Cryopreservation of Gene-Edited Pig Skin at -80°C Prevents Histological Injury for One Month but Not for Three Months
Abstract
Background: The standard treatment for severe burns involves using temporary allografts, but this approach can be
Method: Genetically-modified pig skin, lacking two key carbohydrate xenoantigens and expressing human protective proteins, was harvested for split-thickness grafts. The skin samples were either stored in saline at 4°C (control) or cryopreserved at -80°C and then evaluated at various time-points using histological, electron microscopy, and immunohistochemical methods to assess tissue viability and proliferation.
Results: Both control and cryopreserved skin samples remained intact on day 28. The control group showed no abnormalities at day 56, but unfortunately no cryopreserved sample was available on that day. By day 84, histopathology and electron microscopy of both control and cryopreserved samples indicated structural disarray. Minimal TUNEL staining was noted on day 7, and Ki67 counts remained consistent between days 0, 28, and 84.
Conclusions: These findings support the potential of cryopreserved pig skin as a viable option for grafting within a month of storage. However, we were unable to demonstrate any advantage over storage at 4°C.
Keywords
Cryopreservation, Pig, genetically-modified, Skin, Xenotransplantation
Abbreviations
H&E: hematoxylin and eosin
TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling
Introduction
Standard therapy for severe burns includes wound debridement, excision, and coverage with autologous split-thickness skin grafts1. However, extensive burn injuries often result in a shortage of viable donor sites, making autologous grafts unavailable or inadequate for covering all affected areas. In such cases, allografts from deceased human donors are commonly used to temporarily cover the sites of full-thickness burns, providing benefits by reducing fluid loss and the risk of contamination and infection, and alleviating pain for patients. Nonetheless, drawbacks include the potential risk of pathogen transmission, limited availability, and high cost [1,2].
Pig skin, histologically similar to human skin, could serve as a viable option for temporary wound coverage by promoting granulation tissue formation until an autograft becomes available [3-6]. This option becomes particularly relevant in scenarios like pandemics or wartime when access to tissue banks or live pigs may be limited.
Advances in gene editing enable targeted modifications of the pig genome to prevent hyperacute rejection of pig xenografts and reduce immunological damage [7]. Utilizing pathogen-free environments for animal husbandry is crucial to minimize cross-species contamination and ensure the production of high-quality skin grafts. Extensive research supports the efficacy and reliability of pig skin as a valuable short-term treatment option for burn injuries [8-10].
Porcine skin can be utilized fresh or preserved through methods such as cryopreservation, lyophilization, or chemical dehydration with glycerol, which can affect its viability3,9,10. Establishing a standardized method for storing pig skin is essential for ensuring its viability for future transplantation. Therefore, we conducted a limited pilot study to evaluate the histological and structural appearance of gene-edited pig skin grafts following either (i) storage in saline at 4°C (control) or (ii) cryopreserved at -80°C. Our goal was to identify the period of time that a simple method of cryobanking might store pig skin without obvious histopathological injury.
Materials and Methods
Gene-edited pig 5.1
A genetically-modified pig (22kg) lacking expression of two of the three major carbohydrate xenoantigens (galactose-α1,3-galactose and Sda), with knockout of growth hormone receptors (GHR-KO), and expressing six human protective proteins (CD46, CD55, TBM, EPCR, CD47, and HO-1) was generously provided by our collaborators at Revivicor (Blacksburg, VA, USA). All experimental procedures involving the pig were approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital. The study was conducted in strict accordance with the guidelines provided by the National Institutes of Health for the care and use of laboratory animals.
Skin harvest 5.2
After the pig was euthanized, the skin surface was disinfected using 70% isopropyl rubbing alcohol followed by povidone-iodine scrubs. Split-thickness skin grafts were harvested using an electric dermatome (Zimmer Biomet, Warsaw, IN) set to a thickness of 0.012 inches. The harvested skin was immediately prepared for storage by laying it flat on nylon mesh and cutting it into 3x4 cm pieces. These porcine skin samples, along with the mesh, were carefully rolled into cylinders and placed into storage tubes.
