Hormonal Pathology of the Endometrium and Endometrial Carcinoma
Introduction
Numerous health issues of women are related to hormonal anomalies including sexual development, fertility, menopause and benign and malignant gynecologic tumors.
Endometrial cancer is the most common gynecologic cancer in the USA, with over 66,570 new cases and 13,000 deaths due to the disease, estimated for 2021. Worldwide, mostly in the industrialized countries, the incidence of this neoplasm has risen by about 20% over the two last decades, with over 380.000 new cases predicted for 2021, while other gynecologic malignancies such as uterine cervical cancer have decreased in spectacular proportions.
Hormone therapy is used to improve some physiological effects and to counteract abnormal and deleterious effects of natural hormonal activity. Millions of women all over the world receive hormone therapy at some point of their life such as for young women oral contraceptives, hormonal components in the treatment of infertility, for peri- and postmenopausal women hormone therapy for menopausal symptoms, and hormone mostly adjuvant therapy for benign and malignant gynecologic tumors. Patients with congenital sexual abnormalities as well as persons with gender dysphoria are also receiving hormone therapy.
The structural and physiological effects of hormones include a large spectrum of changes in the uterus, especially in the endometrium. The effect of hormone therapy depends on the duration and nature of the therapeutic agents which are permanently changing due to new pharmaceutical products, new technology as well as changing concepts of therapy.
Endometrial cancers are often causally related to abnormal hormonal stimulation. The carcinogenic potential of estrogenic stimulation and their mitogenic effect are extensively studied but still incompletely understood. The diagnostic interpretation of the commonly taken endometrial biopsies is often difficult, and some therapeutic regimens are controversial. This article includes the description of relevant aspects of endometrial physiopathology; the effects of the most commonly used hormonal therapy and an overview of endometrial carcinogenesis, its diagnosis and therapy.
Hormonal Physiopathology of the Uterus
The uterine tissue is exquisitely sensitive to hormonal influences. It is capable to translate them into functional and structural changes with promptitude and versatility. The complex interplay between pituitary and gonadal functions mediated by a finely tuned feedback mechanism, functionally related to the central nervous system, reaches its target in the uterine tissue. Its ultimate goal is to secure the reproductive role of the uterus. Normal reproduction is based on the cyclic changes taking place in the entire female genital system, and most importantly in the endometrial inner layer of the uterus, a highly differentiated and diversified tissue composed chiefly of glands, stroma and blood vessels. The programming of events in the endometrium is regulated by the presence of receptors, essential to the transfers and translocation of the hormones secreted into the blood stream, and their translation into structural changes at the target tissue level. The menstrual cycle is composed of a preovulatory phase with proliferative endometrium and a post-ovulatory phase with secretory and predecidual endometrium, a potential host for an implanting conceptus. If no fertilized ovum is implanted the endometrium is shedding and a new cycle starts. The most commonly involved gonad-secreted hormones are Estrogens and Progesterone which bound to receptors, forming hormone-receptor complexes. The activated steroid-receptor functions as a transcription factor modulating the specific synthesis of a specific mRNA responsible for the cellular action of the hormone. The receptor-bound hormone is transported into the cellular compartments during normal menstrual cycles, eliciting proliferation and secretion, followed by menstrual shedding in normal menstrual cycles without fertilization [1].
The biologic activity of the steroid hormones is maintained only while the nuclear site is occupied by the hormone- receptor complex, thus duration of exposure and concentration are related to effect. The decrease or absence of estrogenic hormones eventually results in atrophy of the target endometrial tissue, while the excess of the same may produce irregular patterns of proliferation, hyperplasia and neoplasia. Clinically, abnormal hormonal stimulation may be associated with infertility, abnormal vaginal bleeding (prolonged, reduced or irregular), as well as with abnormal benign tissue growth such as leiomyomas, adenomyosis or endometrial polyps or neoplastic changes; peri- and postmenopausal systemic disorders are frequently related to decrease of steroid hormone secretion.
Hormone Therapy Effects on the Uterus
Hormonal therapy is used to counteract the deleterious effects of "natural", non-iatrogenic hormonal effects. Hormone therapy is now widely prescribed, being used by millions of women all over the world. It actually seems that most women use some hormone therapy at least at some point of their life.
Adolescents and young women use oral contraceptives containing predominantly Progesterone, sometime also prescribed for irregular vaginal bleeding. The endometrial tissue undergoes "pseudogestational" changes, with secretory and atrophic glands and decidualized stroma [2].
Steroid ovarian and pituitary hormones are used to treat infertility associated with hormonal inadequacy or included in ovulation stimulation used by reproductive technology. The goal is the creation of normal ovulatory endometrial menstrual cycles, with well-developed proliferative and secretory endometrium [3].
Postmenopausal symptoms are frequently treated with hormone replacement therapy (HRT), despite controversial data on their safety and efficiency. Regimens consist presently of the administration of Estrogens and Progesterone combined in variable dosages; the regimens are permanently shifting due to changing concepts and new pharmaceutical products. Currently the HRT recommended by the Guidelines from the North American Menopause Society consists of transdermal estradiol and micronized Progesterone [4]. The endometrial tissue responds by mostly developing simultaneous proliferative and secretory changes, occasionally hyperplasia and in relatively rare cases endometrial neoplasia. Endometrial hyperplasia and carcinoma may occasionally develop, especially in HRT without Progesterone, as some patients do not tolerate Progesterone side effects. Interpretation of endometrial biopsies may be sometimes difficult as neoplastic changes may be masquerading with decidual pseudo gestational changes [5].
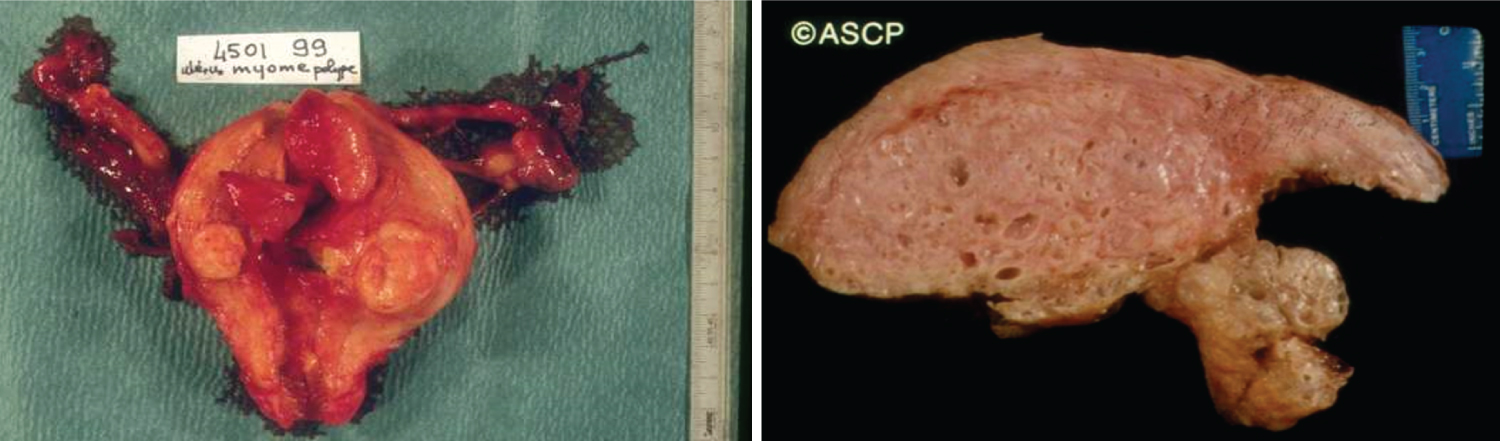
Common benign uterine tumors such as myometrial leiomyomas, adenomyomas, and endometrial hyperplastic polyps benefit from hormone therapy with Progesterone, in addition to surgical resection, by inhibiting and possibly reverting the proliferative myometrial changes. Leiomyomas (fibroids) are caused by the estrogenic effect on the uterine smooth muscle tissue, growing during the reproductive period and shrinking with menopause. This is also possible with hormone therapy consisting of Gonadotropin-releasing (GnRH) analogues (Lupron), reducing considerably, but reversibly, the size of the leiomyomas by inducing an iatrogenic reversible temporary menopause [6].
