Managing Hidradenitis Suppurativa
Abstract
Background
Hidradenitis suppurativa is a debilitating chronic skin disease often failure to recognise by healthcare professionals with an average of diagnostic delay of seven years, that has resulted in misdiagnosis and mismanagement of many patients. Knowledge of the pathogenesis is limited, although it seems to be associated with follicular obstruction, follicular rupture and an associated immune response. The inflammatory cascade leads to recurrent abscesses, suppurative dermal tunnels and fibrosis. Finding a successful treatment approach for hidradenitis has been challenging, in part because of limited research-based information, clinical variation of the disease and the lack of a gold-standard treatment.
Objective
Review the available data about hidradenitis suppurativa, highlighting how to diagnosis and management this disease.
Methods
Using Pubmed and UpToDate databases we selected 43 articles referring to hidradenitis suppurativa, in English language and published within the last 5 years.
Results
The diagnosis of hidradenitis is usually made by the recognition of typical lesions on typical anatomical locations and a history of relapses and chronicity. However, clinical evaluation frequently underestimates the severity and disease involvement, so it is advised the use of imaging techniques, like ultrasound, for more accurate staging and managing of this disease. Hidradenitis management requires a holistic approach, and the main treatment goals are reducing the frequency of new lesions, minimizing pain and suppuration, preventing disease progression and treating existing lesions and scarring. These may require a combination of medical treatment, mainly with antibiotic, hormonal and biologic therapy, and surgical intervention with punch debridement, unroofing or wide excision. Treatment is chosen by the degree of severity, patient tolerance of specific agents, comorbidities, and treatment cost and availability.
Conclusions
Combining surgery with dietary and behavioral changes and medical therapy provides the best chance for preventing the development of new lesions and controlling disease progress.
Keywords
Hidradenitis suppurativa, Dermatology, Surgical dermatology
Introduction
Hidradenitis suppurativa (from the Greek hidros = sweat and aden = glands) [1] is a chronic, inflammatory skin disease of the hair follicle characterized by the development of recurrent inflammatory nodules, abscesses, sinus tracts and scarring, involving the intertriginous regions, as axillary, perianal and inguinal areas [2].
Physical pain, malodor, scars, chronic drainage, and disfigurement are common features of this disorder, which can lead to several emotional reactions, including anger, sadness, anxiety, and depression [3]. A systemic review and meta-analysis by Machado, et al. showed an overall prevalence of depression in 16.9% and anxiety in 4.9% of hidradenitis patients [4].
A study analysing the clinical characteristics of hidradenitis conclude that the most troublesome symptom of hidradenitis was pain, presented in 77.5% of patients, followed by exudation, pruritus, appearance and smell, and the presence of pain was a crucial contributor that adversely affects quality of life [5]. In fact, patients with hidradenitis have a significant reduction in quality of life that exceeds that of other common skin diseases, with a considerable impact on patients' self-esteem [3,6].
The prevalence of hidradenitis suppurativa range from 0.05% to 4.10% [7]. The onset of symptoms typically occurs after puberty, with the average age of onset in the second or third decades of life and with a female predominance [8].
Early hidradenitis lesions often mimic other disorders; misdiagnosis of hidradenitis as recurrent furunculosis or "boils" is common [1]. Differential diagnoses of hidradenitis include carbuncle, furuncle, epidermoid or dermoid cyst, erysipelas, lymphogranuloma venereum, granuloma inguinale, tuberculosis and follicular occlusion tetrad [9]. Diagnostic delay on average is seven years and the failure to recognise hidradenitis suppurativa by healthcare professionals has resulted in misdiagnosis and mismanagement of many patients. Therefore, patients often undergo repeat and unnecessary investigations and procedures [8,10].
At the moment, patients often receive multiple one-week courses of oral antibiotics to treat flares, when this may not alter the natural history of individual lesions. Alternatively, patients have to attend emergency departments to undergo incision and drainage of lesions. There is a need to ensure more joined-up care for these patients, identifying the condition earlier and providing better long-term disease control [11].
Prompt recognition of the correct diagnosis is important. An early and accurate diagnosis facilitates the initiation of a treatment plan aimed at minimizing the risk of progression to disabling, endstage disease [1]. However, finding a successful treatment approach for hidradenitis has been challenging, in part because of the lack of a gold-standard treatment method, limited research-based information, and the nature of clinical variation in the disease [12].
In this study, we will review the available data about hidradenitis suppurativa - pathogenesis, risk factors, clinical features, diagnosis, clinical staging and complications, highlighting how to diagnosis this disease and management it, pointing out the most suitable treatment options to each stage of disease.
Methods
In this review we used the Pubmed and UpToDate databases and searched the MeSH terms "hidradenitis" and "hidradenitis AND surgical treatment". The limits considered were English language and published within the last 5 years. The final update of the search was conducted on July 2019.
In the selection of the articles we analysed the title and the abstract and afterwards the full text of previous selected studies and excluded those who were not related to the theme, considering studies about hidradenitis suppurativa. Additional articles were further investigated through manual search from relevant reference lists. Overall, this research ended up with 43 articles, which comprehends the literary basis of this review.
Results
Pathogenesis
The etiopathogenesis of hidradenitis is not fully understood [13]. The idea that the disorder is primarily caused by an inflammation of apocrine glands is nowadays rejected and follicular occlusion by hyperkeratosis and perifolliculitis seems to be the earliest modification detected in hidradenitis skin [14].
Follicular occlusion with posterior dilatation and rupture, leads to the spill of keratin, corneocytes, hair shaft and sebum products from breached pilosebaceous units into the dermis, which can act as danger-associated molecular patterns that stimulate an immune response [14]. Over time, the initial acute inflammatory response may evolve into chronic foreign body-type granulomatous inflammation [1].
Questions remain about whether dysregulation of the immune system is a contributor. To determine the hidradenitis skin and blood transcriptomes and hidradenitis blood proteome, patient data from previously published studies were analysed and integrated from a cohort of patients with moderate to severe hidradenitis compared to healthy volunteers. In the hidradenitis skin transcriptome (lesional skin compared to non-lesional skin), there was an abundance of immunoglobulins, antimicrobial peptides, and an interferon signature. Gene-sets related to Notch signalling and Interferon pathways were differentially activated in lesional compared to non-lesional skin. Analysis of the hidradenitis skin transcriptome revealed a significantly increased proportion of plasma cells in lesional skin. In the hidradenitis skin and blood transcriptomes and hidradenitis blood proteome, gene-sets related to the complement system changed significantly, with dysregulation of complement-specific differentially expressed genes and proteomes. These data point towards an exaggerated immune response in lesional skin that may be responding to commensal cutaneous bacterial presence and raise the possibility that this may be an important driver of hidradenitis disease progression [15].