Skin storage 5.3
The skin samples were divided into two groups – (i) a Control group, and (ii) a Cryopreserved group. Skin samples in the Control group were stored in conical tubes containing 0.9% normal saline at 4˚C until they were examined on days 0, 1, 2, 4, 7, 14, 28, 56, and 84 following the initial skin harvest.
Skin samples in the Cryopreserved group were placed in Sarstedt 8 mL screw cap tubes, and freezing media was added just before freezing to prevent toxicity at room temperature [11,12]. The freezing media consisted of 60% RPMI, 30% fetal bovine serum, and 10% DMSO. The volume of freezing media (~4-6 mL) was sufficient to cover the skin samples without overfilling the tubes. The samples were then immediately transferred into a CryoMed phase freezer, which initially maintained them at 4˚C before gradually lowering the temperature in a controlled manner to reach -80˚C, minimizing tissue damage during freezing. Samples were stored at -80˚C and evaluated on days 0, 1, 4, 7, 14, 28, and 84 after the initial skin harvest. (Unfortunately, a sample taken on day 56 was mislaid.)
Skin thawing 5.4
Cryopreserved skin samples stored at -80˚C were removed from the freezer, and the caps of the Sarstedt freezing tubes were loosened to equalize the pressure before thawing the samples in a 37˚C water bath. The frozen skin samples were thawed just long enough to safely remove them and the mesh from the media, followed by immediate washing with RPMI through three sequential dilutional washes.
Preparation of skin samples for histopathological examination 5.5
(i) Skin samples were initially fixed in 10% formalin, followed by standard processing for histological examination and embedding in paraffin. Hematoxylin and eosin (H&E) staining was conducted on the slides. (ii) Separate skin samples were fixed in Karnovsky fixative and processed for electron microscopy. (iii) Immunohistochemistry using Ki67 and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was used to evaluate for proliferation and viability of samples. TUNEL stains were assessed by counting positive cells in 10 random 20x (magnification) fields. Ki67 stains were assessed by counting the number of positive cells in 5 random 40x fields.
Results
Two histopathologists, who were blinded to the identity and timing of the samples, conducted independent assessments at various time-points after storage or cryopreservation.
Control group 6.1
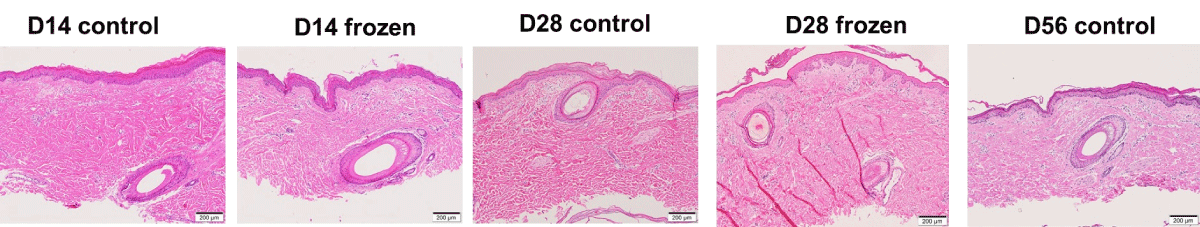
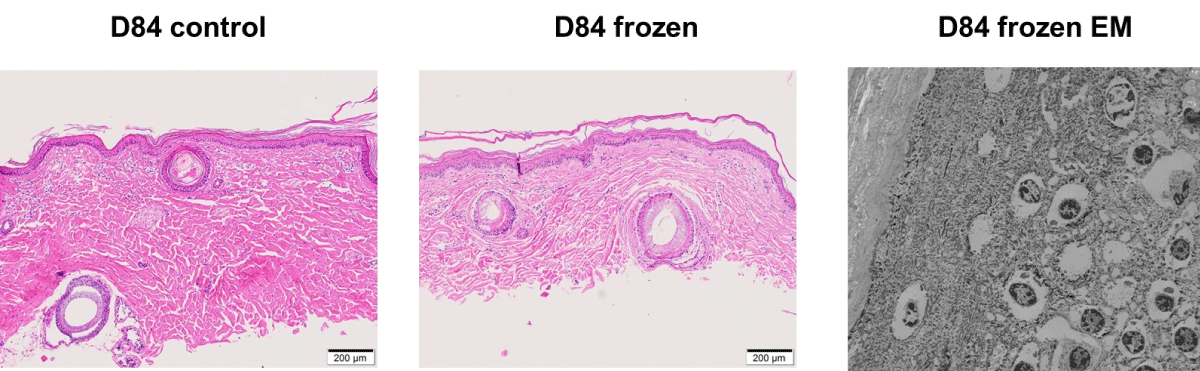
Histologic images from control samples showed intact skin tissue at all time-points up to day 28 (Figure 1). On day 56, histopathological evaluation of skin continued to show no abnormalities. By day 84, histopathology and electron microscopy indicated disarray and focal loss of cell-to-cell junctions in the epidermis, with endothelial swelling observed in capillaries and arterioles (Figure 2).