The management of gender dysphoria includes a plethora of approaches, from hormone therapy and surgical interventions to psychotherapy; nevertheless, primary medical intervention for gender affirmation is hormonal therapy. With estrogens and antiandrogens or testosterone. This treatment is generally safe but can be irreversible, hence updated guidelines of the Endocrine Society on transgender medical care are recommended to be followed [7].
Hormonal therapy is also used in the management of congenital sexual anomalies, a wide category of controversial medical conditions, among which most commonly known are: Agenesis or hypoplasia of a urogenital ridge (ex-Mayer-Rokitansky-Küster-Hauser syndrome), ovarian hypoplasia (aka Turner syndrome), agenesis and dysgenesis of the ovary (histopathologically indistinguishable from Turner syndrome), androgen insensitivity syndrome (aka testicular feminization syndrome), hermaphroditism and pseudohermaphroditism etc. The historic approach for the management of these patients consisted of early surgery for "correcting the external genitalia". However, recently the process of 'gender assignment' considers many factors, including a well-established etiological diagnosis of the "disorders of sexual development" (DSD) [8,9], options for fertility preservation, eventual need for replacement hormonal therapy during puberty and finally, delayed, elective surgical treatments.
In terms of hormonal treatment for different congenital sexual anomalies, there are few generally accepted standard approaches. With the exception of congenital adrenal hyperplasia, hormonal treatment is directed towards pubertal induction (in cases of hypogonadism), started around 10-13 years of age, and hormone replacement therapy started at different ages, with the declared purpose of gender identity reinforcement, secondary sexual characteristics development and psychosexual health promotion; evidently, there are specific details of hormonal treatment for different DSDs.
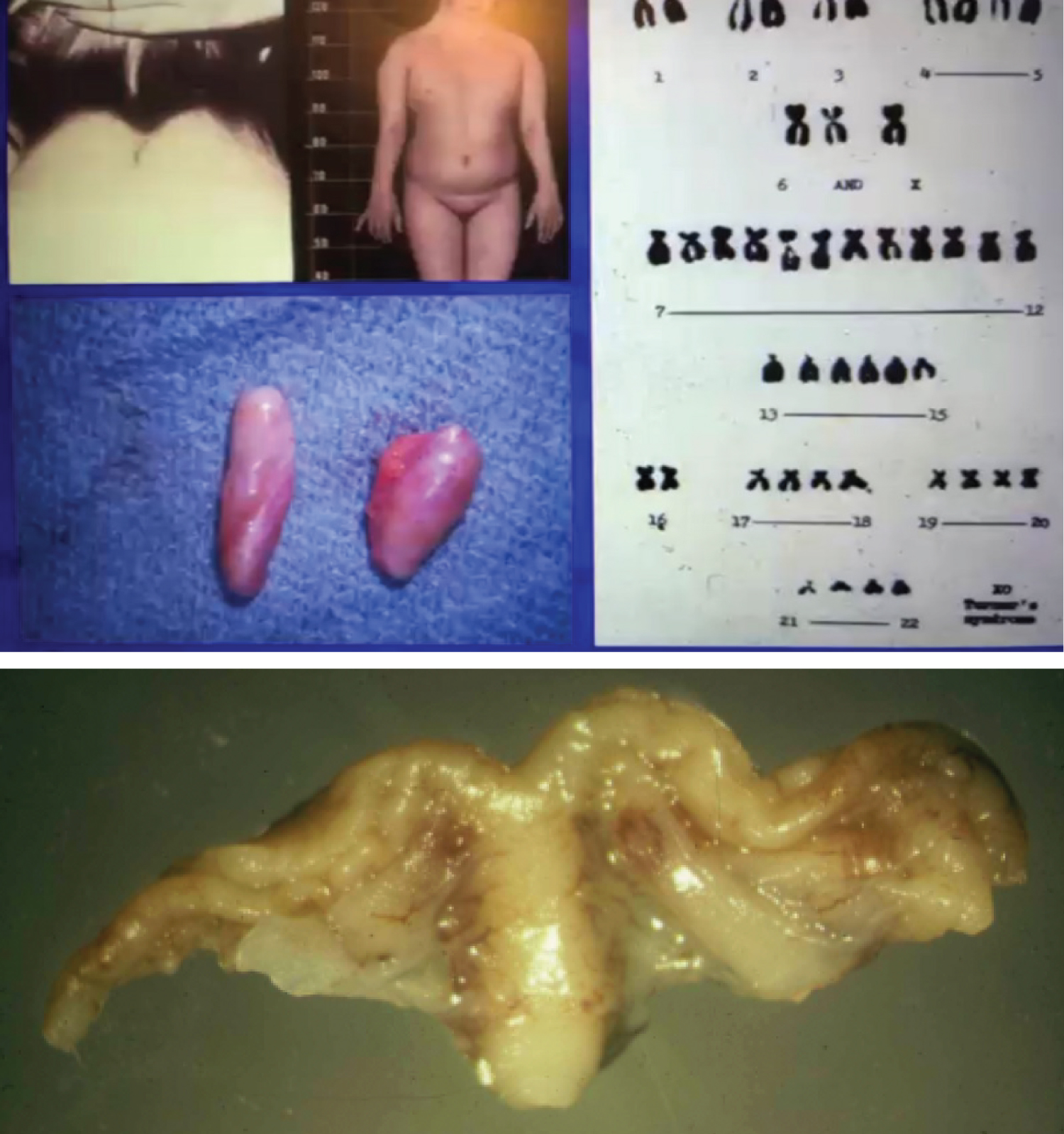
Worth mentioning and with a word of warning is hormonal therapy in cases of Turner syndrome. Clinically, patients with Turner syndrome present with ovarian failure and amenorrhea. Histologically, the typical ovary in monosomy X is a streak gonad (Figure 1). Hormonal treatment for these patients include recombinant growth hormone (if the patient has short stature) and steroid therapy for induction of puberty and replacement (most patients have variable degrees of hypergonadotropic hypogonadism). The hormone replacement therapy includes estrogen and progesterone. Use of estrogen alone is associated with an increased risk of endometrial hyperplasia and neoplasia. If a prolonged estrogen-only therapy is used in pre-pubertal patients with Turner syndrome with an infantile uterus, endometrial carcinoma may develop, frequently of endometrioid type, hormone receptor positive and with a favorable prognosis. Figure 2 shows a case of a 19-year-old patient with Turner syndrome who received long term unopposed estrogen therapy and subsequently developed endometrioid endometrial adenocarcinoma. To decrease the risk of endometrial neoplasia development due to therapy with estrogen alone, progesterone is usually added to the regimen [10].