Important elements of the immune system that have been implicated include complement dysregulation, immunoglobulin (Igs) transcripts, antimicrobial peptides (AMPs), an interferon signature, Toll-like receptor 2 (TLR2), pro-inflammatory cytokines such IL-1, IL-6, IL-17, IL-12/23, and a dysregulated Th17/T-reg cell áxis [15].
Also, a role for immune system dysregulation in hidradenitis is suggested by similarities between hidradenitis and Crohn disease. In fact, Egeberg, et al. investigating the prevalence and risk of inflammatory bowel disease in patients with hidradenitis compared with the general population, concluded that hidradenitis was significantly associated with the presence and risk of new-onset inflammatory bowel disease, which may be due to a shared immunopathogenesis [13].
Risk factors
Genetic susceptibility, mechanical stresses on the skin, smoking, obesity, adipokine dysregulation and insulin/glucose dysregulation, diet, hormonal factors and microbiome are consider as factors that may be associated with the development or exacerbation of hidradenitis [1,10].
Identification of families where hidradenitis was transmitted as an autosomal dominant trait has highlighted the genetic basis of disease susceptibility. However, the percentage of first-degree relatives affected is around 35%, which differs from the 50% expected for a dominant disease [14]. So, environmental factors have also an important role in the development of the disease.
Increased mechanical stress (pressure, friction, shear) on intertriginous skin and other areas (eg: beltlines, brassiere straps, and other sites of clothing friction) likely contributes to the localization of hidradenitis via increasing the chance of follicular occlusion and follicular rupture [1].
Approximately 70% to 75% of patients with hidradenitis smoke and 10% to 15% are past smokers [16]. Stimulatory effects of nicotine and other components of tobacco on follicular occlusion, neutrophil chemotaxis, TNF-alpha production by keratinocytes, and Th17 cells have been cited as potential contributing factors [1].
The prevalence of being overweight or obese may be higher than 75% in patients with hidradenitis [16]. One theory for the association between excess weight and hidradenitis is that dietary choices that increase risk for the disease are those that also contribute to obesity. Also, hormonal changes associated with obesity may result in relative androgen excess and are proposed to increase follicular plugging. Moreover, the comparatively larger size of intertriginous areas, local skin irritation from sweat retention, narrowed follicular orifices secondary to intrafollicular keratin hydration during skin occlusion, and obesity-related increases in levels of circulating proinflammatory cytokines may contribute [1].
An impact of hormones on hidradenitis is suggested by observations of the rarity of hidradenitis in prepubertal children and improvement in hidradenitis during treatment with antiandrogenic agents [1]. In fact, studies have demonstrated that the use of anti-androgenic medications has a favorable response in comparison to antibiotic based therapy alone [12]. In support of a contribution of androgens to hidradenitis, there are reports of female patients in whom treatment with oral contraceptives containing androgenic progestins, intramuscular medroxyprogesterone acetate, or levonorgestrel in an intrauterine device may precipitate or worsen hidradenitis [1]. The role of sex hormones in the pathogenesis of hidradenitis remains unclear. The prevalence of hidradenitis is most often reported to be higher in women and changes in hidradenitis activity have been reported during times of fluctuating hormones such as during premenstrual periods, pregnancy, and menopause [12].
Next-generation sequencing analysis demonstrated a significantly different microbiome in patients with hidradenitis (lesional and nonlesional) compared with that in healthy controls. In lesional skin, microbiome types consisted predominantly of Corynebacterium species or Porphyromonas and Peptoniphilus species, which were not detected in healthy controls. Several taxa, including Propionibacterium, showed a significantly higher relative abundance in healthy controls versus hidradenitis skin. The data suggest that a dysbiotic microbiome may have a role in the development of hidradenitis suppurativa [2]. Dysbiosis could allow the development of a pathobiome or an augmented expression of virulence factors by otherwise harmless commensal bacteria probably driven by host inflammation [14]. However, Ring, et al. [2] showed paucity rather than an enrichment of bacterial aggregates in pre-clinical hidradenitis skin when compared with healthy controls, suggesting that bacterial infection is secondary to the underlying inflammatory process and the inflammation is not caused by an infection [7,14].
Overall, hidradenitis is perceived as a complex disease where environmental factors trigger chronic inflammation in the skin of genetically predisposed individuals [14].
Clinical features
Hidradenitis mostly occurs in intertriginous areas. Hidradenitis can affect the axillae (most common site), inguinal area, inner thighs, anogenital regions, perineum, mammary and inframammary regions, buttocks, trunk, and, occasionally, the scalp and retroauricular areas. Beltlines, waistbands, abdominal folds, and brassiere straps or bands are common locations [1,8].
The primary visible lesions of hidradenitis are inflammatory nodules (eg: elevated, solid, palpable lesions). Most frequently, the first lesion is a solitary, painful, deep-seated inflamed nodule (0.5 to 2 cm in diameter), often in an intertriginous area [1,10]. Up to 50% of patients report a burning or stinging sensation, pain, pruritus, warmth, and/or hyperhidrosis, 12-48 hours before an overt nodule occurs. Mean duration of a single painful nodule is 7-15 days [8]. After a variable period of time, subcutaneous inflammation of contiguous nodules leads to the development of abscesses yielding purulent or serosanguineous drainage, sinus tracks, fistulae and scarring [1,8,10].
Sinus formation, clusters of open comedones (described as tombstone comedones because they reflect end stage damage to the folliculopilosebaceous unit with associated loss of the sebaceous gland and hair), and scarring often appear in long-standing hidradenitis and are the result of recurrent or persistent disease [1,10]. Lymphoedema can occur as a consequence of lymph gland scarring and subsequent obstruction of lymph drainage [10].
The appearance of healed areas ranges from individual, pitted, acneiform scars after resolution of small nodules to dense, fibrotic bands or indurated, thick, scarred plaques affecting the whole affected area. Scars may also be atrophic (particularly on the trunk) or keloidal, and scarring on the buttocks sometimes manifests as multiple pitted scars. In patients with active disease, scarring is accompanied by inflammatory nodules and draining sinuses [1].