Cryopreserved group
Histopathologic evaluation showed morphologically intact and histologically viable skin tissue with adnexal structures and blood vessels preserved at all time-points up to day 28 (Figure 1). Some samples showed focal spongiosis and vacuolization suggesting edema, but the relevance of this to suitability for grafting remains uncertain.
(No cryopreserved sample was available on day 56). By day 84, histopathology and electron microscopy indicated identical adverse changes to those seen in the control samples (Figure 2).
In both groups, the TUNEL stains on formalin-fixed and frozen tissues on day 7 showed minimal staining (3-5 positive cells in 10 random 20x fields), indicating no significant difference. The mean Ki67 counts were comparable between day 0, day 28, and day 84 samples (not shown), with no statistically significant changes over time
Discussion
The histological similarities between pig and human skin have suggested pig skin xenografts as a potential alternative to deceased human donor skin allografts for severely burned patients. Genetically engineered pigs with the α1,3-galactosyltransferase gene-knock out demonstrated enhanced skin graft mean survival of eleven days in non-immunosuppressed baboons [13]. Skin from pigs with multiple gene edits survived as long as allografts after transplantation on non-immunosuppressed squirrel monkeys [14]. Optimal preservation and long-term storage methods for porcine skin, ensuring viability for delayed application, are crucial for achieving desirable outcomes yet have not been extensively studied.
Enhancements in genetically modifying pigs have significantly reduced the immunogenicity of their organ, tissue, and cell grafts and improved their acceptance by a recipient primate's immune system [15]. These modifications offer the potential for prolonged survival of pig skin grafts with minimal or no need for immunosuppressive therapy. These advances have the potential to revolutionize the treatment of severe burns in patients, providing effective coverage for weeks (or ultimately months) following transplantation [14].
Preservation of pig skin for delayed application commonly involves using glutaraldehyde, which aids in disinfection while preserving the structural and mechanical integrity of the xenograft. However, this method does not sustain cell viability [10]. In contrast, cryopreservation methods can maintain both the biological and structural functions of skin tissue and cells [16]. However, inadequate cryopreservation techniques can lead to tissue damage and disrupt the normal metabolic activity of the skin [17,18]. The duration of storage in a frozen state is a critical factor that significantly influences the outcome of xenografts [16]. The present limited pilot study aimed to investigate whether the duration of storage affects the viability and quality of gene-modified pig skin following cryopreservation.
We found no significant differences in histological features between the cryopreserved and control skin samples up to 28 days. Minimal apoptotic activity further supported skin viability under cryopreservation within the first week. While no cryopreserved sample was available on day 56, the control skin samples on day 56 showed no abnormalities, suggesting preserved tissue integrity within this period. However, by day 84, structural deterioration, including epidermal disarray and endothelial swelling, was evident in both control and cryopreserved samples.
The great limitation of our study is the absence of cryopreserved skin available on day 56, and so no conclusion can be drawn on whether cryopreservation has any advantage or disadvantage over storage at 4°C. However, we can tentatively conclude that storage by either method is successful for 28 days but not for 84 days. Nevertheless, to fully ascertain their long-term suitability for clinical use, future investigations should include the evaluation of how long these stored skin samples survive after transplantation into nonhuman primates. The ability to store skin for extended periods without significant loss of tissue integrity would open the door for more flexible and accessible clinical applications in the treatment of severe burns, where timely donor skin availability can be a limiting factor.
