Fitbit Sleep Measures in Children with and without Sleep Disordered Breathing
Abstract
Introduction
Sleep disordered breathing (SDB) is a spectrum of diseases ranging from primary snoring (PS) to obstructive sleep apnea (OSA). Although PS does not characteristically demonstrate gas exchange abnormalities on polysomnography and is usually considered a more benign condition, it still may be associated with significant neurocognitive and behavioral issues, such as hyperactivity, inattentive behavior, aggression and poor school performance. To date, little research has focused on sleep-related movements as a potential measure of sleep disruption in children with SDB. The purpose of this study was twofold: To determine if the Fitbit reliably provides night-to-night data on the number of minutes the child was moving and to compare movement during sleep in children with and without SDB using the Fitbit activity monitor.
Methods
Thirty-six children between 2 and 12 years of age were recruited from the otolaryngology outpatient clinic for the prospective cohort study between January and September 2015. Twenty target children (SDB) had chronic symptoms of snoring and sleep disordered breathing and the remaining 16 controls had no history of snoring or difficulty breathing at night by parent report. A Sleep-Related Breathing Disorder Scale Questionnaire was administered at enrollment. Each child wore a Fitbit at night during sleep for 2 weeks. Group comparisons on total sleep time (TST), movement minutes, and sleep efficiency (SE) were made using mixed models.
Results
After controlling for age, the number of movement minutes differed significantly by SDB/Control group (p = 0.012), with the SDB group averaging about 13 more movement minutes per night.
Conclusion
Our findings verify that children with SDB have more sleep-related movements and highlight the possibility that movement may be a useful marker for sleep fragmentation. Further studies are needed to determine the potential use of Fitbit as a screening device for identifying children with SDB at risk for sleep disturbance.
Keywords
Pediatric, Sleep disordered breathing, Activity monitor, Obstructive sleep apnea, Actigraphy, Polysomnogram
Introduction
Sleep disordered breathing (SDB) is a spectrum of diseases ranging from primary snoring (PS), upper airway resistance syndrome (UARS), to obstructive sleep apnea (OSA). OSA constitutes the more severe end of the spectrum, while PS is the least severe and consists of nightly snoring without apnea, hypoxemia, or hypoventilation. Although PS does not characteristically demonstrate gas exchange abnormalities on polysomnography and is usually considered a more benign condition, it still may be associated with significant neurocognitive and behavioral issues, such as hyperactivity, inattentive behavior, aggression and poor school performance [1-4]. Emerging evidence suggests that children with PS may have increased sleep disruption, movement, and sleep fragmentation [5-8].
The ‘gold standard' for assessing sleep architecture is polysomnography (PSG). However, PSG has limitations, including its availability, cost, invasiveness, and inability to measure night-to-night sleep patterns. In addition, most studies utilizing PSG have not demonstrated large differences in sleep architecture between children with SDB and normal controls [7,9,10]. There is also evidence that children with SDB have a blunted EEG arousal response to respiratory stimuli and cortical arousals during PSG may not be sensitive enough to detect sleep disruption [11,12]. So although PSG may provide a diagnosis for children with severe SDB such as OSA, identifying those with milder disease who are also at risk for neurocognitive and behavioral impairment may be a challenge.
Most recently, nocturnal body movements have been proposed to be a more specific marker of sleep disturbance in children than adults due to age-related differences in sleep architecture and arousal processes [11,13-15]. For some disease states associated with sleep disruption, studies have demonstrated that abnormal sleep-related body movements can occur with particular frequency and distribution [16]. To date, however, little research has focused on sleep-related movements as a potential measure of sleep disruption in children with SDB.
Accelerometer-based activity (actigraphy) monitors have been shown to provide a valid estimate of movements during sleep [17]. They are small wristwatch sized devices that can collect data by an internal accelerometer. The collected data are translated into epochs (30 sec or 1 min) of activity. Using validated algorithms, data is generated about nocturnal body movements and sleep-wake states. The device is small, allows for multiple-day data collection, and can be easily used in a child's natural environment. To date, however, wide use of actigraphy to diagnose sleep abnormalities has not been implemented mainly due to the high cost of each unit, lengthy training, and complicated data abstraction involved.
