Metabolic Dysfunction Associated Steatotic Liver Disease in Individuals with Obstructive Sleep Apnea: A Meta Analysis
Abstract
Background: Obstructive sleep apnea (OSA) is a disorder with recurrent episodes of upper airway obstruction resulting in periods of apnea. Metabolic dysfunction-associated steatotic liver disease (MASLD) is a disorder where accumulation of lipids in the liver can progress to cirrhosis. While the relationship between both OSA and MASLD is explored in primary studies, a comprehensive meta-analysis is needed for quantifying this association.
Methods: Systematic review was conducted across PubMed, Web of Sciences and Scopus to explore the co-occurrence of MASLD in OSA and calculate the pooled odds ratio (OR) of MASLD to OSA. Weighted mean differences (WMD) for liver enzymes between groups were calculated.
Results: A statistically significant increase in risk of MASLD for individuals with OSA compared to controls was observed with an OR of 2.41 [95% CI: 1.857-3.127] for 9 studies compromising 3,433 participants. Individuals with severe OSA had higher pooled OR of MASLD compared with less severe OSA, OR= 2.68 [95% CI: 1.732-4.145] and 2.096 [95% CI: 1.511-2.907]. Significant WMD was found for both ALT and AST compared to controls, WMD = 4.006 [95% CI: 1.561-6.45] and 1.624 [95% CI: 0.855-2.393]. No differences were found regarding study region, exclusion of individuals with prior OSA diagnosis, population type, and method of MASLD detection.
Conclusion: OSA was cross‑sectionally associated with higher odds of MASLD, with risk increasing by OSA severity. Those with OSA had higher liver enzymes compared to controls. These findings may highlight the need for routine screening for MASLD in individuals with OSA
Keywords
MASLD, MASH, OSA, Liver Enzymes, Meta-analysis, Sleep Apnea
Introduction
Obstructive sleep apnea (OSA) is a disorder characterized by multiple episodes of upper airway collapse during sleep [1]. Recurrent attacks of airway obstruction result in sleep interruptions, leading individuals to experience excessive daytime sleepiness, daytime dysfunction, insomnia, and morning headaches [2,3]. OSA is linked to a greater susceptibility of non-sleep-related comorbidities such as coronary artery disease, heart arrhythmias, and gastroesophageal reflux disease [4,5]. Moreover, a three-fold rise in metabolic and cardiovascular diseases occur in individuals with OSA [6].
OSA affects about 9% to 38% population and is increasing both in prevalence and severity with rising rates of obesity [2,7]. Risk factors linked with the development of OSA include smoking, male gender, and old age [8]. OSA can be diagnosed using overnight polysomnography (PSG) by detecting at least five events of apnea or hypopnea per hour [9].
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), is characterized by an abnormally excessive amounts of fats within the hepatocytes with or without alcohol intake. The spectrum of MASLD is wide, from simple fatty infiltration or steatosis to a severe inflammatory state known as metabolic dysfunction associated steatohepatitis (MASH) with worsening liver fibrosis.
Like OSA, MASLD is related to obesity, where individuals with a severe class of obesity are at an increased risk of developing advanced liver disorders [10]. MASLD is also related to other conditions like diabetes mellitus, chronic kidney disease, cardiovascular diseases [11]. The mechanism underlying the development of MASLD is complex and partially understood. Some mechanisms describe a two-hit hypothesis where the first hit is described as an intrahepatocellular accumulation of lipids, followed by subsequent lipid peroxidation and oxidative stress forming the second hit [12]. OSA and underlying intermittent hypoxia could lead to lipid peroxidation with increased oxidative stress and development of an inflammatory state; all of this could aggravate MASLD [13].
Thus, this study aimed to explore the co-occurrence of MASLD across all levels of severity in those diagnosed with OSA, providing comprehensive evidence about the association between OSA and MASLD. The effects of OSA on liver enzymes were also analyzed through a quantitative analytical study of the current literature.
Methods
Identification of eligible studies
Three different databases were searched in a systematic way, including PubMed, Web of Science (WoS), and Scopus. A comprehensive search was developed using different combinations of words or terms related to MASLD (“Non-alcoholic Fatty Liver Disease”, “Non-alcoholic Fatty Liver”, “Non-alcoholic Fatty Liver”, NAFLD, “Non-alcoholic Steatohepatitis”, NASH, MASLD, “Metabolic dysfunction-associated steatotic liver disease”, MASH, and “Metabolic dysfunction-associated steatohepatitis”) and terms related to OSA (“Obstructive Sleep Apnea”, “Sleep Apnea”, “Obstructive Sleep Apnea Syndrome”, OSAHS, “Obstructive Sleep Apnea Hypopnea Syndrome”, and “Obstructive Hypopnea”). Moreover, relevant medical subject headings (MeSH terms) were used when searching PubMed (“Non-alcoholic Fatty Liver Disease"[Mesh], and "Sleep Apnea, Obstructive"[Mesh]). Database filtration for studies published in the English language and non-review articles was done when appropriate prior to retrieval. All studies up to the cut-off date for conducting the database searching, March 20, 2025, were collected.
The articles collected from the three databases were combined, with duplicate articles being marked and removed using End Note software for citation management. The remaining articles were exported into an Excel spreadsheet and were further subjected to a two-stage screening process. This review protocol was preregistered on the Open Science Framework (OSF) on April 23, 2025 (https:// osf.io/up4xr). There was no external funding received for this research.
Development of the inclusion criteria and exclusion criteria
For a study to be regarded as appropriate extraction, it should (1) be observational in design, (2) have both an OSA group and a non-OSA group as controls, (3) provide prevalence data for MASLD in both study groups or provide an odds ratio, (4) follow objective and validated methods for the diagnosis of both MASLD and OSA, and (5) have a full text that is available in the English language. Studies were excluded if they were of inappropriate study design, such as editorials, notes, letters to editors, abstract studies, review articles, single-arm clinical trials, and book chapters. Studies were also excluded if they were not reporting sufficient data to calculate the odds ratio of MASLD in subjects suffering from OSA compared to those with no OSA. To achieve accurate estimates, studies focusing solely on MASH without addressing the wide spectrum of MASLD were not included in the analysis. The entire screening process was done considering the 2020 PRISMA flow diagram for eligibility evaluation [14].
Extraction of the relevant data and quality assessment
After identifying eligible articles, the following items were identified and collected: Family name (last name) of the first author, the year at which the study was published, country of study conduction, the period of study conduction, study population, method of OSA and MASLD diagnosis, and method of assessing OSA severity. For both the OSA and the control group, the total number of individuals and the number of those with MASLD were extracted. The mean and standard deviation (SD) of both ALT and AST were also recorded. For the OSA group, prevalence of MASLD across each category of severity was extracted.
The process of quality assessment was carried out in accordance with the “Newcastle-Ottawa Scale” (NOS) for all the included studies. This inventory has eight different questions with a nine-point maximum score, as one question, comparability, is rated with two points. Studies having a summed score range of 7-9 are flagged as high-quality papers. Articles with a total score of five or six are deemed of intermediate quality. articles scoring below five are regarded as poor-quality [15].
Statistical analysis
The pooled odds ratio (OR) of MASLD in individuals with OSA with a 95% confidence interval was calculated for all the included articles. The I2 test was utilized to measure between-study heterogeneity. Heterogeneity was classified in accordance with the I² index into four groups: insignificant for I² up to 25%, low for I² up to 50%, moderate up to 75%, and high for those greater than 75% [16]. The random effects model was utilized for all the statistical analyses as it accounts for the variability across different study populations and the potential differences in true effect across studies [17]. The random effects model was implemented using the Der Simonian-Laird method to account for between-study heterogeneity. All analyses were performed using Comprehensive Meta-Analysis (CMA) Software, version 3.0.