Diagnosis
The diagnosis of hidradenitis suppurativa is normally based on the patient history and physical examination with recognition of characteristic clinical manifestations [1].
The three main clinical features that support the diagnosis of hidradenitis are:
• Typical lesions - multiple deep-seated painful inflamed nodules, tombstone comedones, sinus tracts, abscesses and/or fibrotic scars;
• Typical anatomical locations - occur in ≥ 1 of the areas for which hidradenitis has a predilection, namely axillae, groins, perineal and perianal regions, buttocks, infra-mammary and inter-mammary folds;
• Relapses and chronicity - a clear history of chronicity and recurrence is essential for the diagnosis, 2 recurrences in intertriginous areas over a period of 6 months or persistent lesions for ≥ 3 months have been used as a qualifier for the diagnosis of hidradenitis [1,7,10,17].
All 3 criteria must be present for the definitive diagnosis, so an observation period may be necessary to establish the diagnostic requirement for recurrence/chronicity [17].
No pathognomonic test exists [17]. Bacterial culture is not recommended in clinical practice unless signs of secondary infection such as surrounding cellulitis or fever are present [16]. A skin biopsy usually is not required. Imaging techniques are not necessary for the diagnosis of hidradenitis suppurativa [1,18]. However, though staging and monitoring of patients with hidradenitis suppurativa have been traditionally based on clinical evaluation, alone usually underestimates the severity and disease involvement [1,18-20]. In fact, physical examination may show important limitations because of its low sensitivity to define the disease’s activity and its poor sensitivity for differentiating between different lesion subtypes or the true extent of inflammatory edema, which are critical determinants for the assessment of severity of hidradenitis according to the clinical criteria [19].
Some non-invasive skin imaging techniques such as ultrasound, magnetic resonance imaging, computed tomography, positron-emission tomography and dermoscopy, have been demonstrated to be useful in the diagnosis and management of hidradenitis, since they may reveal some findings not appreciable at physical examination, suggesting the diagnosis in case of minimal/mild presentation or in non-active long-lasting cases and allowing a more accurate staging, treatment planning and monitoring of this debilitating disease [21].
The use of ultrasound, in particular, can improve the staging and management of hidradenitis, since it has the ability to detect, categorize, and measure lesions, including subclinical ones, in all corporal regions affected by the disease, map the extent of the abnormalities and also be useful for preoperative assessment of the subclinical extent of disease [1,18-22]. This imaging information may allow better selection of the type of medical treatment or the location and extent of the surgical incision. Also, in patients with indications for local or systemic treatments, imaging can be a noninvasive tool for monitoring the response [22].
In this context, Martorell, et al. developed a standardized sonographic report that could summarize the relevant anatomical characteristics and staging of patients to better support therapeutic decisions, considering the identification of sonographic key lesions (pseudocyst, fluid collection, fistulous tract, connected fistulous tracts and hair tracts), measurements of the lesions, evaluation of layer location, lymph node presence, detailed evaluation of blood flow with spectral curve analysis in the periphery of the key lesions and evaluation of both axillary and groin regions plus any other symptomatic or clinical region in all cases [19].
Additionally Wortsman, et al. found that fistulous tracts in hidradenitis are key signs of severity and some factors could affect the reversibility of the anatomic changes and physiopathological process, like the presence of fibrotic scarring, because this may not be easily resolved by current medical treatments, the presence of edema and hypervascularity, which may indicate the level of inflammation to which an individual is exposed, and retained hair tract fragments within fistulous tracts that can also generate a chronic irritation factor in the region associated with difficulties in their reabsorption. So, they developed a sonographic classification into 3 types of fistulae: type 1: low fibrotic scarring (grades 0-1) with high or low edema (grades 0-2); type 2: high fibrotic scarring (grade 2) with low edema (grades 0-1); type 3: high fibrotic scarring (grade 2) with high edema (grade 2). Type 3 concentrated 71% of the cases presenting communicating tracts, and type 2, 29% [18].
Categorizing fistulous tracts in hidradenitis using ultrasound, may support earlier and more precise management. In fact, subclinical fistulous tracts detected on ultrasound can modify sonographic scoring, which can indicate the need to move to more aggressive treatment or switch to surgical management. Therefore, the sonographic categorization of fistulous tracts into 3 types may serve as an additional tool for assessing severity in these cases, and perhaps may help to predict a future early need for, or the response to, more aggressive types of treatment such as biologic drugs or surgery [18].
Overall, imaging techniques are elective medical tests for staging and monitoring patients, which can support therapeutic decisions by providing earlier, objective, deeper, anatomical, and comparative evaluations in this difficult to treat disease [19].
Clinical staging
Several scoring systems to describe the severity of hidradenitis exist but the most commonly used is the Hurley staging classification system [6]. The Hurley clinical staging system is used to divide patients with hidradenitis into three disease severity groups:
Stage I - Abscess formation (single or multiple) without sinus tracts and cicatrization/scarring;
Stage II - Recurrent abscesses with sinus tracts and scarring, single or multiple widely separated lesions;
Stage III - Diffuse or near-diffuse involvement, or multiple interconnected tracts and abscesses across the entire area [17].
Stage I is the most common, affecting 68% of patients, stage II occurs in 28% of patients, and 4% develop stage III [17].
The Hurley classification is useful for rapid classification of hidradenitis severity, but it has serious limitations [17]. In fact, whereas the Hurley staging system only has three categories, it is based on static disease characteristics, and only accounts for the clinical presentation, other more advanced systems exist to categorize treatment effectiveness and quality of life impairment [6,17].
Sartorius, et al. proposed four parameters in the evaluation of treatment effectiveness (Hidradenitis Suppurativa Score): 1) Anatomical region involvement; 2) Number of lesions (abscesses/nodules and fistulae); 3) Longest distance between two relevant lesions; and 4) Clear separation of all lesions by normal skin. A final numerical score is constructed from the analysis of each of these parameters for each anatomical region (axillae, groin, buttocks), with higher scores correlating with higher disease intensity. With this numerical and dynamic Hidradenitis Suppurativa Score, disease intensity can be specified and quantified, and the development of the disease can be measured before and after treatment [6]. However, although the severity of mild hidradenitis can be measured quite rapidly with the Sartorius score, its use may be limited in more severe cases, since distinguishing separate lesions in this cases becomes challenging, particularly when lesions become confluent and sinuses become interconnected [17].
