Removal of Phenol from Acqueous Solution by Adsorption on Senna Singueana and Santaloid Afzelli
Abstract
Phenolic compounds are important industrial wastes, and are classified as hazardous substances contaminating groundwater resources. Therefore, the removal or diminish of these organics compounds in order to reach the permitted levels before discharging becomes a challenging.
Several processes have been developed to remove phenolic compounds from waters, including electrochemical oxidation, redox reactions, membrane separation and photocatalytic degradation. Recently, tendency of phenolic compounds removal involves adsorption and photocatalytic process, using synthetic or natural particles, such as carbon materials and clays. Actually, materials in micrometric scale play an important role in the processes previously mention due to their unique chemical and physical properties. In this research work, comparison is done between the adsorption of capacity of Senna singueana and Santaloid afzelli. These two plant leafs are use as absorbent for the absorption of phenol from already prepared phenol solution. The variables effect used for this study are absorbent dosage, temperature, pH, initial ion concentration and contact time. During this study various adsorption isotherms were used to obtain a perfect fit for the adsorption.
Keywords
Phenolic compounds, Senna singueana, Santaloid afzelli, Removal methods, Adsorption
Introduction
Phenols are organic compounds of great environmental interest. Their determination has been increasing in recent years because of their toxicity, even at low concentrations. Phenolic compounds are often derived from various manufacturing processes such as pharmaceutical, oil refineries, coke plants, and phenolic resin plants [1-4].
Phenolic compounds in portable water emit an unpleasant odor and flavor in concentration as low as 5 µg L -1 and are poisonous to aquatic life, plants and humans. Kumar, et al. [5] have reported that ingestion of phenols in concentrations from 10 to 240 mg L -1 for long periods causes mouth irritation, vision problems, diarrhoea, and excretion of dark urine. They are considered one of the priority pollutants by the US Environmental Protection Agency [1,6].
World Health Organisation (WHO) has established the maximum permissible concentration of phenol in drinking water as 1 mg L -1 [7,8]. As a result, various studies have been conducted for the removal of phenolic compounds before being discharged to receiving sink. Water pollution is one of the most important problems in the world, which represents a risk to the human and environment. The increasing industrial and human activities have caused an increase on the discharge of wastewater into the water resources [9-13]. Phenolic compounds from different industrial activities such as refineries, pesticides, insecticides, pharmaceutical, etc., are found among the main pollutants of water. These compounds are toxic and their degradation is difficult; thus, it is important the development of materials and effective methods that allow the removal of these pollutants from water. Different methods have been used to assist with this problem [14-18]. The use of cheap, renewable and readily available absorbents like Senna singueana and Santaloid afzelli has shown to be quite efficient and promising.
Materials and Methods
Senna singuena and Santaloid afzelli leafs were removed, wash, dried, grinded and sieve to obtain absorbent of large surface area. NaOH was prepared, also H 2 SO 4 was also prepared. Distill water was obtained.
Determination of effect of contact time
The effect of contact time was studied using an initial concentration of 60 mg/l. The time intervals chosen for this experiment were 30, 60, 90, 120, 150 minutes.
Procedure
A 0.5g of senna singueana and Santalloid afzelli was weighed properly and mixed with 100 mls solution of initial concentration 60 mg/l. the mixture was shaken constantly for a period of 30 mins. At the end of the contact time period, the mixture was filtered. The procedure was then repeated for 60, 90, 120 and 150 minutes. The concentration of the filtrate was determined using the UV-visible photo spectrometer.
The difference between the initial and final concentration of the solution was recorded as the amount absorbed for each contact time.
Determination of effect of initial ion concentration
The effect of initial ion concentration on adsorption was studied using 0.5g of the adsorbent. The concentrations chosen for this experiment are 20, 30, 40, 50 and 60 mg/l.
Procedure
A 0.5g of Senna singueana and Santaloid afzelli was mixed with 100 mls solution of initial phenol concentration of 20 mg/l. The mixture was shaken constantly for 30 mins. At the end of the equilibrium time, the mixture was filtered. The procedure was repeated for 30, 40, 50 and 60 mg/l. The concentration of the filtrate was determine using UV-visible photo spectrometer.
The difference between the initial and final concentration was recorded as the amount of phenol absorbed for each concentration.
Determination of effect of absorbent dosage
The effect of absorbent dosage on adsorption was studied using initial concentration of 60 mg/l. The absorbent dosage chosen for this experiment were 1g, 1.5g, 2.0g 2.5g and 3.0g respectively.
Procedure
1g of Senna singueana and Santaloid afzelli was mixed with 100 mls solution of initial concentration of 60 mg/l. The mixture was shaken constantly for 30 mins. The mixture was filtered. The procedure was repeated for 1.5g, 2g, 2.5g and 3.0g. The concentration of the filtrate was determined using UV-visible photo spectrometer.
The difference between the initial and final concentration was recorded as the amount absorbed for each absorbent dosage.