Endometrial Carcinogenesis and Precancerous Changes
Endometrial carcinoma (EC) now the most common gynecological malignancy in the USA is known to be strongly associated with excessive Estrogen (E) secretion. The presence of Estradiol in the serum and its receptor in the nuclear DNA of the endometrial cells stimulates their growth by mitotic division. The mitogenic function of estradiol is essential for the reproductive function of the endometrium. Around or after menopause this function decreases progressively as does the inhibitory function of Progesterone due to failing ovulation. In certain abnormal situations such as ovarian polycystic disease (PCOD) or gonadal hormonally active tumors (Granulosa or Granulosa-theca cell tumors) E is secreted excessively often resulting in abnormal endometrial growth stimulation. After menopause, with the progressive diminution and eventual cessation of ovarian E, due to failure to respond to gonadotropin hormone, FSH and LH which continue to rise, but in the absence of E the endometrium becomes gradually atrophic. Extragonadal E in postmenopausal women is derived from androgen conversion mostly by adipose tissue. Available E varies with the substrate: as the level of fat and the aromatase capacity of fat tissue increase, circulating levels of estrone and estradiol increase. With obesity sex hormone-binding globulin levels decrease providing greater androgen substrate availability and higher concentration of free biologically active E.
Obesity (BMI over 30), nulliparity, late menopause and exogenous estrogen without adequate Progestin opposition are the most common risk factors for EC. Polycystic ovarian disease with anovulatory menstrual cycles may as well generate a continuous and unopposed E production which results in endometrial hyperstimulation with possible hyperplastic and neoplastic changes. It should be mentioned that in younger, premenopausal women obesity is becoming more frequent contributing to the total increase of EC and its precursors.
Endometrial Hyperplasia (EH)
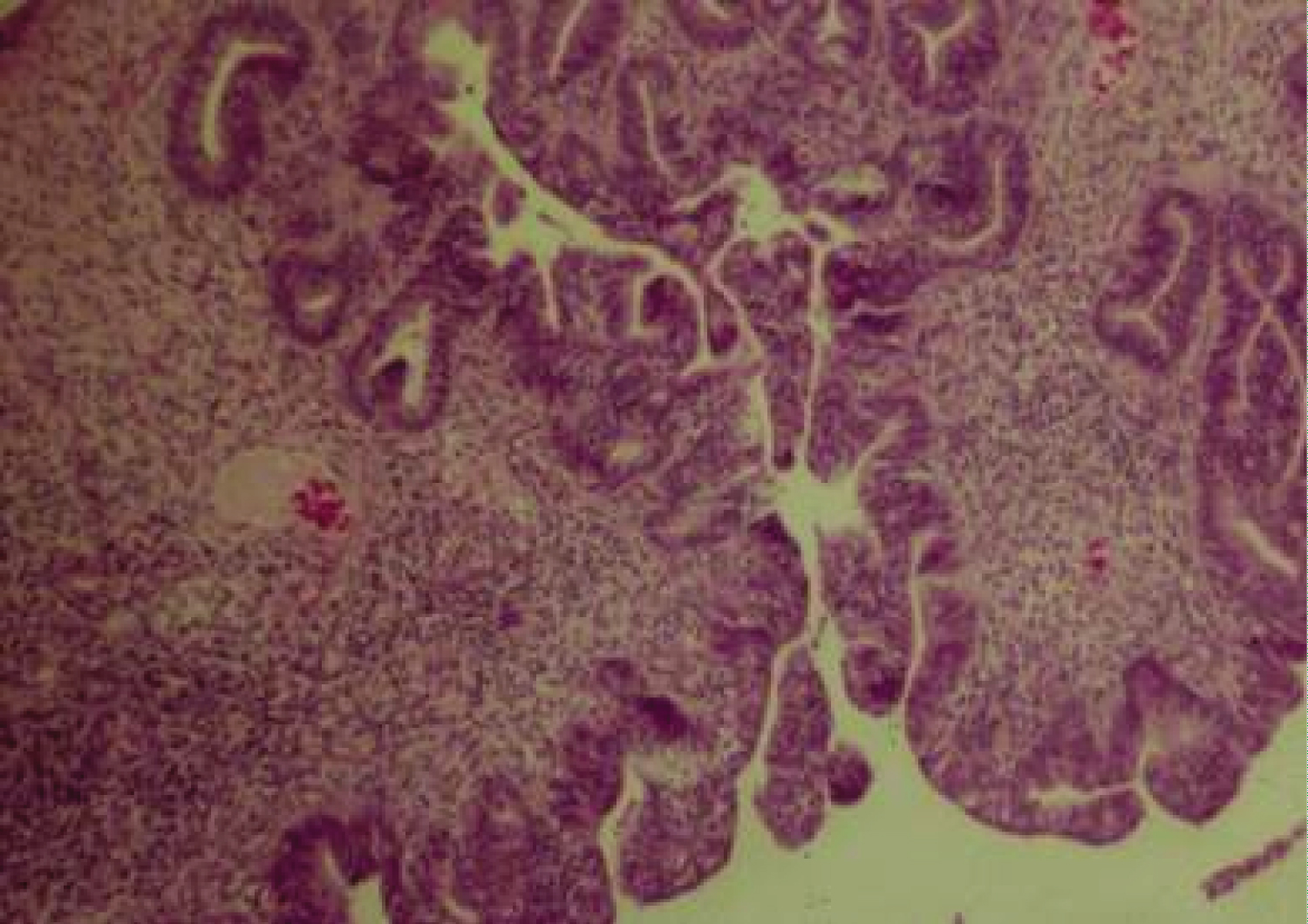
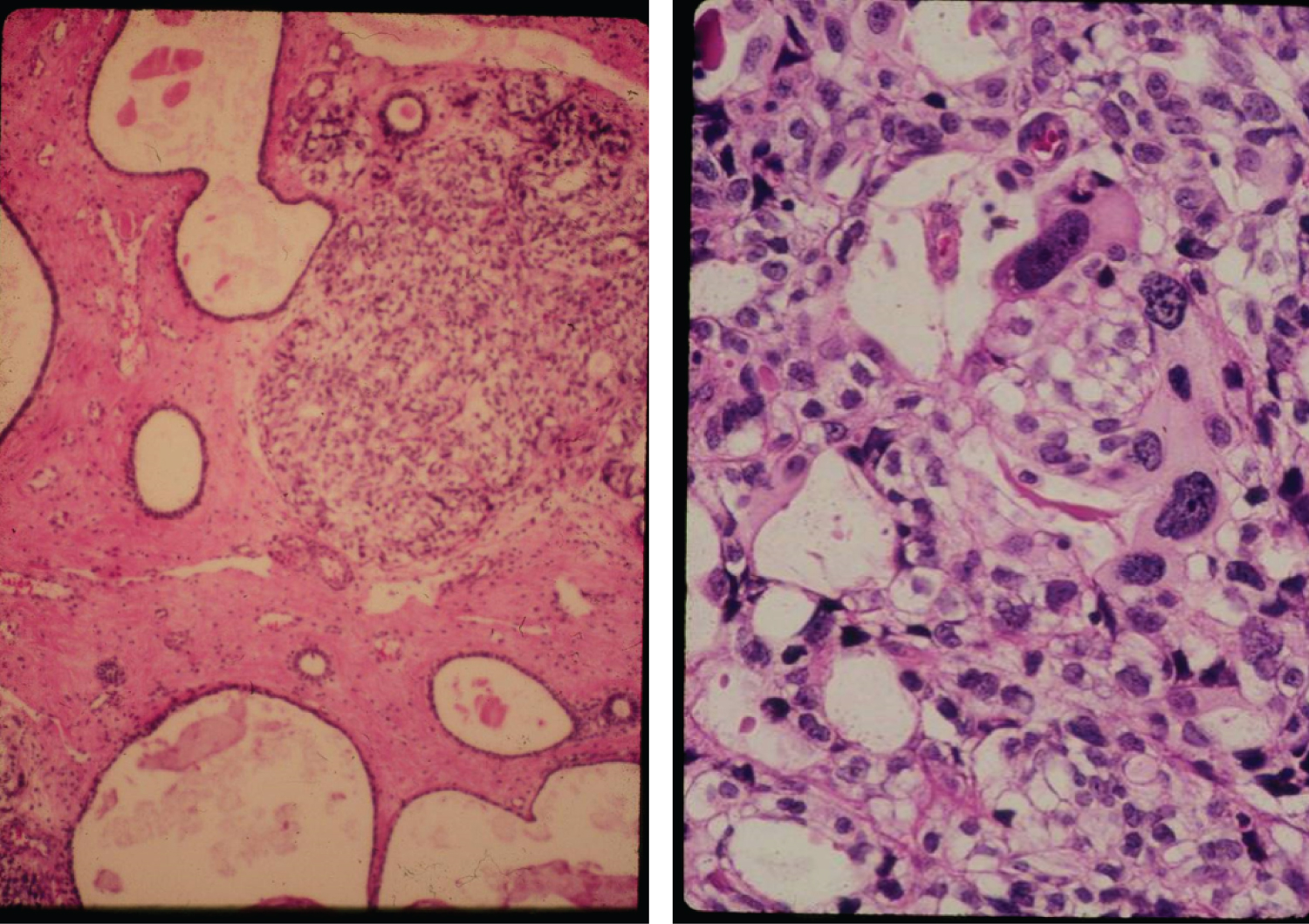