A sensitivity analysis was undertaken to determine whether one study inherently impacted the overall pooled estimate. Both visual interpretation and assessment for symmetry for funnel plots and the Egger’s test p-value assessment were utilized to detect if there is an underlying publication bias. Publication bias is considered absent when the plot is visually symmetrical, and the Egger’s test reported p-value is over 0.05. For studies providing prevalence data of MASLD across different categories of OSA severity, a pooled odds ratio for MASLD in mild/moderate OSA in relation to no OSA and for MASLD in severe OSA in relation to no OSA was calculated. Pooled weighted mean difference (WMD) estimates for ALT and AST were calculated for OSA and no OSA groups. A p-value less than or equal to 0.05 was deemed as a threshold for statistical significance throughout the study.
Results
Study identification and screening
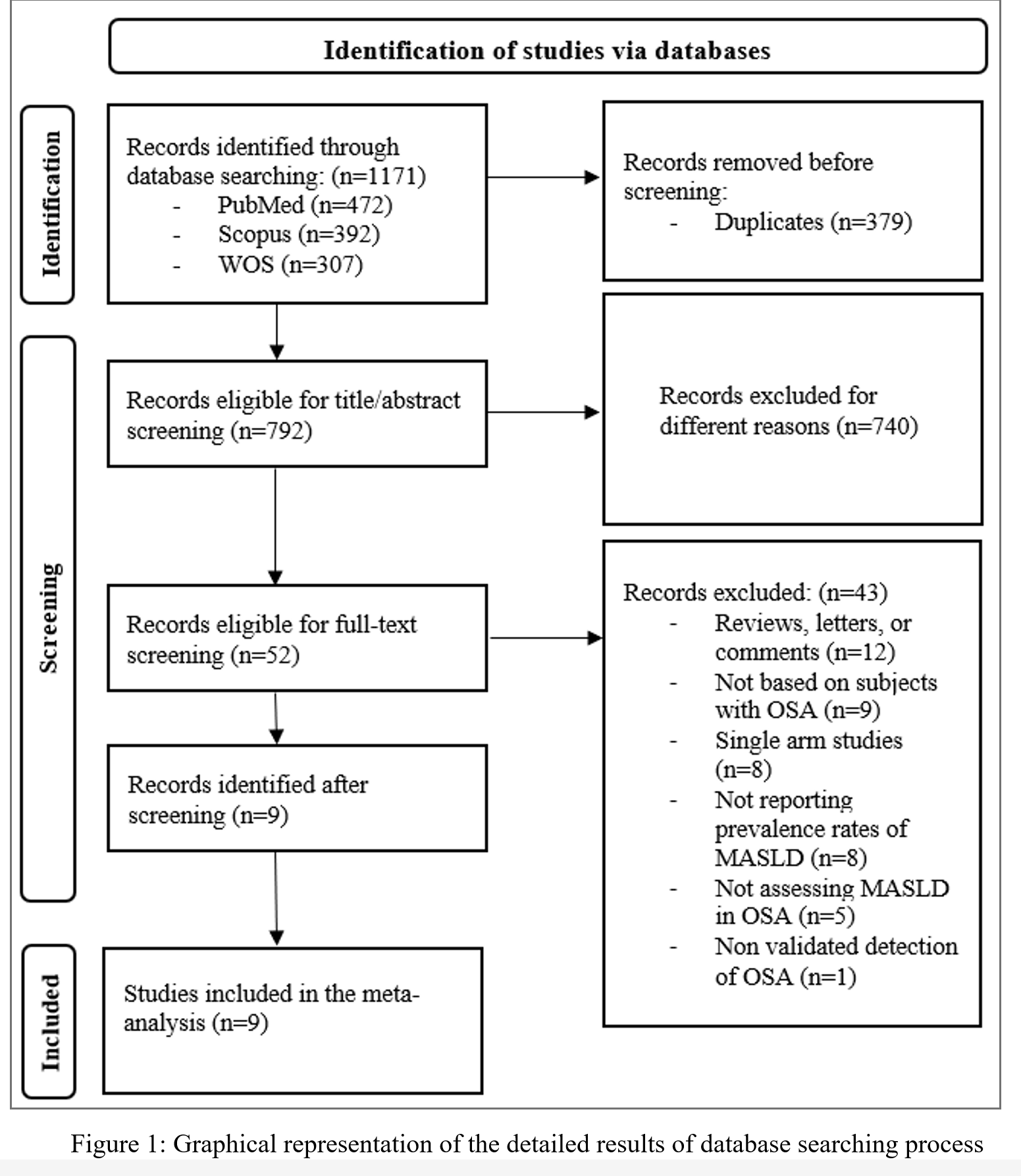
Figure 1 presents the results of database searching and the two-staged screening process as presented with the PRISMA flowchart. In total, 1171 articles were identified from the three databases searched. 379 articles were identified as duplicates and were removed prior to screening, leaving a total of 792 articles eligible for the first stage of screening. 740 articles were excluded for not being related to our analysis by the end of the title and abstract screening. The full texts of the remaining 52 were retrieved and assessed against the previously mentioned criteria for inclusion and exclusion discussed in the methods section. 43 publications were removed for different reasons as illustrated in the PRISMA flowchart, resulting in a total of nine articles deemed appropriate for inclusion in the meta-analysis [18-26].
Characteristics of the studies included in the analysis
Table 1 shows the characteristics of the nine studies included in the meta-analysis. These nine studies included a total of 3433 individuals; 2677 had OSA diagnosed with PSG, and the remaining 756 subjects without OSA are regarded as controls. Nine studies were conducted in four different countries, with China being the most represented country by five studies, followed by Spain, India, the USA, and South Korea, with one study from each. The smallest OSA cohort was 32, as reported by Tomar, et al. while the largest was 967, as reported by Huang, et al. [19,22]. Five studies provided prevalence data of MASLD across different severities of OSA. For all the included studies, the severity of OSA is measured in accordance with the AASM criteria, whereas the cut-off values for mild, moderate, and severe OSA in accordance with the Apnea Hypopnea Index (AHI) are 5-15 events/hour, 15-30 events/hour, and >30 events/hour, respectively [27,28].
MASLD diagnosis was a subject of variability across the studies. Four studies utilized abdominal ultrasonography for detection of MASLD; two studies utilized fatty liver index (FLI) and hepatic steatosis index (HSI), which depend on non-invasive clinical and laboratory parameters; one study used liver attenuation index (LAI), which is a CT-based assessment; and two studies used liver biopsies either alone or in alteration with US when available. Four of the nine studies excluded participants who had a previous diagnosis of OSA.
Regarding the study population, six studies involved individuals from the general population, one study involved only older individuals (≥60 years), another study involved obese individuals to be subjected to bariatric surgery, and the remaining study was conducted on individuals with chronic liver disease. Table S1 shows the detailed results with the final score for the quality assessment for the nine included studies in accordance with the NOS scale; six articles were of high quality, while the other three articles were of intermediate quality.