Acknowledgements
Work on xenotransplantation in the authors’ laboratory was supported in part by Department of Defense grant WB1XWH-20-1-0559 and in part by NIH NIAID U19 grant AI090959.
Conflict of Interest Statement
DA is CEO of Revivicor, Blacksburg, VA, USA, and DKCC is a consultant to eGenesis Bio, Cambridge, MA, USA, but the opinions expressed in this article are those of the authors and do not necessarily represent the views of the companies. No other author has a conflict of interest.
References
- Jeschke MG, Van Baar ME, Choudhry MA, et al. (2020) Burn injury. Nat Rev Dis Primers 6: 11.
- MacNeil S (2007) Progress and opportunities for tissue-engineered skin. Nature 445: 874-880.
- Chiu T, Burd A (2005) “Xenograft” dressing in the treatment of burns. Clin Dermatol 23: 419-423.
- Leto Barone AA, Mastroianni M, Farkash EA, et al. (2015) Genetically modified porcine split-thickness skin grafts as an alternative to allograft for provision of temporary wound coverage: preliminary characterization. Burns 41: 565-574.
- Bromberg BE, Song IC, Mohn MP (1965) The use of pig skin as a temporary biological dressing. Plast Reconstr Surg 36: 80-90.
- Yamamoto T, Iwase H, King TW, et al. (2018) Skin xenotransplantation: Historical review and clinical potential. Burns 44: 1738-1749.
- Cooper DKC, Pierson RN (2023) Milestones on the path to clinical pig organ xenotransplantation. Am J Transplant 23: 326-335.
- Nathan P, MacMillan BG (1976) Burn wounds: selection and preservation of skin, natural products, blood, and blood products for burn therapy. CRC Crit Rev Clin Lab Sci 7: 1-31.
- Busby SA, Robb A, Lang S, et al. (2014) Antibiotic susceptibility and resistance of Staphylococcus aureus isolated from fresh porcine skin xenografts: risk to recipients with thermal injury. Burns 40: 288-294.
- Hermans MHE (2014) Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: is there a clinical difference? Burns 40: 408-415.
- Pegg DE (2007) Principles of cryopreservation. Methods Mol Biol 368: 39-57.
- Erol OD, Pervin B, Seker ME, et al. (2021) Effects of storage media, supplements and cryopreservation methods on quality of stem cells. World J Stem Cells 13: 1197-1214.
- Leonard DA, Mallard C, Albritton A, et al. (2017) Skin grafts from genetically modified α-1,3-galactosyltransferase knockout miniature swine: A functional equivalent to allografts. Burns 43: 1717-1724.
- Hara H, Foote JB, Hansen‐Estruch C, et al. (2023) In vitro and in vivo immune assessments of genetically‐engineered pig skin grafts in New World (squirrel) monkeys. Xenotransplantation 30: e12832.
- Lei T, Chen L, Wang K, et al. (2022) Genetic engineering of pigs for xenotransplantation to overcome immune rejection and physiological incompatibilities: The first clinical steps. Front Immunol 13: 1031185.
- Holzer PW, Lellouch AG, Moulton K, et al. (2022) Clinical impact of cryopreservation on split thickness skin grafts in the porcine model. J Burn Care Res 41: 306-316.
- Ge L, Huang Z, Wei H (2011) Skin Graft Preservation. In: Spear M, ed. Skin Grafts - Indications, Applications and Current Research. InTech.
- Jaiswal AN, Vagga A (2022) Cryopreservation: A Review Article. Cureus 14: e31564.
Corresponding Author
Zahra Habibabady, MD, Center for Transplantation Sciences, Department of Surgery, Massachusetts General Hospital/Harvard Medical School, 149 13th Street, Charlestown, MA 02129, USA, Tel: 410-440-3633
Copyright
© 2025 Habibabady Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.