EH is most common in early postmenopause, often manifested by postmenopausal vaginal bleeding which on endometrial biopsy may display irregular glandular proliferation, glandular crowding and outpouching, epithelial cell piling up with or without atypical nuclear changes. EH without atypia is usually reversible with Progesterone therapy (84%) and spontaneously (60%). EH with atypia is often diagnosed in older women and is characterized by an atypical glandular pattern with piled up epithelial cells, lost cell polarity and atypical nuclei varying in size, shape and texture (Figure 3). Mitotic activity resulting from estrogenic stimulation may be present in both hyperplastic lesions, typical and atypical, as seen also in normally cycling proliferative endometrium. Atypical endometrial hyperplasia is a precancerous condition and also classified into intraepithelial glandular neoplasia and intraepithelial carcinoma as both represent an early noninvasive stage of EC, the first being possibly reversible with Progesterone therapy. The differential diagnosis is subtle and therapeutic decisions should be made in consultation with gynecologic pathologists. Atypical hyperplasia has a 36% progression rate to malignancy, even with Progestins in 27% of cases [11].
The management of EH depends on the histopathologic diagnosis especially the evaluation of the malignant potential, fertility requirements and patient preferences as well as medical comorbidities. All therapeutic strategies should include removal of intrinsic or extrinsic sources of Estrogen. Hormone Replacement Therapy (oral, patch, vaginal ring with systemic dose) with Estrogen without an opposing Progestin in a woman with a uterus result in a markedly increased risk of EH or EC.
For EH without atypia the diagnostic D&C can also be therapeutic; Progestin or oral contraceptives are usually effective. Gonadotropin releasing Hormone (GnRH) agonists, ovulation induction with aromatase inhibitors may be used as alternatives to Progestins. For EH with atypia there is a risk of finding an EC in the hysterectomy specimen of 14-57%, therefore hysterectomy is indicated to be performed as soon as possible. Progestin therapy is an option for women who wish to preserve fertility or do not tolerate surgery. Megestrol 160 mg per day in divided doses is the drug of choice [12].