Pooled odds ratio estimates
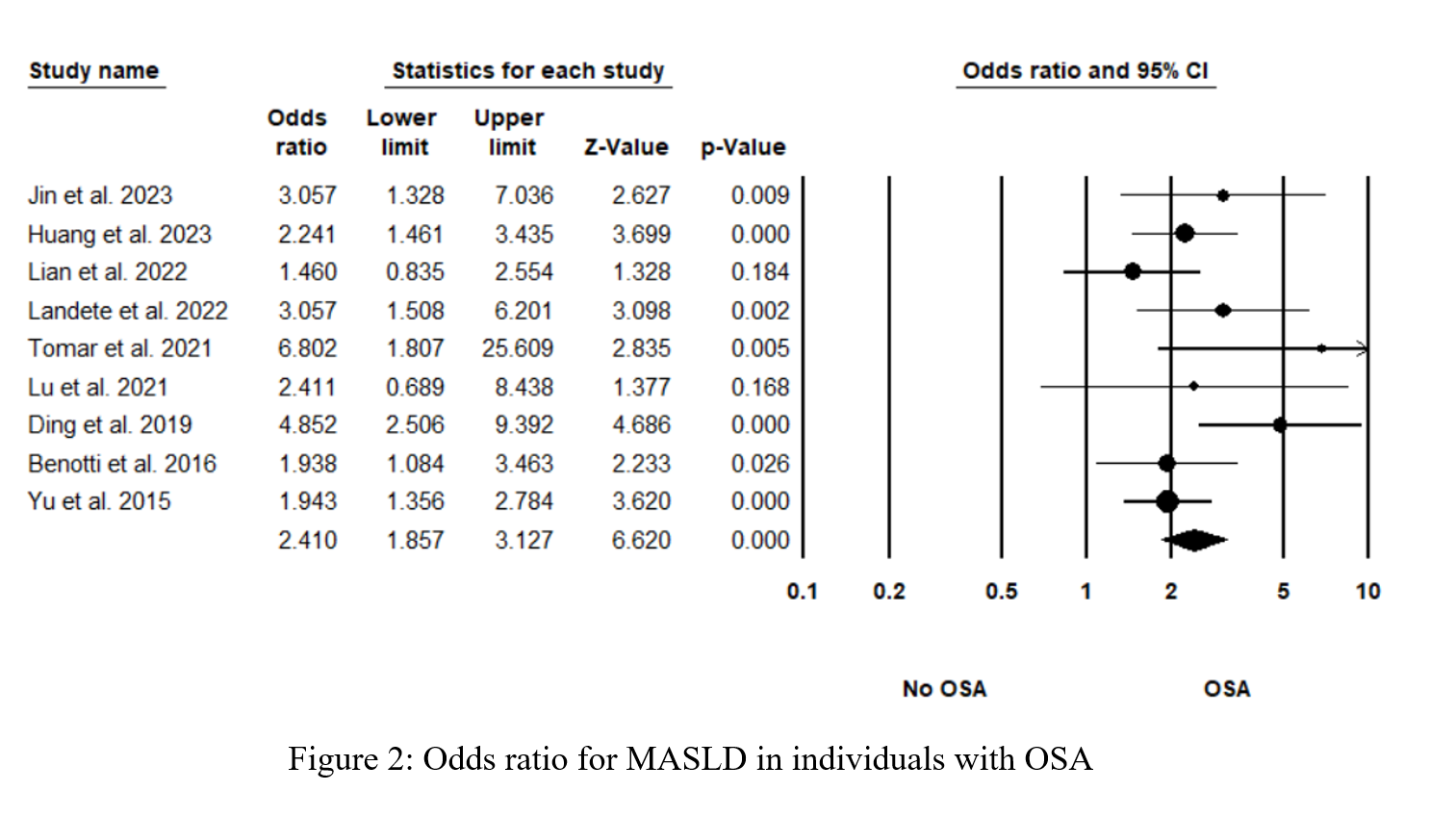
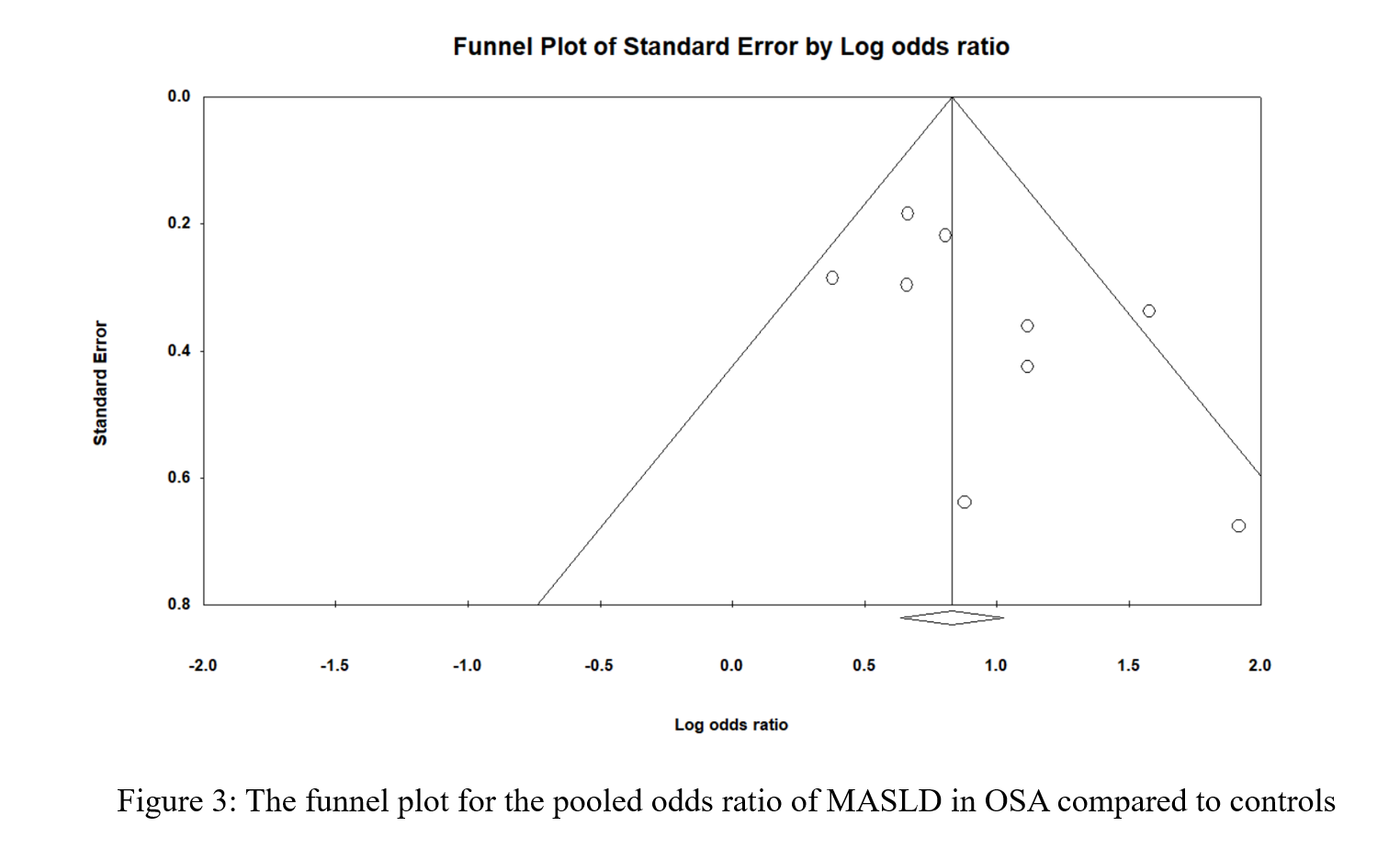
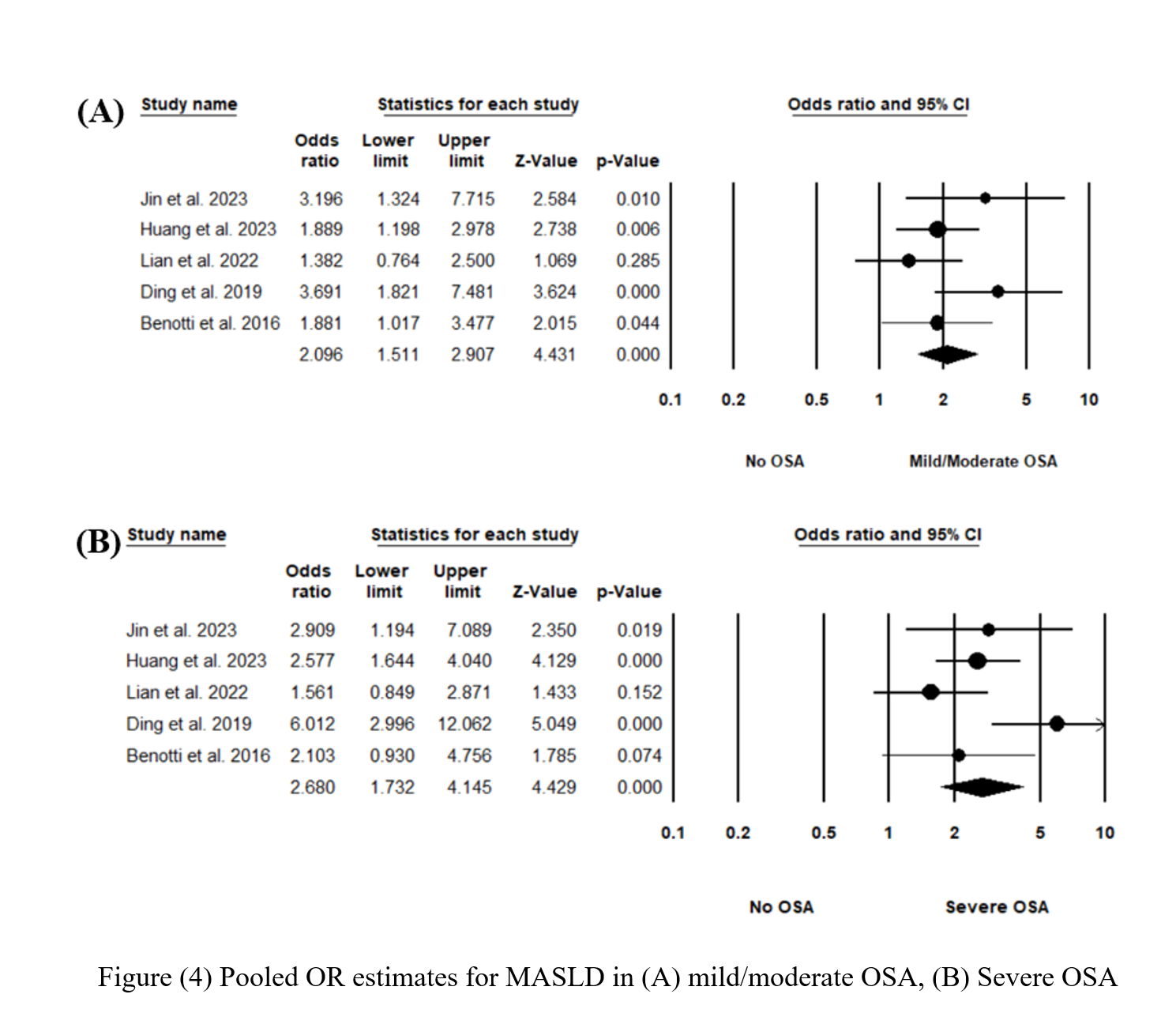
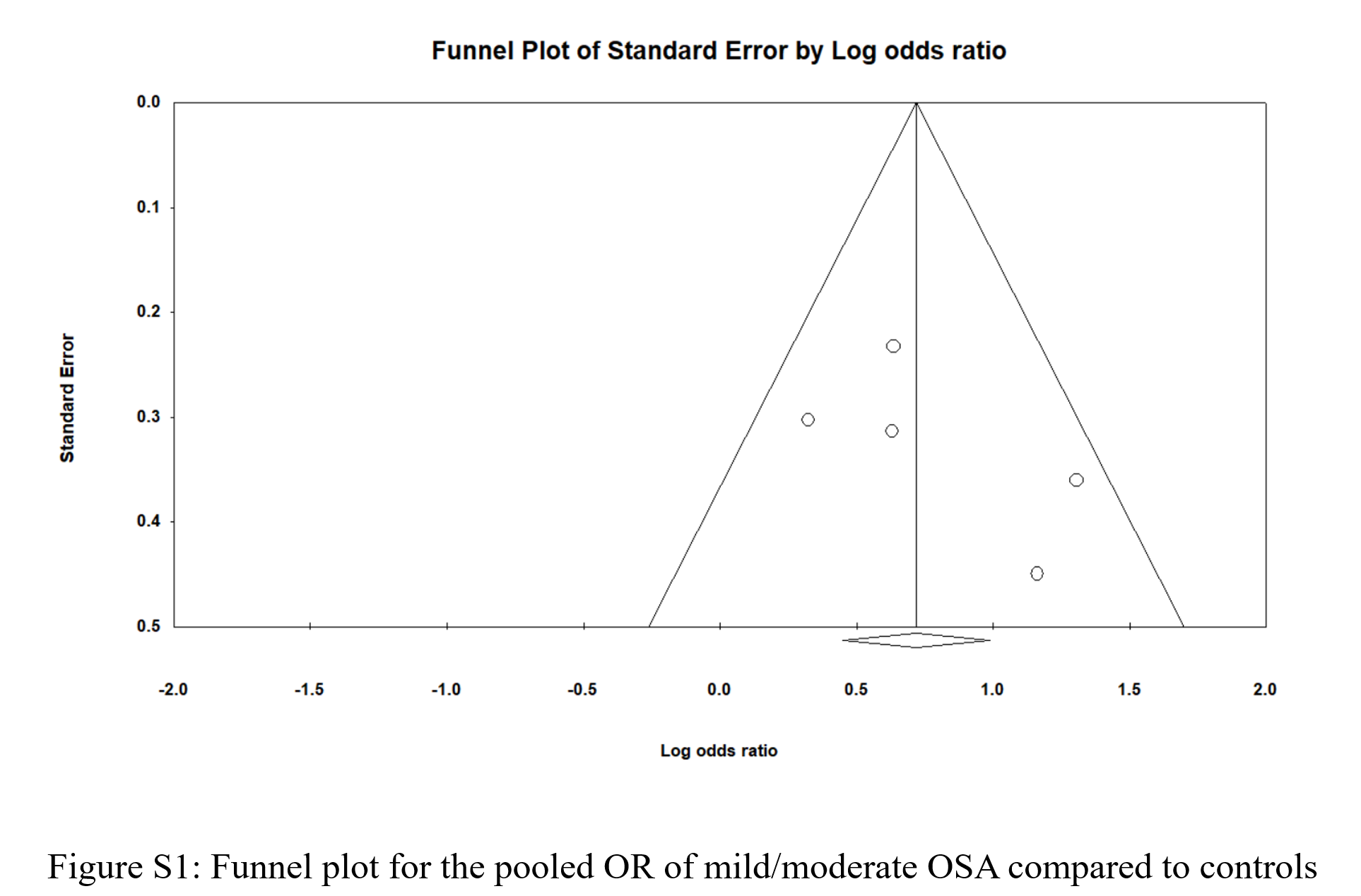
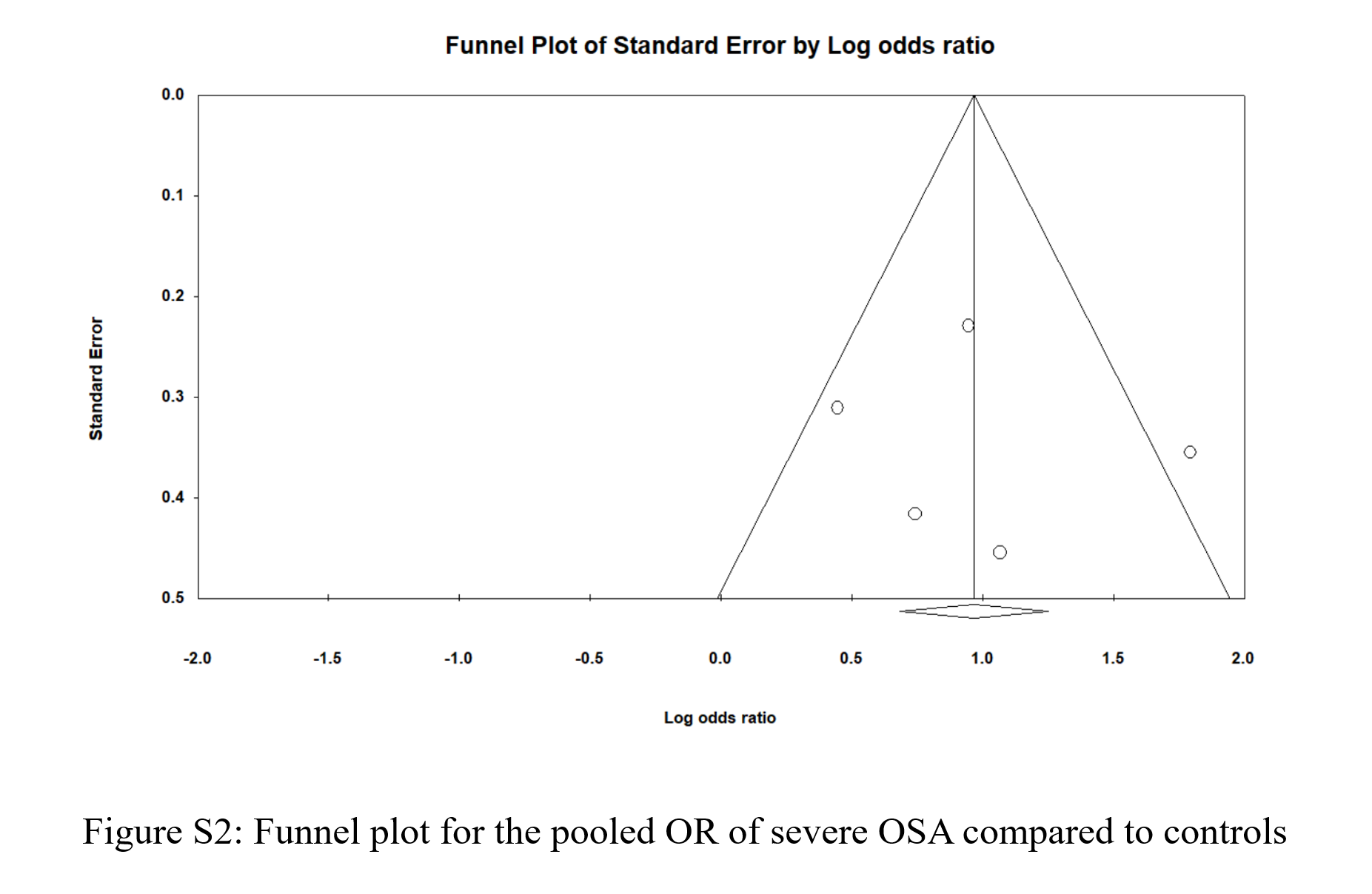
Figure 2 shows the pooled odds ratio (OR) for MASLD across all levels of OSA severities. As based on 2677 individual OSA and 756 controls with no OSA, the pooled OR was 2.41 [95% CI: 1.857-3.127], p < 0.0001, I 2 = 34.87%. Sensitivity analysis was executed by sequentially excluding one study and observing the variations in the pooled estimates; none of the included studies significantly affected the pooled estimates (Figure S1). The potential presence of publication bias was examined using the funnel plot visual interpretation and the Egger’s test p-value. Figure 3 shows the funnel plot, which is visually symmetrical; Egger’s