The Physician Global Assessment is another score that has been developed for use in a phase II trial, with 6 defined stages:
Clear - No inflammatory or non-inflammatory nodules;
Minimal - Only the presence of non-inflammatory nodules;
Mild - Fewer than 5 inflammatory nodules without abscesses and draining fistulas or 1 abscess or draining fistula without additional inflammatory nodules;
Moderate - Fewer than 5 inflammatory nodules, or 1 abscess or draining fistula and ≥ 1 inflammatory nodules, or 2 to 5 abscesses or draining fistulas and fewer than 10 inflammatory nodules;
Severe - Two to 5 abscesses or draining fistulas and ≥ 10 inflammatory nodules;
Very severe - > 5 abscesses or draining fistulas [17].
The Hidradenitis Suppurativa Severity Index was created by Kerdel, et al. and incorporates categorical objective parameters with categorical subjective patient-reported parameters [17].
The most recently developed and validated hidradenitis-specific score is the Hidradenitis Suppurativa Clinical Response that provides a meaningful clinical endpoint in hidradenitis, defined as a) > 50% reduction in inflammatory lesion count; b) No increase in abscess count and c) No increase in draining fistula count [6,17].
Complications
Long-standing, poorly controlled hidradenitis may lead to significant physical and emotional consequences. Complications include:
• Strictures and contractures;
• Lymphatic obstruction, lymphedema of limbs and genitalia;
• Malaise, depression, and suicide;
• Long-term effects of chronic inflammation including anemia, hypoproteinemia, and amyloidosis;
• Infectious complications (eg, lumbosacral epidural abscess, sacral bacterial osteomyelitis);
• Arthritis;
• Squamous cell carcinoma;
• Anemia;
• Fistulae into the urethra, bladder, rectum, and peritoneum [1].
Medical treatment
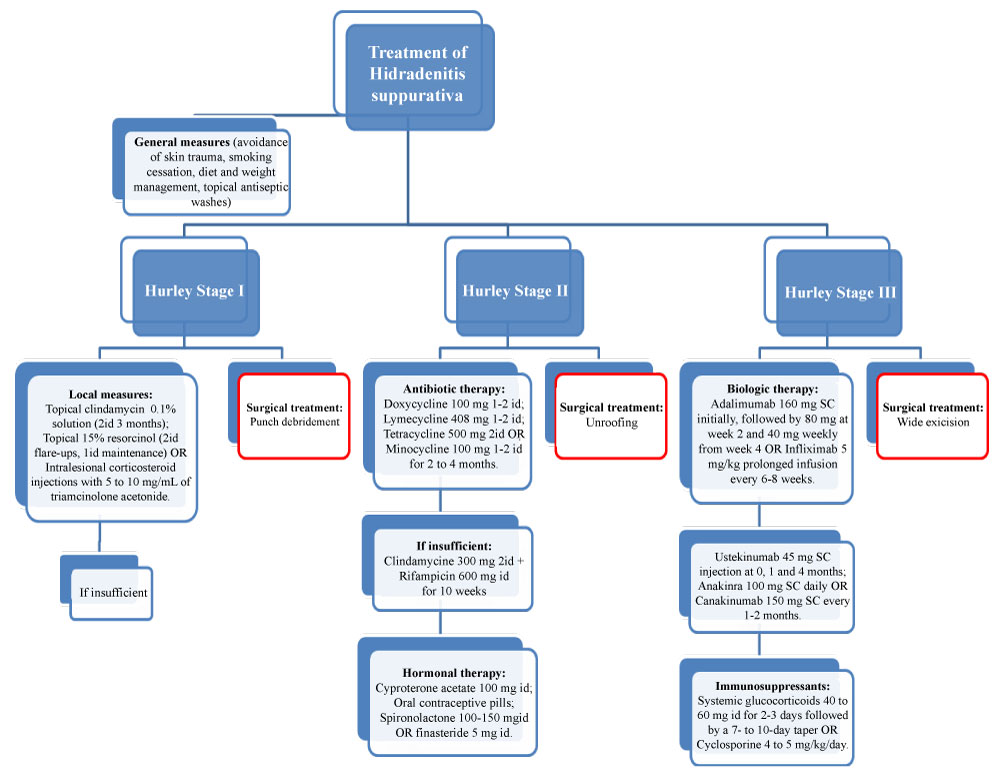
Hidradenitis is a complex disorder that requires a holistic approach (Figure 1). Patient education about the complex nature of the disease and its comorbidities is essential to ensure treatment compliance [10]. Interventions for hidradenitis target one or more of three major goals:
• Reduce the frequency of new lesions, minimizing pain and suppuration;
• Prevent disease progression by limiting the formation of scarring;
• Treat existing lesions and scarring, a goal which may require a combination of medical and surgical intervention [23].
More than 50 interventions exist for treatment of hidradenitis suppurativa, including patient self-management strategies, topical therapy, oral systemic agents, biologic therapies, surgery, and laser and light interventions. Disease severity, patient tolerance of specific agents, comorbidities, and treatment cost and availability guides treatment choices [23].
General measures in all hidradenitis stages
• Self-management - Patient self-management is an important part of the approach to hidradenitis. Patients should be offered individualized self-management strategies: Avoidance of skin trauma, Smoking cessation, Weight management, Topical antiseptic washes for decreasing bacterial colonisation (Figure 1) [9,10,23]. Cessation of smoking and weight loss seem to lead to a reduction in the severity of the disease and some patients go into remission [10].
• Management of comorbidities - Patients with hidradenitis may be at increased risk for alcohol dependence, nicotine addiction, depression, cardiovascular risk, hypertension, diabetes, hyperlipidaemia, obesity, metabolic syndrome, rheumatologic conditions, autoimmune conditions, follicular syndromes, polycystic ovarian syndrome, thyroid disease, and malignancies [10,24].
• Pain management - Pain from hidradenitis nodules and abscesses may cause sleep disturbance, limit function, and induce psychologic distress. Nonsteroidal anti-inflammatories can be used to treat both pain and inflammation. Additional analgesia, including opioid analgesia, may be needed [23].