Commercially available electronic activity monitors (such as those manufactured by Fitbit, Jawbone, and Nike) have become accessible to the public and their popularity and use continue to grow (Figure 1). These small, relatively inexpensive (average price $100) devices improve on standard accelerometers by providing automated feedback and interactive tools via mobile device or personal computer. Their low cost, wide reach and apparent effectiveness make these activity-monitoring devices appealing for clinical and research applications. Use of these devices for research in children with SDB is beginning to be explored. The purpose of this study was twofold: To determine if the Fitbit reliably provides night-to-night data on the number of minutes the child was moving and to compare movement during sleep in children with and without SDB using the Fitbit activity monitor. We hypothesized that children with SDB would demonstrate an increased frequency of nocturnal movements. We hypothesized that the Fitbit could provide a reliable estimate of movement during sleep for children with and without SDB, thus providing stable estimates of movement from night to night in children.
Methods
Thirty-six children between 2 and 12 years of age were recruited for the prospective cohort study from an otolaryngology outpatient clinic between January and September 2015. Children were excluded if they routinely slept with a parent/sibling, did not have a Fitbit-compatible syncing device, were taking any systemic medications that could affect sleep or respiration, were born prematurely, or had any significant cardiac, neurologic or developmental disease or disorder. Twenty target children (SDB) had chronic symptoms of snoring and the remaining 16 controls had no history of snoring or difficulty breathing at night by parent report. Controls included siblings of patients or those seen in the otolaryngology clinic for follow up from tympanostomy tube placement, tonsillectomy (for reasons other than SDB), epistaxis, recurrent acute otitis media and branchial cleft cyst. Four children whose parents did not complete an acceptability questionnaire and refused to wear the Fitbit were withdrawn from the study.
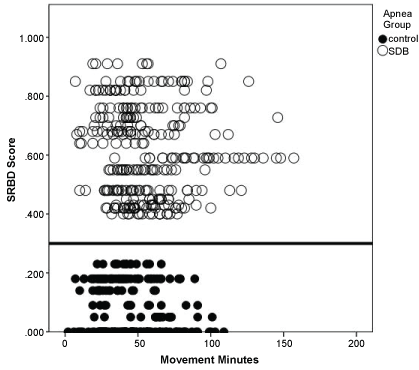
The study was approved by the Children's Hospital Los Angeles Institutional Review Board. Written informed consent was obtained from all parents and assent from children over the age of seven. Information was extracted from participant medical records including date of birth, height and weight, gender, medications, surgical and medical history, and ethnicity. At enrollment, the Sleep-Related Breathing Disorder Scale Questionnaire (SRDB) was administered to all participants. The SRBD Scale consists of 22 closed response question-items validated against polysomnography, Multiple Sleep Latency Test results, and adenotonsillectomy outcomes. Scores > 0.33 suggest a high risk for a pediatric sleep-related breathing disorder [18,19].
All parents received a Fitbit Flex™ and were instructed to place it on the child's non-dominant wrist at bedtime, remove it in the morning, and sync every day. Parents were also instructed to complete a standardized sleep journal, which was used to validate missing or inconclusive data. Children wore the Fitbit every night for at least two weeks at which time the Fitbit was returned and a device acceptability questionnaire administered.
Data analysis
The Fitbit provides a number of variables for each night of sleep: Number of minutes in bed, total minutes of sleep time, the count of awake or movement episodes, and the number of minutes of awake or movement. The Fitbit codes sleep at one-minute intervals by tracking frequency and intensity of movement with a three-dimensional accelerometer system. A subject's sleep within the one-minute interval is categorized as "sleep", "movement", or "awake" based on a proprietary algorithm that scores the intensity of body movement. Data were extracted from Fitbit by Fitabase, a research platform from a third party developer (Small Steps Labs LLC) and exported for analysis in Excel and SPSS. Total sleep time was the sum of the number of minutes each night coded as "sleep". Sleep efficiency was defined as the percentage of minutes in bed coded as asleep (total sleep minutes divided by minutes in bed). Minutes categorized as "movement" were summed for each child and for each night. Sleep runs (measured as time asleep without movement, equal to or greater than 2 hours) were manually recorded from the Fitabase data. The sleep runs were recorded per night of sleep and then an average calculated from the total number of nights.