test p-value was 0.106, thus the absence of publication bias was confirmed. Figure 4 shows the pooled OR for MASLD in individuals with mild or moderate OSA and in individuals with severe OSA (Figure S2). The pooled OR of MAFLD in mild or moderate OSA was 2.096 [95% CI:1.511-2.907], p < 0.0001, I² = 27.97%.
Table 2 shows the results of subgroup analysis for different categorical variables, including study region, method of MASLD diagnosis, study population, and study exclusions with a previous OSA diagnosis. The pooled OR for studies from Asia was 2.46 [95% CI: 1.79-3.39] with moderate heterogeneity, which was slightly higher than the OR pooled from two studies conducted in western countries (Spain and the USA) of 2.37 [95% CI: 1.32-4.27]; however, the difference was not statistically significant. Using conventional methods for MASLD diagnosis, including US or biopsy, showed higher pooled estimates when compared to other non-invasive methods, including HSI, FLI, and LAI (OR = 2.53 [95% CI: 1.78-3.59]) with moderate heterogeneity compared to the OR of 2.29 [95% CI: 1.40-3.76] with no heterogeneity. However, the differences did not show statistical significance. For the three studies including special populations, which were participants over 60 years, obese participants, and hepatic patients, the pooled OR was 2.73 [95% CI: 1.56-4.78]. This was not significantly higher than the pooled OR of studies involving the general population of 2.35 [95% CI: 1.70-3.23]. Four studies, excluding participants with a previous OSA diagnosis prior to PSG, had a pooled OR of 2.93 [95% CI: 1.97-4.35]. This was not significantly higher than that for studies that did not mention such a parameter (OR = 2.09 [95% CI: 1.52-2.89]).