Hurley stage I (mild disease)
• Local measures - local therapy combined with the general approaches described above is the preferred treatment of mild hidradenitis (Figure 1). Useful local measures include topical clindamycin 0.1% solution (category of evidence IIb, strength of recommendation B [25]), applied to affected areas twice daily for three months, intralesional corticosteroid injections with 5 to 10 mg/mL of triamcinolone acetonide (category of evidence IV, strength of recommendation D [25]), and topical 15% resorcinol (category of evidence III, strength of recommendation C [25]), applied to affected areas twice daily during flare-ups and once daily during maintenance treatment. Resorcinol is a chemical peeling agent with keratolytic and anti-inflammatory properties that reduces pain and promotes healing [7,9,10,23]. In a prospective case series of 36 patients, triamcinolone was injected into hidradenitis lesions, with pain, erythema, edema, suppuration and lesion size being significantly reduced [26].
• Antibiotic therapy - When these measures are insufficient, oral tetracyclines may be beneficial (Figure 1), suppressing neutrophil migration, chemotaxis, and inhibiting matrix metalloproteinase, with a favorable adverse effect profile [7,9,10,23]. Short courses (eg: seven days) of penicillin-type antibiotics do not appear to alter the natural history of an acute hidradenitis lesion. A strategy endorsed by the European hidradenitis guidelines is to give a more prolonged course of an oral tetracycline such as lymecycline or doxycycline (category of evidence IIb, strength of recommendation B [25]). The intent of treatment is to accelerate the resolution of early, painful inflammatory lesions and to prevent or reduce the frequency of new lesions [9,10,23].
Hurley stage II
• Antibiotic therapy - antibiotics may help to control skin bacterial load and are also used for their anti-inflammatory effects. Oral tetracyclines (category of evidence IIb, strength of recommendation B [25]) are a key treatment for mild to moderate hidradenitis (Figure 1). A common regimen for adults is 100 mg of doxycycline given once to twice daily. Alternative tetracycline regimens include lymecycline (408 mg once or twice daily), tetracycline (500 mg twice daily), and minocycline (100 mg once or twice daily). Treatment is generally continued for 2 to 4 months [9,10,23]. In a study of 20 patients, treated with 100 mg of minocycline daily combined with 0.5 mg of colchicine twice daily for 6 months and then a maintenance regimen of 0.5 mg of colchicine twice daily for 3 months, all patients started to show signs of improvement within the first 3 months of therapy and continued to improve over the next 6 months [27]. Combination therapy with clindamycin 300 mg twice daily and rifampicin 600 mg daily (category of evidence III, strength of recommendation C [25]) for 10 weeks has been shown to be effective for hidradenitis in several case series and is an option for patients who fail to respond to oral tetracyclines (Figure 1) [9,10,23]. However, has limited long term efficacy. A prospective study in which oral clindamycin and rifampicin were given to patients for 12 weeks with 1-year follow-up reported an initial clinical response in 19 of 26 patients immediately following the treatment then decreasing to 7 patients at 1 year [28]. Dapsone, a sulfone drug with immunomodulatory and antibacterial properties that is utilized for the treatment of multiple neutrophil predominant skin diseases, may be effective in mild to moderate hidradenitis (category of evidence IV, strength of recommendation D [25]), particularly in the early neutrophil-mediated phase of new lesions. Erythromycin and cephalosporins have also been used for long-term antibiotic therapy as well as the combination therapy with rifampin, moxifloxacin, and metronidazole [9,10,23].
• Hormonal therapy - Androgens may contribute to the development of hidradenitis. Examples of antiandrogenic therapies (category of evidence IV, strength of recommendation D [25]) that may improve the disease include cyproterone acetate (100 mg daily), oral contraceptive pills, spironolactone (100-150 mg/day), and finasteride (5 mg/day) (Figure 1) [9,23]. A study showed 75% improvement, with 59% experiencing complete resolution with treatment on finasteride [12]. A case series with hidradenitis treated with spironolactone demonstrated notable results, with the majority presenting improvements in their hidradenitis symptoms and complete resolution of disease. Multiple studies have demonstrated that spironolactone and cyproterone acetate may be equivalent in efficacy [12].
• Oral retinoids - The antiproliferative and immunomodulatory effects of oral retinoids can control the inflammatory nature of hidradenitis. Use of acitretin (category of evidence III, strength of recommendation C (25)) for 9 to 12 months resulted in lesion resolution, pain relief, and persistent improvement even after discontinuing the therapy. However, it is not recommended in women of child-bearing age, as they should not conceive for 3 years even after treatment cessation [9].
Hurley stage III (severe and refractory disease)
• Biologic therapy - Patients who do not respond sufficiently to oral antibiotics, oral retinoids, or hormonal therapies may benefit from biologic treatments, in particular, adalimumab (category of evidence Ib, strength of recommendation A [25]) or infliximab (category of evidence Ib/IIa, strength of recommendation B [25]) - TNF-α inhibitors [23] (Figure 1). Adalimumab is recommended in hidradenitis at a dose of 160 mg SC initially, followed by 80 mg at week 2 and 40 mg weekly from week 4 [29]. Infliximab is given by prolonged infusion every six to eight weeks, with dosing based on body weight (5 mg/kg) [10]. The two phase 3, placebo-controlled trials - PIONEER I and II, that supported the approval of adalimumab in hidradenitis reported clinical response > 50% in about 41-58% of the study patients [30,31]. These studies indicated that patients with hidradenitis who received a short duration of adalimumab treatment experienced better combined efficacy and similar safety compared with placebo [31].
• Conventional immunosuppressants - Occasionally, systemic glucocorticoids (category of evidence IV, strength of recommendation D (25)) 40 to 60 mg per day for two to three days followed by a 7- to 10-day taper, or cyclosporine (category of evidence IV, strength of recommendation D [25]), 4 to 5 mg/kg per day orally, are prescribed for hidradenitis (Figure 1), however, evidence on the efficacy of these treatments for hidradenitis is limited. In addition, because these drugs may induce severe adverse effects, they are rarely utilized for long-term therapy [23].