Height and weight were used to calculate body mass index (BMI), which was converted to a BMI z-score to adjust for gender and age. BMI percentile was categorized according to the CDC categories: Underweight < 5th percentile, Healthy greater than 5th but less than 85th percentile, Overweight equal to or greater than 85th but less than 95th percentile, and Obese ≥ 95th percentile. The dates for each Fitbit recording were coded by the day of the week and then categorized into weekend (Friday, Saturday, and Sunday) and weekday (Monday through Thursday) nights. Finally, the SRDB was scored by summing the number of "Yes" responses (value 1) and dividing by the total number of non-missing items. The SRDB score represents the proportion of sleep disordered breathing symptoms the child has out of a total possible 22 symptoms by parental report. The SRDB score was further broken down into the following symptom subscales: Snoring, Breathing, Sleepiness, and Behavioral (inattention/hyperactivity) issues [18]. Analysis was performed using both total SRDB scores and subset scores.
The intensive longitudinal Fitbit data were analyzed using SPSS 24.0 Mixed Model Analysis. The majority of both SDB and control groups recorded at least 14 nights of sleep with the Fitbit. Based on the sample size of the consecutive nights of Fitbit recordings and an initial exploratory Mixed Model analysis of the repeated measures covariance structure, the maximum number of Fitbit nights used in the final analyses was 14 nights for both groups. The best fitting model used a first order autoregressive model for the repeated measures covariance structure and included covariates. The14-night average of sleep runs was analyzed using an ANOVA. Differences between the groups on the descriptive variables were analyzed using the t-test and the p-value was set at < 0.05 for all statistical tests.
Results
Children in the target SDB group were younger on average than those in the control group. They also exhibited higher SRDB scores than controls (p < 0.001, Table 1), validating the initial categorization of the children based on parental perceptions of snoring. We confirmed those perceptions with the SRDB score. All children in the SDB group scored greater than the 0.33 cut-off score defining sleep disordered breathing and all control children scored < 0.33 (Figure 2). For either group, there did not appear to be any significant correlation between total SRDB scores and movement minutes or sleep runs (Table 2). Examining the SRDB subscale scores, a significant relationship was noted between Snoring and Breathing subscales and movement minutes, with increased movement related to higher subscale scores. No association was found between SRDB subscales and sleep runs (Table 2).
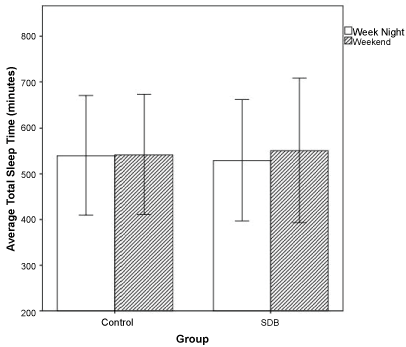
Overall, a difference in total sleep time (TST) by age (p = 0.0001) was noted, with older children sleeping fewer number of minutes each night. A comparison of mean TST between BMI groups demonstrated that, compared to underweight (average = 566 minutes) and healthy children (average = 542 minutes), overweight (average = 511 minutes) and obese (average = 526 minutes) children slept the fewest minutes per night (p = 0.037, Table 3). There was no significant difference in TST between SDB and control groups, but a trend towards decreased TST in the SDB group (Table 3). There was a significant difference between SDB and control groups by weekend vs. weekday night (p = 0.045, Figure 3). The control group tended to sleep the same number of minutes regardless of weekend or weekday night. The SDB group tended to sleep a greater number of minutes on a weekend night relative to a weekday night.
Although children in the control group appeared to have greater sleep efficiency than those in the SDB group, this difference was not significant. However, sleep efficiency differed significantly by BMI group; the healthy BMI group had the highest sleep efficiency (90%), while the obese group had the lowest sleep efficiency (88%) (p = 0.009, Table 3). The interaction of Apnea Group and BMI group was significant (p = 0.009). A visual inspection of means showed that overweight children in the SDB group had the greatest reduction in sleep efficiency compared to overweight children in the comparable control group (Figure 4). Age was not significantly associated with sleep efficiency. The mean sleep efficiency for all subjects did not exhibit significant variability from night to night across the 14 nights, indicating stability of the Fitbit measurements.