Liver enzymes in relation to OSA state
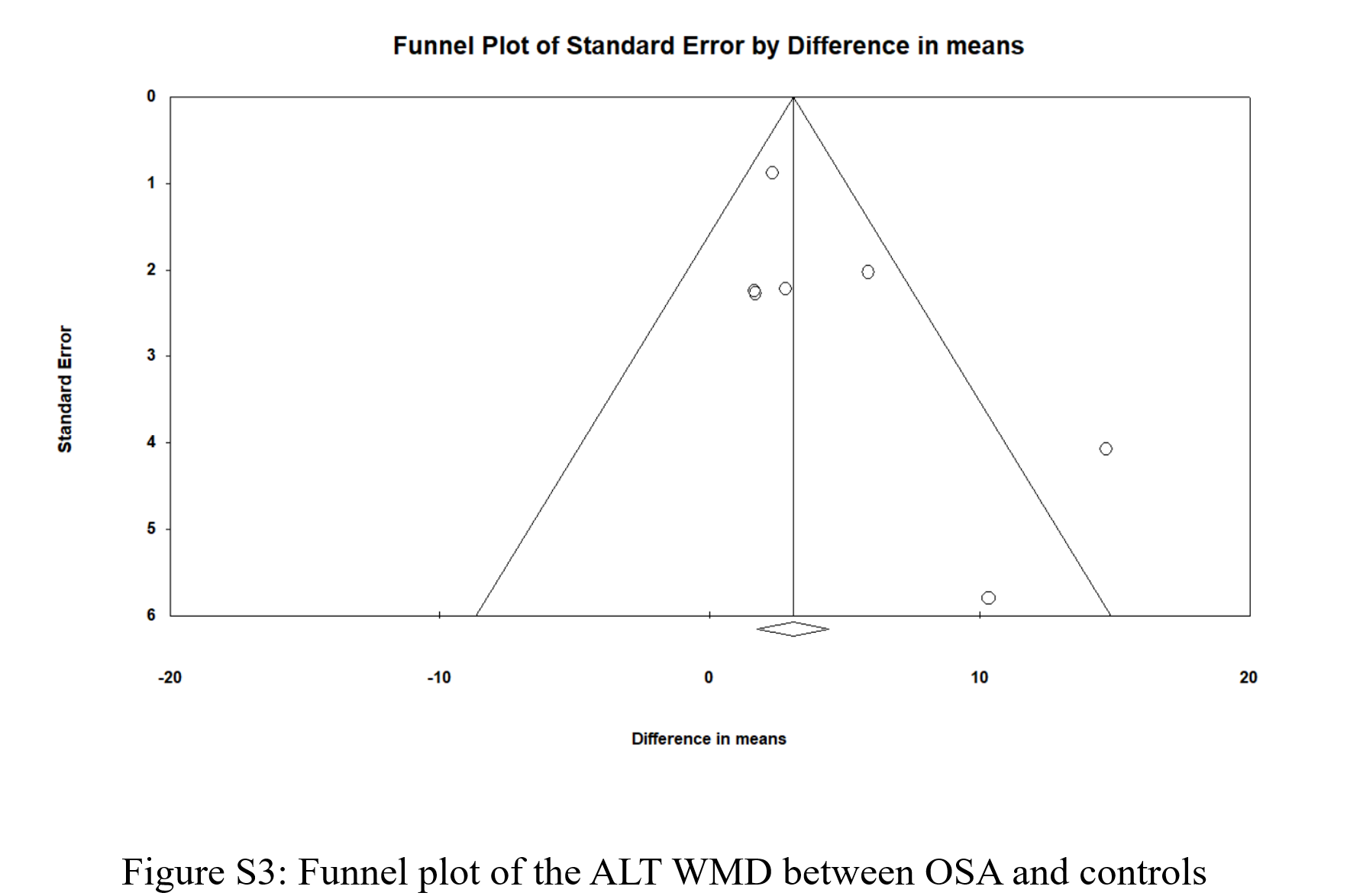
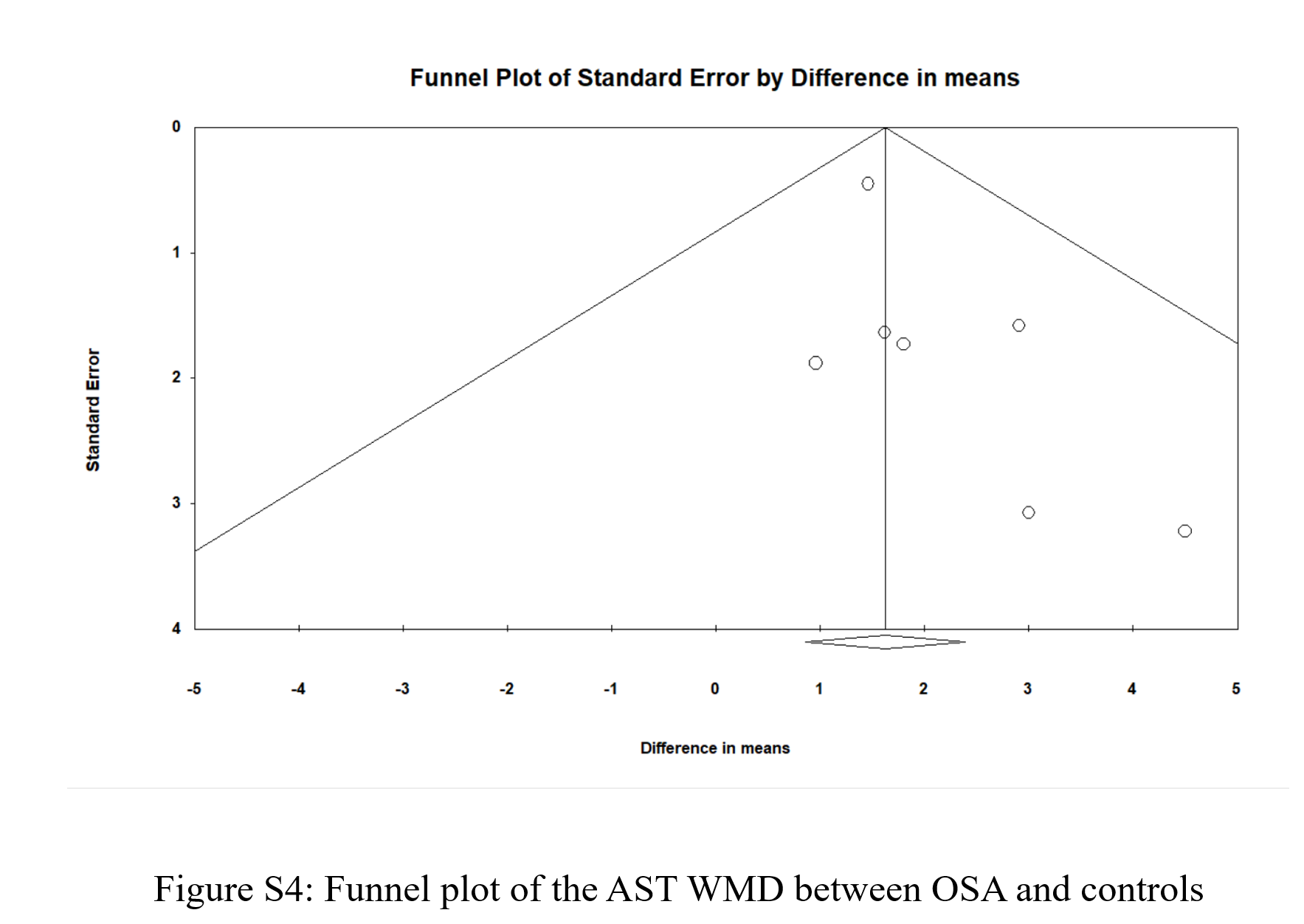
Seven studies provided ALT and AST levels for individuals with and without OSA. As shown in (Figure 5), the WMD for ALT was 4.006 [95% CI: 1.561-6.45], p = 0.001, I2 = 54.199%, and the WMD for AST was 1.624 [95% CI: 0.855-2.393], p < 0.0001, I2 = 0%. Figure S3 and Figure S4 show the funnel plots for ALT and AST, respectively; both were visually symmetrical. The p-value for the Egger’s test was 0.107 for ALT and 0.109 for AST. Thus, there was no strong evidence of publication bias.
Discussion
This meta-analysis was done to investigate the co-ocHtcurrence of MASLD in individuals with different severities of OSA. Extracted from nine studies conducted in four different countries with 2677 subjects and 756 controls, subjects with OSA had a statistically significant higher odds of having MASLD compared to controls. The odds ratio for severe OSA in relation to healthy controls was higher than that for individuals with mild or moderate OSA. Additionally, a statistically significant difference in liver enzymes, ALT and AST, was observed with higher ALT and AST values in individuals with OSA.