Determination of effect of temperature
The effect of temperature on adsorption was studied using 0.5g of the absorbent and initial concentration of 60 mg/l, the temperature chosen for this experiment are 30, 35, 40, 45 and 50 °C.
Procedure
0.5g of Senna singueana and Santaloid afzelli was mixed with 100 mls solution of initial ion concentration of 60 mg/l. The mixture was shaken constantly for 30 mins after which it was placed in water bath at a temperature of 30 °C for about 30 mins. The mixture was then removed from the water bath and filtered. The procedure was repeated for temperature of 35, 40, 45 and 50 °C. The concentration of the filtrate was determined using UV-visible photo spectrometer.
The difference between the initial concentration and final concentration was recorded as the amount absorbed for each temperature.
Determination of effect of pH
The effect of pH on adsorption was studied using 0.5g of the absorbent and an initial ion concentration of 60 mg/l. The pH values chosen for this experiment are pH, 3, 5, 7, 9 and 11.
Procedure
Phenol solutions of 60 mg/l were prepared. 1M nitric acid or 1M of sodium hydroxide was added drop wise until the desired pH of 3, 5, 7, 9 and 11 was obtained. 0.5g of Senna singueana and Santaloid afzelli was measured accurately and mixed with the phenol solutions of varying pH. The mixture was shaken constantly for 30 mins. The mixture was then filtered and the concentration of the filtrate was determined using UV-visible photo spectrometer.
The difference between the initial and final concentration was recorded as the amount of absorbed for each pH.
Calculation of the Percentage of Phenol Absorbed
The percentage adsorption of phenol was calculated for each experiment using the formula
Where R = percentage adsorption of phenol
Ci = initial phenol concentration in mg/l
Ce = final phenol concentration in mg/l
Calculation of amount absorbed
The amount of phenol absorbed was also calculated for each experiment performed using the formula.
Amount absorbed = Ci-Ce
Where Ci = initial phenol concentration in mg/l
Ce = final phenol concentration in mg/l
Thermodynamic treatment of sorption
Thermodynamic considerations of an adsorption process are necessary to conclude whether the process is spontaneous or not. Gibb’s free change, , is the fundamental criterion of spontaneity. Reactions occur spontaneously at a given temperature if is negative. The thermodynamic parameter Gibb’s free energy, for the adsorption process is calculated using the following equations:
Where R is the universal gas constant (8.314 j/mol/k) and T is the absolute temperature in K. Kc is calculated using the formula below:
Where CAE is the amount of phenol absorbed in mg/l,
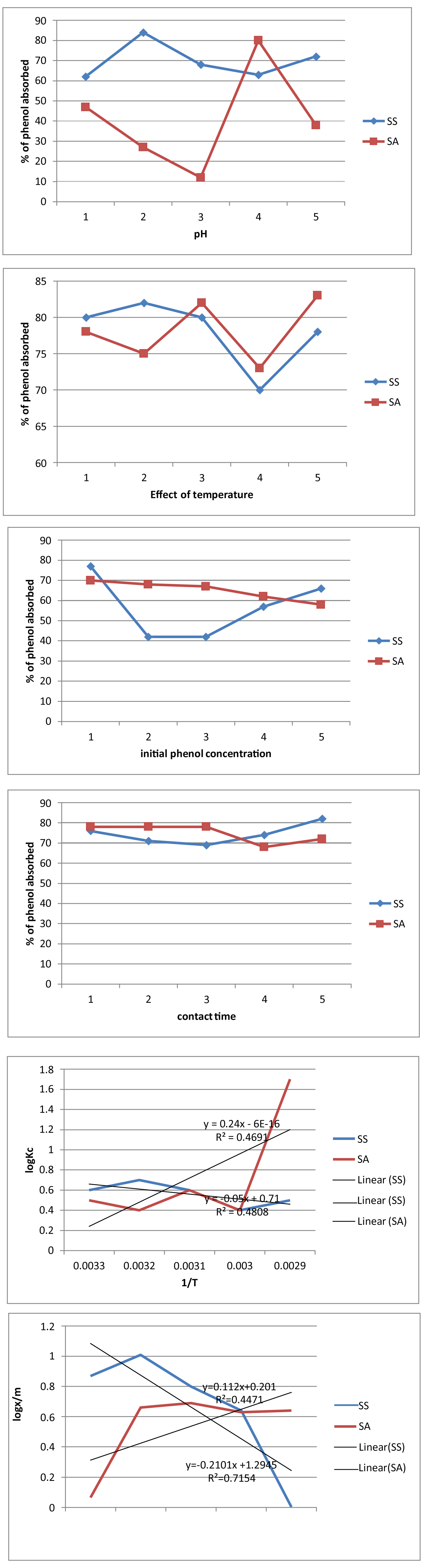
Ce is the equilibrium concentration in solution in mg/l and Kc is the thermodynamic equilibrium constant (Table 1 and Figure 1).
Results and Conclusion
In this study, Senna singueana and Santaloid afzelli were used as absorbent for the removal of phenol from distilled water solution. Batch experiments were carried out to study the effect of variables on the rate of adsorption. Such as contact time, pH, temperature, Initial ion, concentration and absorbent dosage. It was found from the study to be effective in the removal of phenol [19,20].