• Emerging therapies - Ustekinumab (IL-12/IL-23 antibody) 45 mg SC injections at 0, 1 and 4 months, anakinra (IL-1 receptor antagonist) 100 mg SC daily, and canakinumab (monoclonal antibody to IL-1β) 150 mg SC every 1-2 months, are biologic therapies that may be of benefit for patients with severe and refractory hidradenitis based upon limited data (Figure 1) [23,32]. In a open-label study with 17 patients receiving ustekinumab treatment at weeks 0, 4, 16 and 28, moderate-to-marked improvement of the modified Sartorius score was achieved in 82% of patients at week 40 and the Hidradenitis Suppurativa Clinical Response 50 in 47% [33]. A double-blind, randomized, placebo-controlled clinical trial with a 12-week treatment phase and a 12-week follow-up phase, with 20 patients, comparing anakinra with placebo, decreased disease activity score was reported in 67% of the anakinra group (compared to 20% in the placebo group) and clinical response was achieved in 78% of the anakinra group (compared to 30% in the placebo group) [34].
• Alternative therapies - Metformin 500 mg daily to 500 mg three times daily, oral zinc (category of evidence III, strength of recommendation C [25]) 90 mg/day for at least 6 months, and laser or light therapies (photodynamic therapy, psoralen + ultraviolet A - PUVA, long-pulsed neodymium:yttrium-aluminum-garnet laser - Nd:YAG (category of evidence Ib, strength of recommendation A [25]), intense pulse light - IPL (category of evidence IV, strength of recommendation D [25]) and carbon dioxide laser ablation) may be useful as adjunctive or alternative therapies [16,23,32]. Metformin though primarily an antidiabetic drug, has found to be a potential treatment option in hidradenitis because of its role in improving hyperinsulinemia and its antiandrogenic properties [35].
Surgical treatment
In the setting of refractory hidradenitis, surgery may be needed to remove active foci of disease and eliminate scarred tissue sequelae. Surgery aids in the control of inflammation through several mechanisms by removing epithelialized sinus tracts and associated debris that act as foreign bodies under the skin and removing and draining inflammatory material. Arresting these processes prevents the progressive invasion and sinus formation that lead to scarring [36].
Surgical procedures may be performed in any Hurley stage of disease, since punch debridement or limited unroofing on individual inflammatory nodules or sinus tracts, up until wide excision in severe cases typically reserved for Hurley stage III disease. The approach to treat lesions is individualized and becomes more aggressive with higher-stage hidradenitis, particularly if medical therapies have not been successful at controlling the disease [1,23,36]. Surgery alone does not alter disease biology, understanding the trade-offs between extent of excision, surgical morbidity, and reducing the risk of future lesions is an important surgical judgment [16].
As surgery is an invasive procedure that will result in additional scarring and also carries a high recurrence rate, prior to proceeding, the risks, benefits, and alternatives should be discussed with the patient [9,36].
Surgical procedures:
• Punch debridement - punch debridement (mini-unroofing - category of evidence III, strength of recommendation C [25]) is indicated for small nodules, being the treatment of choice for acute inflammatory nodules, typically in patients with mild or moderate hidradenitis (ie, Hurley stage I or II) (Figure 1). Punch debridement (mini-unroofing) is centered around a single folliculopilosebaceous unit to evacuate a newly inflamed nodule. The objective of punch debridement is to remove the fractured folliculopilosebaceous unit in the initial punch with its associated sebaceous glands and the area of the follicular unit that contains the stem cells, hypothesized to be responsible for inducing growth of the proliferative mass and the subcutaneous sinuses and sinus tracts. The surgical removal of the entire involved folliculopilosebaceous unit in this manner eliminates the potential for lesion recurrence and prevents the development of the invasive proliferative gelatinous mass that results from follicular rupture and likely leads to sinus formation. Punch debridement involves the use of a 5- to 7-mm circular punch to deeply excise the acutely inflamed folliculopilosebaceous unit within an inflammatory nodule with a small amount of surrounding tissue. This is followed by aggressive debridement using digital pressure and then curettage or simple grattage (scrubbing) with gauze wrapped around a cotton-tipped swab. The wound is allowed to heal by secondary intention [36].
• Unroofing - Surgical unroofing (also described as deroofing - category of evidence IV, strength of recommendation D [25]) is indicated for the treatment of inflamed nodules, abscesses, and sinus tracts typically found with moderate and severe hidradenitis (ie, Hurley stage II or III) (Figure 1). The technique consists of careful unroofing and debridement of sinuses and inflamed tissue under local or regional anesthesia, sinus tracts or the inflamed cavity are entered through the overlying skin or through a sinus opening and opened widely with scissors, explored with scissor tips or a malleable metal probe, and serially unroofed until no residual tracts of inflammatory activity remain. The active proliferative inflammatory mass that is attempting to repopulate the area with new folliculopilosebaceous unit must be completely removed by rough gauze grattage (scrubbing) or by sharp curettage with a spoon curette, such as a 6 × 8 mm #0 oval or a Volkmann bone curette. Any residual epithelialized sinus floor may be left exposed to assist healing by secondary intention, or it may be dissected away [36]. A similar procedure referred to as STEEP (skin-tissue-saving excision with electrosurgical peeling) has been described as an alternative to wide excision for Hurley stage II or III disease that preserves normal tissue while completely removing lesional tissue [36,37]. In STEEP procedure fat is maximally spared by performing successive tangential excisions of lesional tissue until the epithelialized bottom of the sinus tracts has been reached, while wide excisions generally reach into the deep subcutaneous fat [38]. In this technique, performed mostly under general anesthesia, the roof of the tract is incised electrosurgically with a wire loop tip coupled to an electrosurgical generator. Consecutive tangential excisions are made until the floor of the sinus tract is reached and the entire area is clear of lesional and fibrotic tissue. The wound margins are probed for the presence and subsequent removal of residual sinus tracts. Lastly, the electrosurgical generator is used to achieve hemostasis, steroids are injected to prevent the formation of hypergranulation tissue, and the wound is left to heal by secondary intention [37,39]. Carbon dioxide laser excision (category of evidence Ib, strength of recommendation A [25]) and marsupialization (laser vaporization of the wound base and edges to create a pocket-like defect with smooth, rounded edges) with healing by secondary intention can also be used to perform the unroofing of nodules, abscesses, or sinus tracts [16,36].
