLa cells through AKT. PLoS One 6: e19679.
- Gronowitz JS, Hagberg H, Källander CF, et al. (1983) The use of serum deoxythymidine kinase as a prognostic marker, and in the monitoring of patients with non-Hodgkin's lymphoma. Br J Cancer 47: 487-495.
- Bach LA, Fu P, Yang Z (2013) Insulin-like growth factor-binding protein-6 and cancer. Clin Sci (Lond) 124: 215-229.
- He L, He J, Zhao X (2016) Expression of VEGF-D in epithelial ovarian cancer and its relationship to lymphatic metastasis. Asia Pac J Clin Oncol 12: e161-e166.
- Hajitou A, Baramova EN, Bajou K, et al. (1998) FGF-3 and FGF-4 elicit distinct oncogenic properties in mouse mammary myoepithelial cells. Oncogene 17: 2059-2071.
- Kim SY, Kim MS, Lee MK, et al. (2015) PPARγ induces growth inhibition and apoptosis through upregulation of insulin-like growth factor-binding protein-3 in gastric cancer cells. Braz J Med Biol Res 48: 226-233.
- Zhang Z, Sun T, Chen Y, et al. (2016) CCL25/CCR9 Signal promotes migration and invasion in hepatocellular and breast cancer cell lines. DNA Cell Biol 35: 348-357.
- Kai H, Kadono T, Kakinuma T, et al. (2011) CCR10 and CCL27 are overexpressed in cutaneous squamous cell carcinoma. Pathol Res Pract 207: 43-48.
- Jablonska E (1997) Release of soluble IL-6 receptor (IL-6sR) in comparison with release of soluble TNF receptors (sTNF-Rs) by PMNs and WBC derived from breast cancer patients. Cancer Lett 119: 79-85.
- Kolachi NF, Kazi TG, Afridi HI, et al. (2012) Investigation of essential trace and toxic elements in biological samples (blood, serum and scalp hair) of liver cirrhotic/cancer female patients before and after mineral supplementation. Clin Nutr 31: 967-973.
- Satarug S (2012) Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma. Curr Drug Metab 13: 257-271.
- Iciek M, Marcinek J, Mleczko U, et al. (2007) Selective effects of diallyl disulfide, a sulfane sulfur precursor, in the liver and Ehrlich ascites tumor cells. Eur J Pharmacol 569: 1-7.
- Kim SH, Lee IC, Ko JW, et al. (2015) Diallyl disulfide prevents cyclophosphamide-induced hemorrhagic cystitis in rats through the inhibition of oxidative damage, MAPKs, and NF-κB pathways. Biomol Ther (Seoul) 23: 180-188.
- Di CX, Han L, Zhang H, et al. (2015) Diallyl disulfide attenuated carbon ion irradiation-induced apoptosis in mouse testis through changing the ratio of Tap73/ΔNp73 via mitochondrial pathway. Sci Rep 5: 16020.
- Nwokocha CR, Owu DU, Nwokocha MI, et al. (2012) Comparative study on the efficacy of Allium sativum (garlic) in reducing some heavy metal accumulation in liver of wistar rats. Food Chem Toxicol 50: 222-226.
- Lawal AO, Ellis EM (2011) The chemopreventive effects of aged garlic extract against cadmium-induced toxicity. Environ Toxicol Pharmacol 32: 266-274.
- Obioha UE, Suru SM, Ola-Mudathir KF, et al. (2009) Hepatoprotective potentials of onion and garlic extracts on cadmium-induced oxidative damage in rats. Biol Trace Elem Res 129: 143-156.
- Murugavel P, Pari L (2007) Effects of diallyl tetra sulfide on cadmium-induced oxidative damage in the liver of rats. Hum Exp Toxicol 26: 527-534.
Corresponding Author
Dr. Caroline Odewumi, Department of Biological Sciences, Florida A&M University, 1530 SMLK Boulevard, 501 Jones Hall, Tallahassee, FL 32307, USA.
Copyright
© 2018 Odewumi C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.