For the effect of pH, it was observed that as the pH, moves to weakly acidic medium, the adsorption increases. Therefore it can be imply that he adsorption of phenol unto Senna singueana and Santaloid afzelli is favored at low pH.
It was also observed from the effect of contact time that a minimum contact time of 120 minutes is required for the removal of 81.5% of phenol from the solution.
For the effect of temperature, the adsorption decreased as the temperature increased. This implied that the adsorption of phenol onto Senna singueana and Santaloid afzelli is favored at low temperatures.
For the effect of initial ion concentration, it was observed that the adsorption decreased as the initial ion concentration increased from 20-60 mg/l with maximum adsorption of 77% at concentration of 20 mg/l.
It was observed from the effect of absorbent dosage that as the adsorbent dose increased the adsorption increased. This implied that an increase in adsorbent dose has effect on the adsorption. Also a minimum of 6g of the adsorbent will be enough to obtained 100% adsorption of phenol from the solution.
Adsorption equilibrium data fitted well into Langmuir and Freundlich isotherms. Although Langmuir isotherm provided the best fit. This implied that the adsorption of phenol was more of a mono layer adsorption and the adsorbed molecules do not interact with each other.
Recommendation
This study showed that Senna singueana and Santaloid afzelli is readily available and it also has the potential for removing industrial waste from aqueous solution. Also the process is environment friendly and may also provide an affordable technology for small and medium scale industry in Nigeria.
References
- Uddin MT, Islam MS, Abedin MZ (2007) Adsorption of phenol from aqueous solution by water hyacinth J Eng Appl Sci 2: 11-17.
- Ahmaruzzaman M (2008) Adsorption of phenolic compounds on low-cost adsorbents: a review. Adv Colloid Interface Sci 143: 48-67.
- Yamasaki H, Makihatal Y, Fukunaga K (2008) Preparation of crosslinked ?-cyclodextrin polymer beads and their application as a sorbent for removal of phenol from wastewater. J Chem Technol Biotechnol 83: 991-997.
- Okasha AY, Ibrahim GH (2010) Phenol removal from aqueous systems by sorption of using some local waste materials. EJEAF Che 9: 796-807.
- Kumar SD, Subbaiah VM, Reddy AS, et al. (2009) Biosorption of phenolic compounds from aqueous solutions onto chitosan-abrus precatorius blended beads. J Chem Technol Biotechnol 84: 972-981.
- Yan J, Jianping W, Jing B, et al. (2006) Phenol biodegradation by the yeast candida tropicalis in the presence of m-cresol. Biochem Eng J 29: 227-234.
- Kumaran P, Paruchuri YL (1996) Kinetics of phenol biotransformation. Water Res 31: 11-22.
- Atieh MA (2014) Removal of phenol from water different types of carbon-a comparative analysis. APCBEE Procedia 10: 136-141.
- Kilic M, Apaydin-Varol E, Putin AE (2011) Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: Equilibrium, kinetics and thermodynamics. J Hazard Mater 189: 397-403.
- Coulter V (2009) Advanced Organic Chemistry. (1 st edn), Chandni Chowk, Delhi: Global Media.
- Khraisheh M, Wu L, Al-Muhtaseb AH et al. (2012) Phenol degradation by powdered metal ion modified titanium dioxide photocatalysts. Chem Eng J.
- Castro-Muñoz R, Yañez-Fernández J, Fila V (2016) Phenolic compounds recovered from agro- food-by-products using membrane technologies: An overview. Food Chem 213: 753-762.
- Bellona C, Drewes JE, Xu P, et al. (2004) Factors affecting the rejection of organic solutes during NF/RO treatment-a literature review. Water Res 38: 2795-2809.
- Choi BG, Kim DI, Hong S (2016) Fouling evaluation and mechanisms in a FO-RO hybrid process for direct potable reuse. J Memb Sci 520: 89-98.
- Carey FA (2001) Organic Chemistry. 4th ed. Dubuque, Iowa: McGraw-Hill Companies.
- Baruah JB (2011) Chemistry of phenolic compounds: State of the art. New York: Nova Science Publishers,
- Kennedy LJ, Vijaya JJ, Kayalvizhi K, et al. (2007) Adsorption of phenol from aqueous solutions using mesoporous carbon prepared by two-stage process. Chem Eng J 132: 279-287.
- Cheung WH, Szeto YS, McKay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98: 2897-2904.
- Balistrieri LS, Murray JW (1981) The surface chemistry of goethite (-FeOOH) in major ion sea water. Am J Sci 281: 788-806.
- Khalid M, Joly G, Renaud A, et al. (2004) Removal of phenol from water by adsorption using zeolites. Indus Eng Chem Res 43: 5275-5280.
Corresponding Author
E.S. Egga, Chemistry Department, Faculty of Natural Sciences, University of Jos, Nigeria.
Copyright
© 2023 Egga ES, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.