• Wide excision - Wide excision (category of evidence IIb, strength of recommendation B [25]) aims at complete removal of lesions and may be needed to manage an area of chronic or extensive hidradenitis (Hurley stage III) (Figure 1), when more conservative medical and surgical measures fail. Surgery, which entails wide excision of the entire affected area with margins beyond the clinical borders of disease (often a lateral margin of 1 cm), combined with continued aggressive medical management is the treatment most likely to achieve the best results. Tissue should be removed until only normal-appearing subcutaneous fat remains. Removing more than the epidermis, its appendages, sinuses, and associated inflammation and scar tissue is only necessary when a true fistula (to a deep structure) is encountered. The best method of skin closure after wide excision is controversial and largely dependent on the size of the excision. Some prefer healing by secondary intention (category of evidence IIb, strength of recommendation B [25]) for even very large excisions. Although effective, this requires prolonged recoveries that can be painful, having the risk of infection, joint contractures and scarring. Local excision with primary closure (eg: Pollock procedure - category of evidence III, strength of recommendation C [25]) may allow faster healing and is quite useful for smaller excisions, but if the disease is not adequately excised, active disease can then present at the periphery of the excision. With more extensive surgical excision, closure of the skin defect with advancement flaps (category of evidence Ia/IIa, strength of recommendation A/B [25]) or split-thickness skin grafting (category of evidence III, strength of recommendation C [25]) may be needed to accelerate wound healing. Regional or free flaps provide thicker coverage with a more natural, less scar-like appearance, but they can be bulky and require thinning as a secondary procedure [16,36,39].
• Incision and drainage - Routine incision and drainage of individual nodules is not an effective or appropriate method for managing hidradenitis and should not be performed to treat solid, inflamed nodules. Nevertheless, incision and drainage may be needed for the immediate relief of pain when fluctuant abscesses are present, but provides only short-term relief, and because it does not clear the actively growing tissue, lesions treated in this manner tend to recur (recurrence rates approach 100%). For drainage, wide circumferential local anesthesia is administered followed by incision. Pus is eliminated using digital pressure or saline rinses. Packing the wound for a few days is usually needed to prevent premature superficial closure while the wound fills in from below [7,16,36,37,39].
Surgical complications
Most patients show good tolerance to surgery, but still the option of healing is accompanied by the possibility of complications and long hospital stays [40].
Complications from surgery can include bleeding, infection-delayed healing, and new disease at the periphery. Resection wounds are highly contaminated, and, if wound complications occur, a combination of intravenous antibiotics and negative pressure wound therapy can be used to manage them [36].
Even after extensive surgery, recurrence is a major concern in patients with hidradenitis suppurativa [40]. A systematic review by Mehdizadeh, et al. estimated average recurrences of 22 percent with local incision, 27 percent with unroofing, and 13 percent with wide excision (15 percent for primary closure, 8 percent for flap closure, and 6 percent for graft closure) [41].
In a study of long-term follow-up (min 1.08 years: max 6.25 years) after radical surgery postoperative recurrences of hidradenitis were seen in 54.2%. Of these 29.2% developed local recurrences (same anatomical region), 16.7% locally in combination with distant recurrences (other anatomical region), and 8.3% had distant recurrences exclusively. Most recurrences (inflamed nodules) were detected in a < 1-cm margin around the operative field. Reviewing the local recurrences according to the anatomical region, the authors’ patient group showed most local recurrences in the inguinal area. On average, recurrences occurred 1.7 years after the operation (min 3 weeks; max 5 years). Surgery under tumescence local anesthesia showed symptoms in 40.6% compared with 28.6% under general anesthesia [40].
However, at least 1 year after wide excision, complete recovery was observed in 45.8% patients with hidradenitis [40].
Although the use of the term "recurrence", some authors consider that the disease does not "recur" after wide excision but can develop in other areas, either around the previous excision or in other anatomic areas [36].
If disease comes back adjacent to areas that were widely excised, the patient could be referred for medical management and try to space surgeries at least three months apart. If medical management is not successful, in most cases small excisions with direct closure can be used to treat any new disease [36].
Conclusion
Successful treatment of hidradenitis requires lifelong attention to prevent new lesions in all stages of the disease. Measures to prevent new hidradenitis lesions include dietary and behavioral changes and topical or systemic medications. Surgical interventions may become necessary to manage nodules, abscesses, sinus tracts, and scars [36].
In fact, surgery has been a mainstay of hidradenitis management for some time, and is often used for patients with extensive Hurley stage III disease, with the best results being achieved with wide local excision [42].
However, the disease often recurs, and this has led to a recent interest in the use of targeted biologic therapy combined with surgical treatment in the management of hidradenitis. A retrospective study of 21 patients undergoing both surgery and biological treatment (infliximab and ustekinumab) or surgery alone found significantly lower recurrence rates and disease progression, as well as a longer disease-free interval in the combined cohort. Patients who underwent combined treatment had a recurrence rate of 19%, new disease developed in 18% and the disease-free interval was approximately 1 year longer. Surgery-only patients had a recurrence rate of 38.5% and new disease developed in 50% [43]. Also, in a longitudinal observational study analysing the impact of surgical intervention in hidradenitis with adjunctive biologic therapy (including TNF-α and IL-12/23 inhibitors), biologic therapy was associated with a more rapid decline in disease activity, with the greatest effect in patients who also underwent surgery, concluding that patients who received surgery with biologic therapy were most likely to achieve a 75% reduction in active nodule count, than those who received surgery or biologic therapy alone or neither [42]. Combining surgery with medical therapy and dietary restrictions and behavorial changes, like smoking cessation, weight management, avoidance of skin trauma and topical antiseptic washes, provides the best chance for preventing the development of new lesions and controlling disease progress [9,10,23,36].
Disclosure
The authors did not receive any funding for this study. They have no financial disclosures and report no conflicts of interest.
References
- Ingram John R (2019) Hidradenitis suppurativa: Pathogenesis,clinical features, and diagnosis. UpToDate.
- Ring HC, Thorsen J, Saunte DM, et al. (2017) The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol 153: 897-905.
- Kouris A, Platsidaki E, Christodoulou C, et al. (2016) Quality of life and psychosocial implications in patients with hidradenitis suppurativa. Dermatology 232: 687-691.
- Machado MO, Stergiopoulos V, Maes M, et al. (2019) Depression and anxiety in adults with hidradenitis suppurativa: A systematic review and meta-analysis. JAMA Dermatol.
- Matusiak Ł, Szczęch J, Kaaz K, et al. (2018) Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol 98: 191-194.
- Yao Y, Thomsen SF (2017) The role of interleukin-17 in the pathogenesis of hidradenitis suppurativa. Dermatol Online J 23.