Endometrial Carcinoma
As mentioned before, endometrial carcinoma (EC) is presently the most common malignant gynecological tumor having increased considerably in frequency over the past two decades, while most other malignant tumors have decreased due to diagnosis of precursor, precancerous stages and preventive measures related to lifestyle, nutrition and genetic counseling. In the case of endometrial neoplastic proliferation, demographic and economic factors seem to play an important role as prolonged longevity and obesity represent the major risk factors of this cancer frequently occurring in elderly and overweight patients. The etiopathogenic factors, hyperestrogenism associated with abnormal metabolic diseases (obesity and diabetes) elicit endometrial proliferation due to the mitogenic effect of estrogens in postmenopausal women whose lifespan is prolonged in most industrialized countries. The other potential etiopathogenic factor of EC in postmenopausal women is the iatrogenic intake of estrogens as hormone replacement therapy (HRT), a much-disputed subject especially at the early 21st century when the WHI (Women's Health Initiative Study) of 27.000 postmenopausal women over 10 years yielded surprising results on the deleterious effects of HRT. More recent studies reassessed the evaluation of HRT concluding that prescribed early after menopause inception, in lower doses and associated with Progesterone, HRT is basically safe.
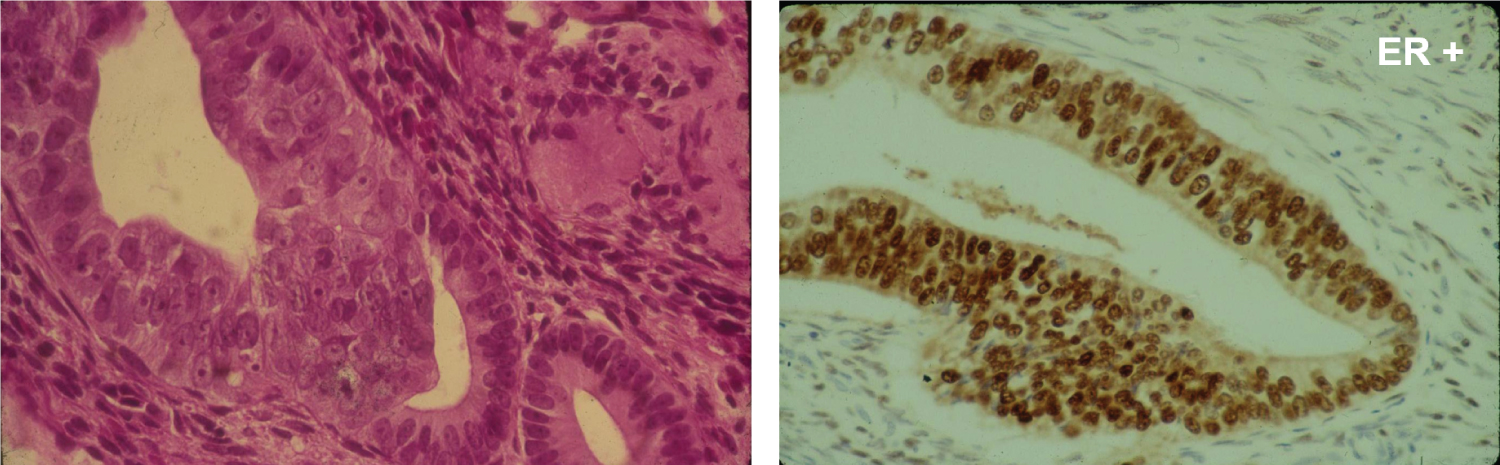
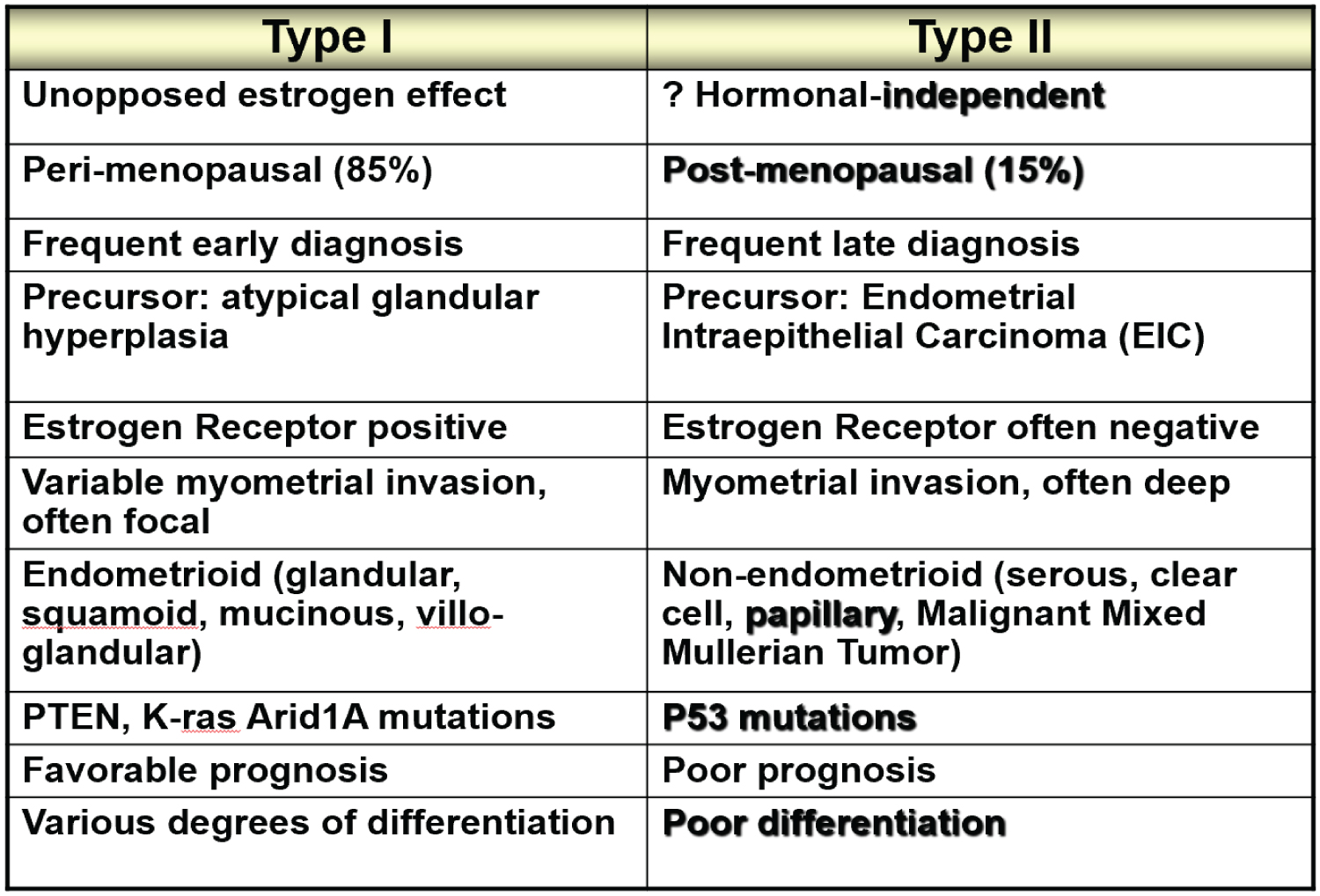
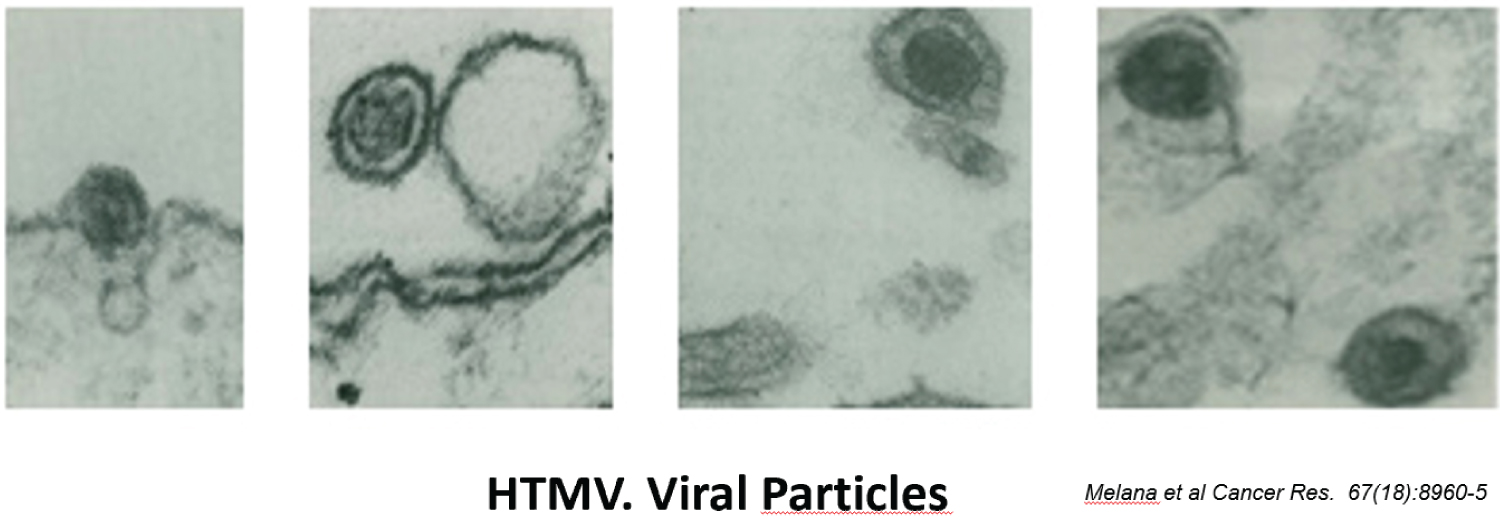
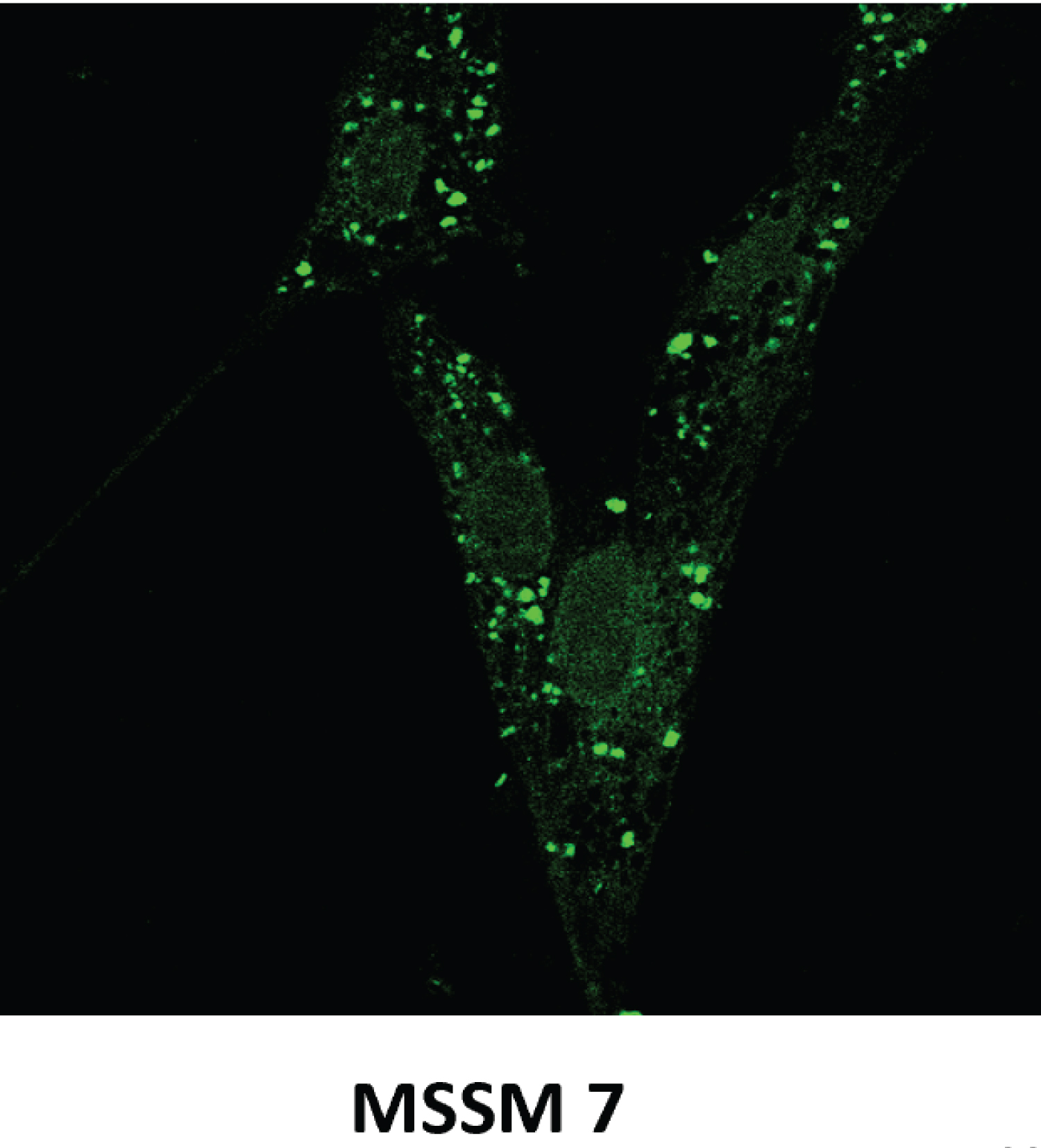
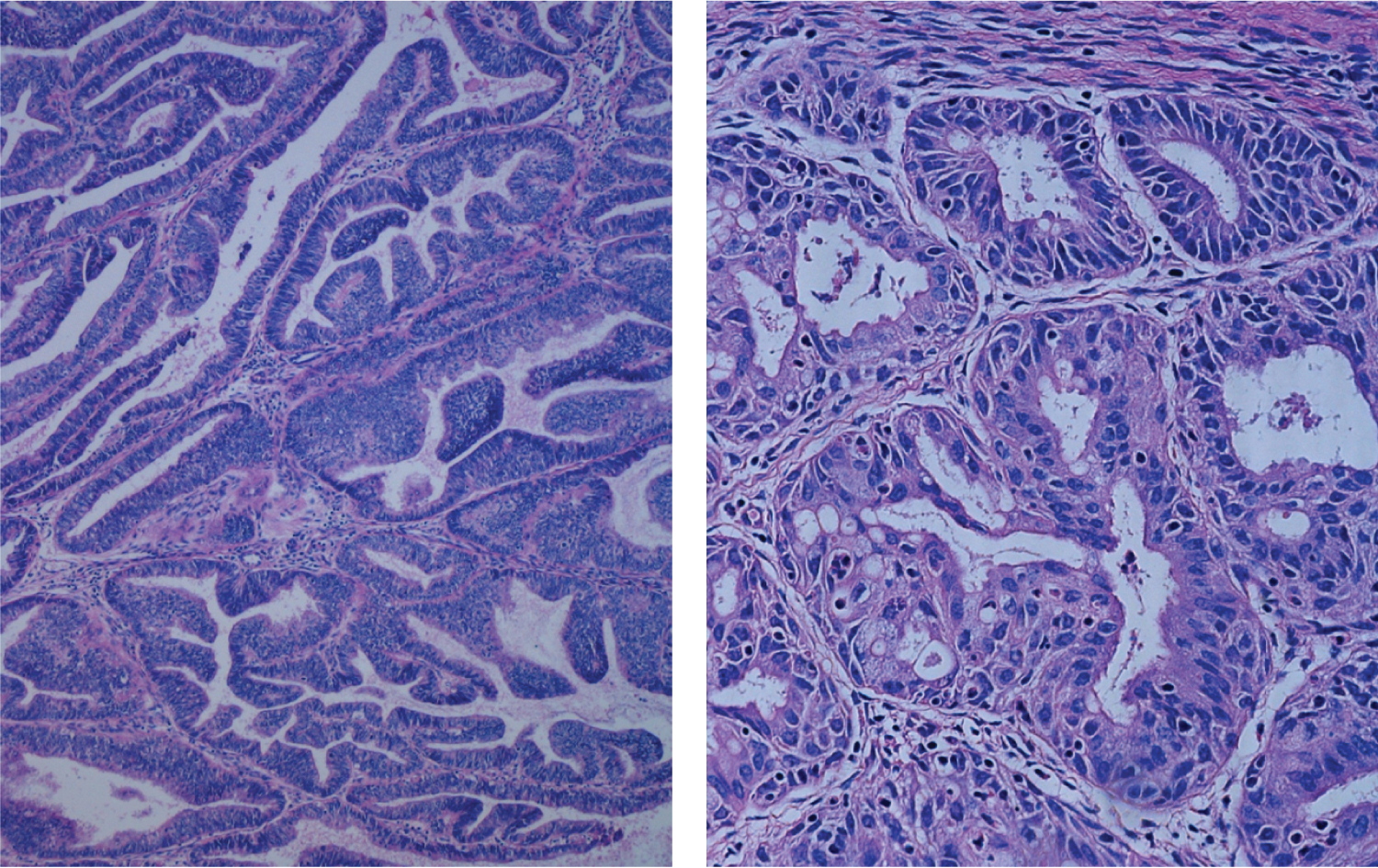