The pooled OR for MASLD in relation to OSA was 2.41 with moderate heterogeneity across the studies and no publication bias was found. This association is comparable to the findings of an earlier meta-analysis in which the pooled OR for the presence of NAFLD as detected by histological or radiological methods were 2.01 and 2.99, respectively [5]. Similarly, the findings of another meta-analysis showed a significant correlation with both the development of different stages of MASLD [13].
Prior studies have noted OSA’s link with metabolic dysfunction owing to OSA-associated chronic intermittent hypoxia and its downstream effects [29]. Physiologically, autonomic imbalance and sympathetic over activity were noted in states of chronic intermittent hypoxia which may lead to hepatic steatosis [30]. This sympathetic over activity involves an increase in cortisol and catecholamine release, thus reducing insulin sensitivity and preventing glucose uptake, leading to an increase in gluconeogenesis [31,32].
Furthermore, increases in oxidative stress could be explained by the increase in the formation of reactive oxygen species associated with the activation of Kupffer cells in addition to the recruitment of phagocytic cells into the hepatic parenchyma [33,34]. Insulin resistance and elevated insulin levels can stimulate phosphoinositide 3-kinase, which can lead to apoptosis and hepatocellular inflammation, thus progressing from simple steatosis to steatohepatitis [35]. Also, sleep fragmentation and intermittent hypoxia results in the activation of “hypoxia inducible factors” (HIFs). Activation of these factors leads to activation of different genes that take part in the in uptake of free fatty acids, suppression of lipid synthesis, and beta-oxidation, thus leading to steatosis [36,37].
Five of the included studies provided estimates of MASLD across different categories of OSA severity. Individuals with severe OSA had higher odds of MASLD (OR = 2.68) compared with those with mild or moderate OSA (OR = 2.096). This is comparable to a previous meta-analysis investigating the relationship between MASLD and OSA through liver enzyme parameters. When individuals with severe OSA were pooled separately from those with mild OSA, the WMD for both ALT and AST was greater [38].
In addition to sleep fragmentation, patients with both OSA and MASLD have shorter sleep durations with delayed onset of sleep, resulting in poor sleep quality [39]. Moreover, shorter sleep durations and irregularities were found to be associated with MASLD [40]. The observed higher OR for severe OSA compared to that for mild or moderate OSA reflects a higher prevalence rate of MASLD disorders in those with severe OSA. As the OSA increases in severity, the individual is subjected to more sleep fragmentation owing to the recurrent episodes of hypoxia, which may be an important contributor to the progression of MASLD [41]. Moreover, the AHI was found to be a predictor for the occurrence of significant fibrosis in MASLD patients with OSA, resulting in an increased disease severity [42]. So, both the occurrence and severity of OSA may be linked with the severity of MASLD.
When discussing modalities of diagnosing MASLD, liver biopsy is regarded as the most accurate method. But given its invasiveness, imaging by US is used as a non-invasive alternative [43,44]. Recent advances in CT also has enabled accurate assessment of both hepatic structure with better detection of hepatic lesions [45]. MASLD could also be diagnosed by a formula incorporating ALT, AST, body mass index (BMI), history of type II diabetes mellitus, and female sex with a cut-off value of 36 for detection [46]. Using HSI with this cut off value yielded a sensitivity of 65.8% and specificity of 54.3% [47]. FLI is calculated from an algorithm incorporating BMI, triglyceride, and gamma-glutamyl transferase levels, with a score greater than 60 for the detection of MASLD. Although this method was validated in previous studies, it was noted as a non-reliable alternative to imaging techniques [48,49].
When grouping diagnostic modalities for MASLD a non-significant difference in the pooled OR was observed. This suggests that the diagnostic method did not significantly impact the relationship between OSA and MASLD. A subgroup analysis for studies based on whether participants had a previous OSA diagnosis was excluded. A non-significant higher pooled OR was found for studies that excluded those with a known OSA diagnosis; this may be a result of receiving previous treatments or lifestyle modifications in studies that did not exclude participants with a previous OSA diagnosis, thus reducing oxidative stress and MASLD progression and an overall positive association.
As pooled from seven studies providing data regarding ALT and AST levels for those with and without OSA, a statistically significant WMD for both ALT and AST was found. This is comparable to the findings of a previous meta-analysis in which individuals with OSA showed a statistically significant difference in both ALT and AST values compared to controls, with the observed relationship being independent of both BMI and diabetes mellitus [50]. This is also comparable to the findings of a previous meta-analysis examining OSA in pediatric populations in which both mild and severe OSA showed a significant association with elevations in ALT and AST [37]. These observations suggest that OSA may directly contribute to liver injuries in general and MASLD without a link to other conditions like obesity and diabetes mellitus.
Although this work provides key insights about the association between OSA and MASLD, with investigation of the alterations in liver enzymes in individuals with OSA, several limitations are evident. First, all nine studies included were retrospective or prospective cross-sectional studies; thus, the causal and temporal link between OSA and MASLD could not be assured. All included studies were of high or intermediate quality based on NOS scores, and no evidence of small-study effects was found in funnel plots or Egger’s test, supporting the robustness of the pooled results. Second, not all the included studies reported MASLD prevalence in relation to OSA severity groups. Third, the matching for all confounding factors that could affect the OR estimates like BMI, Diabetes mellitus, and sex was not explicitly present in some of the included studies. Fourth, moderate heterogeneity was reported for some of the reported estimates.
In the light of these limitations, we recommend future researchers conduct prospective studies to observe the occurrence of MASLD in those with OSA; we also recommend providing an in-depth investigation of the potential confounding effects of coexisting diabetes mellitus or obesity on the association between OSA and MASLD. We also recommend integrating diagnostic tests for MASLD into those with OSA for earlier detection and better management of the disease before advancing into NASH and liver fibrosis. Additionally, the power of Egger’s test is limited in analyses with fewer than 10 studies, which restricts the reliability of publication bias assessment. Residual confounding from unmeasured or uncontrolled factors, particularly obesity and diabetes mellitus, may influence the observed associations, given their strong ties to both OSA and MASLD.
In conclusion, this analysis highlighted the association between OSA and MASLD, with greater risk of MASLD as the severity of OSA increases. Significant differences in liver enzymes for OSA compared to controls were also observed. Future longitudinal studies with control of possible confounding variables are needed to confirm the independent association between OSA and MASLD. Given the increased odds of MASLD in OSA patients, clinicians may consider baseline screening for hepatic steatosis in individuals diagnosed with moderate-to-severe OSA. Non-invasive tools such as FIB-4, FLI, or HSI could be used at the time of diagnosis or follow-up visits to facilitate early detection and guide further hepatological evaluation.
Disclosure Statement
Funding
No funding was obtained for this study.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Kapur VK, Auckley DH, Chowdhuri S, et al. (2017) Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 13: 479-504.
- Senaratna CV, Perret JL, Lodge CJ, et al. (2017) Prevalence of obstructive sleep Apnea in the general population: A systematic review. Sleep Med Rev 34: 70-81.
- Gomase VG, Deshmukh P, Lekurwale VY (2023) Obstructive sleep Apnea and its management: A narrative review. Cureus 15: e37359.
- Sorajja D, Gami AS, Somers VK, et al. (2008) Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest 133: 927-33.
- El Hage Chehade N, Fu Y, Ghoneim S, et al. (2023) Association between obstructive sleep apnea and gastroesophageal reflux disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 38: 1244-1251.
- Gottlieb DJ, Punjabi NM (2020) Diagnosis and management of obstructive sleep Apnea: A Review. JAMA 323: 1389-1400.
- Musso G, Cassader M, Olivetti C, et al. (2013) Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev 14: 417-431.