- Saunte DML, Jemec GBE (2017) Hidradenitis suppurativa: Advances in diagnosis and treatment. JAMA 318: 2019-2032.
- Napolitano M, Megna M, Timoshchuk EA, et al. (2017) Hidradenitis suppurativa: From pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol 10: 105-115.
- Lee EY, Alhusayen R, Lansang P, et al. (2017) What is hidradenitis suppurativa? Can Fam Physician 63: 114-120.
- Vekic DA, Cains GD (2017) Hidradenitis suppurativa - management, comorbidities and monitoring. Aust Fam Physician 46: 584-588.
- Ingram JR (2016) Hidradenitis suppurativa: An update. Clin Med (Lond) 16: 70-73.
- Clark AK, Quinonez RL, Saric S, et al. (2017) Hormonal therapies for hidradenitis suppurativa: Review. Dermatol Online J 23.
- Egeberg A, Jemec GBE, Kimball AB, et al. (2017) Prevalence and risk of inflammatory bowel disease in patients with hidradenitis suppurativa. J Invest Dermatol 137: 1060-1064.
- Tricarico PM, Boniotto M, Genovese G, et al. (2019) An integrated approach to unravel hidradenitis suppurativa etiopathogenesis. Front Immunol 10: 892.
- Hoffman LK, Tomalin LE, Schultz G, et al. (2018) Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PLoS One 13.
- Alikhan A, Sayed C, Alavi A, et al. (2019) North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol 81: 76-90.
- van der Zee HH, Jemec GB (2015) New insights into the diagnosis of hidradenitis suppurativa: Clinical presentations and phenotypes. J Am Acad Dermatol 73: 23-26.
- Wortsman X, Castro A, Figueroa A (2016) Color doppler ultrasound assessment of morphology and types of fistulous tracts in hidradenitis suppurativa (HS). J Am Acad Dermatol 75: 760-767.
- Martorell A, Wortsman X, Alfageme F, et al. (2017) Ultrasound evaluation as a complementary test in hidradenitis suppurativa: Proposal of a standarized report. Dermatol Surg 43: 1065-1073.
- Lyons AB, Zubair R, Kohli I, et al. (2019) Preoperative ultrasound for evaluation of hidradenitis suppurativa. Dermatol Surg 45: 294-296.
- Lacarrubba F, Musumeci ML, Martorell A, et al. (2018) Role of the imaging techniques in the diagnosis and staging of hidradenitis suppurativa. G Ital Dermatol Venereol 153: 20-25.
- Ximena Wortsman (2018) Diagnosis and treatment of hidradenitis. JAMA 319: 1617.
- Ingram JR (2019) Hidradenitis suppurativa: Treatment. UpToDate.
- Miller IM, McAndrew RJ, Hamzavi I (2016) Prevalence, risk factors, and comorbidities of hidradenitis suppurativa. Dermatol Clin 34: 7-16.
- Gulliver W, Zouboulis CC, Prens E, et al. (2016) Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord 17: 343-351.
- Riis PT, Boer J, Prens EP, et al. (2016) Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): A case series. J Am Acad Dermatol 75: 1151-1155.
- Armyra K, Kouris A, Markantoni V, et al. (2017) Hidradenitis suppurativa treated with tetracycline in combination with colchicine: A prospective series of 20 patients. Int J Dermatol 56: 346-350.
- Dessinioti C, Zisimou C, Tzanetakou V, et al. (2016) Oral clindamycin and rifampicin combination therapy for hidradenitis suppurativa: a prospective study and 1-year follow-up. Clin Exp Dermatol 41: 852-857.
- Gupta AK, Studholme C (2016) Adalimumab (Humira) for the treatment of hidradenitis suppurativa. Skin Therapy Lett 21: 1-4.
- Kimball AB, Okun MM, Williams DA, et al. (2016) Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 375: 422-434.
- Giamarellos-Bourboulis EJ, Sobell J, Ryan C, et al. (2017) Infection-free clinical response among patients with hidradenitis suppurativa who were treated with adalimumab: Results from Two Phase 3 Studies. Wounds 29: E98-E102.
- Barlev D, Eisen DB, Alikhan A (2015) Hidradenitis suppurativa: A review with a focus on treatment data. Skin Therapy Lett 20: 1-8.
- Blok JL, Li K, Brodmerkel C, et al. (2016) Ustekinumab in hidradenitis suppurativa: Clinical results and a search for potential biomarkers in serum. Br J Dermatol 174: 839-846.
- Tzanetakou V, Kanni T, Giatrakou S, et al. (2016) Safety and efficacy of anakinra in severe hidradenitis suppurativa: A randomized clinical trial. JAMA Dermatol 152: 52-59.
- Bubna AK (2016) Metformin - for the dermatologist. Indian J Pharmacol 48: 4-10.
- Orgill Dennis P (2019) Surgical management of hidradenitis suppurativa. UpToDate.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. (2018) Surgical procedures for hidradenitis suppurativa. Cutis 102: 13-16.
- Blok JL, Spoo JR, Leeman FW, et al. (2015) Skin-Tissue-sparing Excision with Electrosurgical Peeling (STEEP): A surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. Eur Acad Dermatol Venereol 29: 379-382.
- Janse I, Bieniek A, Horváth B, et al. (2016) Surgical procedures in hidradenitis suppurativa. Dermatol Clin 34: 97-109.
- Walter AC, Meissner M, Kaufmann R, et al. (2018) Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg 44: 1323-1331.
- Mehdizadeh A, Hazen PG, Bechara FG, et al. (2015) Recurrence of hidradenitis suppurativa after surgical management: A systematic review and meta-analysis. J Am Acad Dermatol 73: 70-77.
- Shanmugam VK, Mulani S, McNish S, et al. (2018) Longitudinal observational study of hidradenitis suppurativa: impact of surgical intervention with adjunctive biologic therapy. Int J Dermatol 57: 62-69.
- DeFazio MV, Economides JM, King KS, et al. (2016) Outcomes after combined radical resection and targeted biologic therapy for the management of recalcitrant hidradenitis suppurativa. Ann Plast Surg 77: 217-222.
Corresponding Author
Elisabete Campos, Department of Surgery, Centro Hospitalar S. João, Alameda Professor Hernâni Monteiro, 4200-319 Porto, Portugal, Tel: +351961899951.
Copyright
© 2019 Campos E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.