EC is classified in two major types, I and II (Figure 4). The majority, about 80%, is Type I and represent EC associated mostly with hyperestrogenism, diagnosed in earlier postmenopausal stage with histologically mostly well differentiated endometrioid carcinoma and often associated changes suggestive of cancer precursors (EH). EC Type I is most often diagnosed in early stages, therefore amenable to treatment and resulting in low mortality. Type II is less often diagnosed in most academic medical institutions, affecting more elderly post-menopausal women, frequently diagnosed in late stages of the disease, histologically often non-endometrioid and poorly differentiated cancers. Endometrial hyperplasia, as often seen in hyperestrogenism, is usually absent. Nontumoral endometrium, if present, is atrophic. The prognosis of type II EC is generally poor; most tumors are often invasive and metastatic at the time of diagnosis. The clinical background of the patients is different in the two groups: the known risk factors for EC commonly present in Type I are rarely present in Type II. Patients diagnosed with Type I are on average, younger, recently postmenopausal, have 1 or 2 or no children, sometime a history of infertility, and are often overweight, present with a history of irregular vaginal bleeding and sometime prior diagnosed endometrial biopsies with glandular hyperplasia. There also may be a history of Estrogen intake in this group of patients. Patients in Type II EC are often first seen in Emergency rooms with severe vaginal bleeding, are most commonly elderly late postmenopausal women, some with many children, usually not overweight. Lab tests usually confirm the hyperestrogenic profile of Type I EC patients: High serum estradiol and strong Estrogen Receptors in the examined endometrial biopsied tissue, as compared to Type II EC biopsies revealing positivity of cancer markers such as PT53 and mostly absent Estrogen and Progesterone Receptors. The carcinogenic factors of EC Type II are still poorly understood they are similar to ovarian serous carcinoma with yet unknown etiology, may include hereditary, genetic factors as in Lynch syndrome, viral cofactors as Human Mammary Tumor Virus (HMTV) demonstrated present in Type II but not in type I EC (Figure 5 and Figure 6) [13].