- Kwon M, Kim J, Kim SA (2023) Factors related to obstructive sleep Apnea according to age: A descriptive study. Healthcare (Basel) 11: 3049.
- McNicholas WT (2008) Diagnosis of obstructive sleep Apnea in adults. Proc Am Thorac Soc 5: 154-160.
- Seth A, Orkin S, Yodoshi T, et al. (2020) Severe obesity is associated with liver disease severity in pediatric non-alcoholic fatty liver disease. Pediatr Obes 15: e12581.
- Younossi ZM, Blissett D, Blissett R, et al. (2016) The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 64: 1577-1586.
- Parikh MP, Gupta NM, McCullough AJ (2019) Obstructive sleep Apnea and the liver. Clin Liver Dis 23: 363-382.
- Jin S, Jiang S, Hu A (2018) Association between obstructive sleep Apnea and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Sleep Breath 22: 841-851.
- Page MJ, McKenzie JE, Bossuyt PM, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: n71.
- Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25: 603-605.
- Higgins JP, Thompson SG, Deeks JJ, et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557-560.
- Borenstein M, Hedges L, Rothstein H (2007) Meta-analysis: Fixed effect vs. random effects. Meta-analysis 1: 30.
- Jin YX, Wang BY, Wang XL, et al. (2023) Relationship between obstructive sleep apnea and liver abnormalities in older patients: A cross-sectional study. Int J Clin Pract 2023: 9310588.
- Huang J, Chen L, Li X, et al. (2023) Association between metabolic-associated fatty liver disease and obstructive sleep Apnea: A cross-sectional study. Nat Sci Sleep 15: 49-57.
- Lian N, Wu J, Wang B, et al. (2022) Risk factors of non-alcoholic fatty liver disease and liver fibrosis in non-obese patients with obstructive sleep Apnea. Nat Sci Sleep 14: 2143-2149.
- Landete P, Fernández-García CE, Aldave-Orzaiz B, et al. (2022) Increased oxygen desaturation time during sleep is a risk factor for nash in patients with obstructive sleep apnea: A prospective cohort study. Front Med (Lausanne) 9: 808417.
- Tomar A, Bhardwaj A, Choudhary A, et al. (2021) Association of obstructive sleep apnea with nocturnal hypoxemia in metabolic-associated fatty liver disease patients: a cross-sectional analysis of record-based data. J Family Med Prim Care 10: 3105-3110.
- Lu YB, Weng YC, Huang YN, et al. (2021) Novel screening model of obstructive sleep apnea for snorers with suspected NAFLD undergoing liver sonography. BMC Pulmonary Medicine 21: 1-9.
- Ding H, Huang JF, Xie HS, et al. (2019) The association between glycometabolism and nonalcoholic fatty liver disease in patients with obstructive sleep apnea. Sleep Breath 23: 373-378.
- Benotti P, Wood GC, Argyropoulos G, et al. (2016) The impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity (Silver Spring) 24: 871-877.
- Yu JH, Ahn JH, Yoo HJ, et al. (2015) Obstructive sleep apnea with excessive daytime sleepiness is associated with non-alcoholic fatty liver disease regardless of visceral fat. Korean J Intern Med 30: 846-855.
- Hudgel DW (2016) Sleep Apnea severity classification - revisited. Sleep 39: 1165-1166.
- Berry RB, Budhiraja R, Gottlieb DJ, et al. (2012) Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep Apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med 8: 597-619.
- Preshy A, Brown J (2023) A bidirectional association between obstructive sleep Apnea and metabolic-associated fatty liver disease. Endocrinol Metab Clin North Am 52: 509-520.
- Adori Monika, Bhat Sadam, Gramignoli Roberto, et al. (2023) Hepatic Innervations and Nonalcoholic Fatty Liver Disease. Semin Liver Dis 43: 149-162.
- Ryan S (2017) Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol 595: 2423-2430.
- Kritikou I, Basta M, Vgontzas AN, et al. (2016) Sleep apnoea and the hypothalamic-pituitary-adrenal axis in men and women: Effects of continuous positive airway pressure. Eur Respir J 47: 531-540.
- Cai J, Hu M, Chen Z, et al. (2021) The roles and mechanisms of hypoxia in liver fibrosis. J Transl Med 19: 186.
- Jaeschke H (2011) Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol 1:173-179.
- Pal SC, Eslam M, Mendez-Sanchez N (2022) Detangling the interrelations between MAFLD, insulin resistance, and key hormones. Hormones (Athens) 21: 573-589.
- Rankin EB, Rha J, Selak MA, et al. (2009) Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol 29: 4527-4538.
- Cai H, Bai Z, Ge RL (2021) Hypoxia-inducible factor-2 promotes liver fibrosis in non-alcoholic steatohepatitis liver disease via the NF-κB signalling pathway. Biochem Biophys Res Commun 540: 67-74.
- Chen J, Cao Y, Li Z, et al. (2023) Association between the severity of obstructive sleep Apnea and the risk stratification of acute pulmonary embolism. Clin Appl Thromb Hemost 29: 10760296231175654.
- Bernsmeier C, Weisskopf DM, Pflueger MO, et al. (2015) Sleep disruption and daytime sleepiness correlating with disease severity and insulin resistance in Non-Alcoholic fatty liver disease: A comparison with healthy controls. PLoS One 10: e0143293.
- Wu Qingcui, Song Fuman, Huang Huijie, et al. (2025) Sleep duration, midpoint, variability, irregularity and metabolic dysfunction-associated steatotic liver disease. Behavioral sleep medicine 23: 1-14.
- Wang L, Liu H, Zhou L, et al. (2024) Association of obstructive sleep Apnea with non-alcoholic fatty liver disease: Evidence, mechanism, and treatment. Nat Sci Sleep 16: 917-933.
- Agrawal S, Duseja A, Aggarwal A, et al. (2015) Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int 9: 283-291.
- Brunt EM, Wong VW, Nobili V, et al. (2015) Nonalcoholic fatty liver disease. Nat Rev Dis Primers 1:15080.
- Wang D, Miao J, Zhang L, et al. (2024) Research advances in the diagnosis and treatment of MASLD/MASH. Ann Med 57: 2445780.
- Vernuccio F, Cannella R, Bartolotta TV, et al. (2021) Advances in liver US, CT, and MRI: moving toward the future. Eur Radiol Exp 5: 52.
- Fennoun H, Mansouri SE, Tahiri M, et al. (2020) Interest of hepatic steatosis index (HSI) in screening for metabolic steatopathy in patients with type 2 diabetes. Pan Afr Med J 37: 270.
- Chang JW, Lee HW, Kim BK, et al. (2021) Hepatic steatosis index in the detection of fatty liver in patients with chronic hepatitis b receiving antiviral therapy. Gut Liver 15: 117-127.
- Lonardo A, Ballestri S, Bedogni G, et al. (2021) The fatty liver index (FLI) 15 years later: A reappraisal. Metab Target Organ Damage 1:10.
- Motamed N, Faraji AH, Khonsari MR, et al. (2020) Fatty liver index (FLI) and prediction of new cases of non-alcoholic fatty liver disease: A population-based study of northern Iran. Clin Nutr 39: 468-474.
- Sookoian S, Pirola CJ (2013) Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: A meta-analysis. Obes Surg 23: 1815-1825.
Corresponding Author
Sarvanand Patel, MD, Southern California Permanente Medical Group- Los Angeles Medical Center 4867 W Sunset Blvd. Los Angeles, CA 90027, USA, Tel: 951-290-0199.
Copyright
© 2025 Patel S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.