The pathologic examination of EC Type II often reveals invasion of the EC through the wall of the uterus, extension of the tumor to neighboring tissues, lymph nodes and to distant organs (metastases).
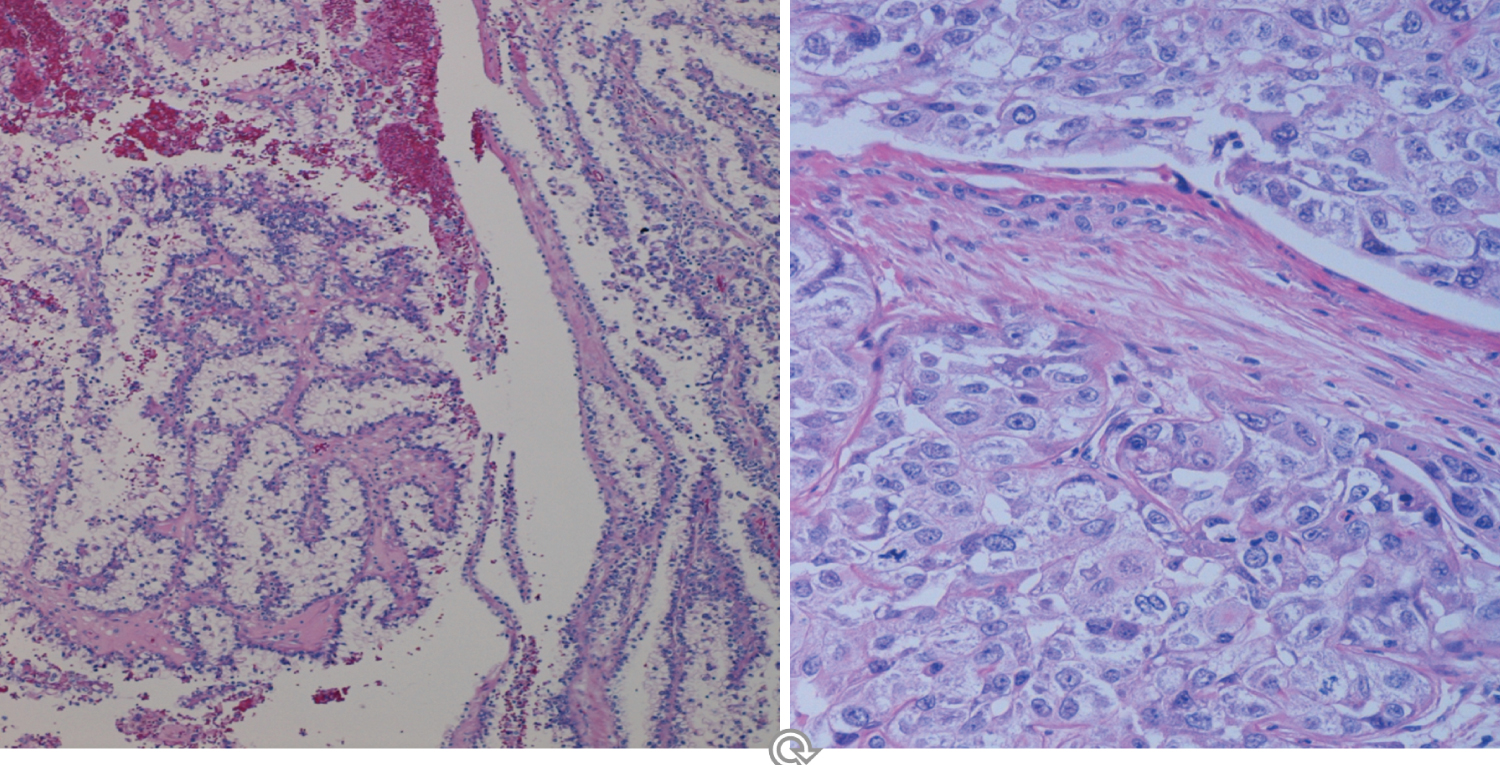
The histologic examination of the endometrial tissue from biopsies and surgically removed uteri was considered the "gold standard" of the diagnosis based on the microscopic tissue architecture and cell characteristics. Type I EC is composed of mostly well differentiated glands and nuclei resembling somehow normal proliferative endometrium, with areas of endometrial hyperplasia and strong Estrogen Receptor positivity, while Type II EC is mostly a non- endometrioid carcinoma with microscopic patterns displaying irregular proliferation of undifferentiated tumor tissue with various patterns, some resembling ovarian serous carcinoma. It therefore appears that Type I EC is a hormonally related neoplasm resulting from a neoplastic development due to estrogenic stimulation while Type II EC is a non-endometrioid carcinoma, a more aggressive tumor possibly arising in atrophic, less or not hormonally stimulated endometrium as hyperplastic changes due to hyperestrogenism are usually absent in the tumor (Figure 7 and Figure 8).
The profound differences between the two types of EC request different management. The diagnosis however may be difficult in some cases, especially when the follow-up tumor behavior is not consistent with the one predicted by the initial diagnosis. Recurrence of a low-grade Type I tumor after surgery as a high-grade Type II EC, less differentiated and more aggressive tumor occurs occasionally. It seems that epigenetic factors can change the biological progression to a more aggressive tumor phenotype.
The FIGO (International Federation of Gynecology-Obstetrics) adopted a surgical-pathological classification into prognostic groups based on the extent of disease and tumor grades (degree of differentiation). Older patients and African American patients are generally diagnosed in higher stages and higher grades. An important prognostic factor is the presence of extrauterine tumor invasion and lymph node metastases [14].
EC type I with a history of prolonged estrogen exposure is most often diagnosed in earlier stages (Stage I-II), with tumor confined to the inner layer of the uterus, the endometrium, or invading the inner half of the myometrial wall, and histologically of endometrioid type with well to moderately well glandular differentiation. This type of tumor is fortunately the statistically most common uterine neoplasm (80%), with a relatively good prognosis after surgical resection in early stages (95% 5-year survival). While surgery is the therapy of choice, patients who are younger, desire pregnancy or have medical conditions incompatible with surgery, have the option of adjuvant therapies such as radiotherapy and hormone therapy, the latter if the diagnosed tumor was Estrogen-Receptor positive. MPA (Medroxy Progesterone Acetate) and Megace are currently used in these cases. Despite the risk of prolonged and unopposed Estrogen intake, the patients usually appear to have a better prognosis as stage and histologic grade are lower in Estrogen users and their survival rates are higher.
Type II EC, high grade and histologically non-endometrioid, is less common statistically (about 20%), and is usually diagnosed in Stage III-IV having a worse prognosis and a higher mortality rate than Type I EC. In addition to surgical resection consisting of radical hysterectomy, adjuvant therapy, radiation, chemotherapy and more recently immunotherapy are used. Hormone therapy appears to be effective when combined with targeted therapeutic agents known to disrupt Estrogen Receptor pathways.
The conventional Pathological diagnostic characteristics of EC type I are: Low histologic grading, most commonly well differentiated endometrioid EC, with squamoid or tubal metaplasia, or secretory carcinoma, associated precursor endometrial hyperplasia and low staging with absent deep myometrial invasion and negative lymph nodes. Type II EC is characterized by poorly differentiated, non-endometrioid, clear cell, papillary serous, undifferentiated, mixed epithelial-stromal EC with high surgical staging: deep myometrial and extrauterine invasion and lymph nodes involvement. While most type I EC displays abundant Estrogen Receptors (ER) in the tumor cells, type II EC is usually negative for ER, and positive for a number of tumor markers, the most common being p53.
Therapeutic decisions involving treatment options are basically surgery for most cases and surgery plus adjuvant therapy for most type II EC. Hormonal therapy represents an option for type I as mentioned and for some recurrent type II EC. Presently type I EC 5-year survival is 95% while the survival of recurrent type II EC is 17%.
Recent progress of molecular biology studies added significant information to the knowledge of the submicroscopic mechanisms of malignant endometrial tissue proliferation. The emerging division of four molecular subtypes of EC appears to offer a more objective and reproducible evaluation of most cases of EC. A better risk stratification of both EC and ovarian carcinoma can be obtained by adding molecular studies such as mismatch DNA repair, p53 abnormalities, and microsatellite instability. It seems that EC NSMP (no specific molecular profile, with normal p53 expression/p53wt) is the most common molecular subtype having a low number of somatic copy number alterations, a low number of mutational burden, high levels of Estrogen and Progesterone Receptors and no p53 abnormalities. For immunotherapy, the study of immune checkpoint blockade and lymphoid cell infiltrates may be relevant as well. For breast cancer hormonal therapy is effective with combined targeted therapeutic agents known to disrupt Estrogen Receptor pathways. It is therefore recommended to integrate molecular parameters to standard pathologic reports using the 2020 ESTRO/SGO/ESP for risk stratification and treatment algorithm [15].
Tamoxifen Therapy for Breast Cancer and the Endometrium
Tamoxifen is a non-steroidal synthetic methylene estrogen derivative used successfully in the treatment and prevention of breast cancer. It binds to estrogen receptors in a manner similar to that of estradiol decreasing the unbound tamoxifen- receptor complex to nuclear DNA. This results in an antiestrogenic effect which can also be obtained by Aromatase Inhibitors, without the side effect produced by Tamoxifen on the Endometrium. While usually acting as an Estrogen antagonist, in some cases, the uterus responds to Tamoxifen therapy as an Estrogen agonist developing leiomyomas, adenomyosis and more often, endometrial polypous hyperplasia (Figure 9). Occasionally, in older patients who received Tamoxifen therapy for over 5-years, EC developed in the polyps. So, while Tamoxifen acts as an estrogen antagonist on breast tissue it is an antagonist-agonist of Estrogen in the endometrium. The largest study of endometrial tissue in patients treated with Tamoxifen for Breast Cancer (700 cases) reported 24% endometrial polyps and 4.7% (33 cases) of EC of which 22 were Type II EC (Figure 10). It has been suggested that adding intrauterine levonorgestrel may prevent the estrogen-agonist effects of Tamoxifen on the uterus. Recently Tamoxifen was replaced in some cases by Aromatase Inhibitors in the prevention and treatment of Breast Cancer [16,17].
Conclusions
The hormonal effects on the uterus are a fascinating example of the unique biological organization of reproduction. Progress in the knowledge of the finely tuned mechanisms of stimulation, inhibition, and feedback between hormones along with advanced technology reached spectacular achievements in human reproduction and opened new horizons in the prevention and cure of neoplasms of the reproductive organs. However, the uterus, fallopian tube and ovaries are still harboring lethal neoplasms the study of which remains to be addressed in joint preventive/clinical/pathological/molecular approaches.
References
- Bazer FW, Roberts RM, Thatcher WW (1979) Actions of hormones on the uterus and effect of conceptus development. J Anim Sci 49: 35-45.
- Deligdisch Shor L (2020) Hormone therapy effects on the uterus. In: L. Deligdisch-Shor, A Mares Miceli, "Hormonal pathology of the uterus". (1st edn). Adv Exp Med Biol 1242: 145-177.
- Barad D (2020) "Hormonal effects in reproductive technology with focus on diminished ovarian reserve". In: L. Deligdisch-Shor, A. Mares Miceli, "Hormonal pathology of the uterus". (1st edn). Adv Exp Med Biol 1242: 13-36.
- Paciuc J (2020) "Hormone therapy in menopause". In: L. Deligdisch-Shor, A. Mares Miceli, "Hormonal pathology of the uterus". (1st edn), 89-115.
- Deligdisch Shor L (2020) Hormone therapy effects on the uterus. In: L. Deligdisch-Shor, A. Mares Miceli, Hormonal pathology of the uterus. (1st edn), 158-167.
- Deligdisch L, Hirschmann S, Altchek A (1997) Pathologic changes in gonadotropin releasing hormone agonist analogue treated uterine leiomyomata. Fertil Steril 67: 837-841.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. (2017) Endocrine treatment of gender-dysphoric/gender-incongruent persons: An Endocrine, Society clinical practice guideline. J Clin Endocrinol Metab 102: 3869-3903.
- Witchel SF (2018) Disorders of sex development. Best Pract Res Clin Obstet Gynaecol 48: 90-102.
- Lee PA, Nordenström A, Houk CP, et al. (2016) Global disorders of sex development update since 2006: Perceptions, approach and care. Horm Res Paediatr 85: 158-180.
- Mares Miceli A, "Replacement hormone therapy for gender dysphoria and congenital sexual anomalies". In: L. Deligdisch-Shor, A. Mares Miceli, "Hormonal pathology of the uterus". (First edition), Springer 2020, 135-137,
- Deligdisch L, Cohen CJ (1985) Histologic correlates and virulence implications of endometrial carcinoma associated with adenomatous hyperplasia. Cancer 56: 1452-1455.
- Javadian P, Nezhat F (2020) Endometrial cancer and its precursors. In: L. Deligdisch-Shor, A. Mares Miceli, Hormonal pathology of the uterus. (First edition), 69.
- Deligdisch L, Marin T, Melana S, et al. (2013) Human mammary tumor virus (HMTV) in endometrial carcinoma. Int J Gynecol Cancer 23: 1423-1428.
- Javadian P, Nezhat F (2020) "Endometrial cancer and its precursors". In: L. Deligdisch-Shor, A. Mares Miceli, "Hormonal pathology of the uterus", First edition, 60.
- Jamieson A, Bosse T, Mc Alpine J (2021) The emerging role of molecular pathology in directing the systemic treatment of endometrial cancer. Ther Adv Med Oncol 13: 1-14.
- Magriples U, Naftolin F, Schwartz PE, et al. (1993) High-grade endometrial carcinoma in tamoxifen-treated breast cancer patients. J Clin Oncol 11: 485-490.
- Deligdisch L, Kalir T, Cohen C J, et al. (2000) Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Selected for Breast Diseases: A Yearbook Quarterly 2001, publ, Mosby Yearbook Inc, Gynecol Oncol 78: 181-186.
Corresponding Author
Liane Deligdisch Schor, MD, Professor of Pathology and Obstetrics-Gynecology, The Mount Sinai Icahn School of Medicine, New York, USA.
Copyright
© 2022 Deligdisch Schor, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.