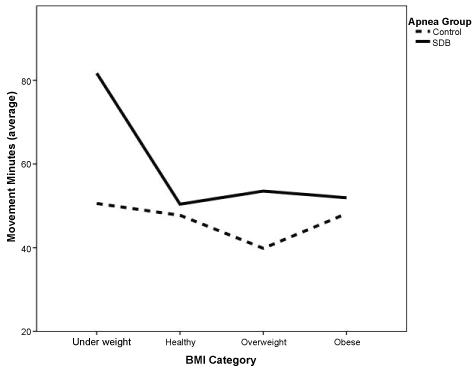
Testing our hypothesis of movement minutes and SDB group, age was inversely related to the number of movement minutes each night (p = 0.026), older subjects tended to have fewer movement minutes per night than did younger subjects. After controlling for age, the number of movement minutes differed significantly by SDB/Control group (p = 0.012, Table 3), with the SDB group averaging about 13 additional movement minutes per night relative to the Control group average (Figure 5), as hypothesized. A significant interaction between Apnea Group and BMI (p = 0.033) showed that underweight children had the greatest elevation in movement minutes in the SDB group (Figure 6) relative to children in the Control group. Movement minutes also differed significantly by the child's sex (p = 0.034); girls had higher number of movement minutes each night compared to boys (Table 3). The number of movement minutes did not differ significantly across the 14 nights of measurement, again demonstrating the reliability of the Fitbit for assessing movement minutes (Table 4).
The average number of sleep runs across all measured nights for each child showed a significant difference between SDB/Control groups (p = 0.038, Table 3). The SDB Group had just 1.0 sleep run per night compared to the Control Group that had an average of 1.3 sleep runs per night (Table 3). Age, BMI and sex were not significant.
Of the 30 parents who completed the acceptability questionnaire (17 in SDB group and 13 controls) only a few reported that they had concerns about the Fitbit being lost, stolen or syncing or working properly (Table 5). Eleven parents (36%) were concerned about completion of the home sleep journal and six reported having initial difficulties with syncing the Fitbit. A few parents reported problems with Fitbit use, with most of those (20%) reporting difficulty getting their child to wear it at night. Five parents reported that the child took the Fitbit off occasionally during the night. In contrast, two parents reported that their children "thought it was cool" and would remind them to put it on them at night.
Almost all parents reported that they liked using the Fitbit (94% SDB parents, 84% control parents). Parents thought the Fitbit provided them useful information about their child's sleep (Table 5). Overall, parents felt it either confirmed what they suspected about their child's sleep or illuminated the frequency of sleep disruption. About 40% of parents provided comments indicating that they found the information about their child's sleep of value. One parent remarked that she could "see whether [the child] was getting enough sleep… [she] let [the child] sleep more on the weekends because of this". Another parent commented that they "did not realize how restless he was".
Discussion
To our knowledge, this is the first investigation to study sleep measures in children utilizing the Fitbit Flex™ in a home environment. Observing the sleep patterns of children who snore and do not snore for 14 consecutive nights showed a number of clear differences between the groups. Our results indicate that there is a measurable increase in minutes of movement among children with SDB when compared to normal controls. Children in the SDB group also had fewer sleep runs than the control group. Although not surprising, these results indicate that children with SDB have more sleep disruption. Our study also revealed that the SDB group slept fewer minutes per night and slept more on weekend nights relative to weekday nights. This finding suggests that children with SDB may compensate for their disturbed sleep by sleeping longer hours, particularly on the weekend when there is no need for early arousals for school or daycare. Weekend catch up sleep, independent of other variables, has been shown to be associated with poor performance on objective attention tasks in adolescents [20].
Several previous studies have investigated the Fitbit's ability to detect sleep wake states and reported a diminished capacity for the device to measure sleep duration and efficiency when compared to PSG [21-24]. Despite these findings, the present study differs in its emphasis on movement and sleep runs, rather than sleep-wake states. It is also unique in its performance of multi-night measures. Our study demonstrates robust and consistent measurement of total sleep time, sleep efficiency and sleep related movement captured by Fitbit from night to night. The Fitbit exhibited remarkable stability within subjects and this implies that in the home environment, there is little variability in the sleep patterns of children from night to night (Table 4). Our parental questionnaire also highlighted the feasibility and satisfaction with use of the Fitbit. The majority of parents and their children had no difficulty with learning to use and wear the device and many parents found that the Fitbit provided valuable feedback about their child's sleep quality.
Other investigators have evaluated nocturnal movements and studied them in the context of SDB. Osterbauer, et al. [25] demonstrated a significant correlation between the same Fitbit Flex model used in this study and PSG measured movements in children with SDB. Lamprecht, et al. [11] explored the temporal relationship between PSG cortical arousal events and actigraphy measured body movements and concluded that specific movements could identify EEG arousals that may have greater impact on sleep quality. Coussens, et al. [16] established that children with SDB had a higher number of PSG nocturnal movements compared to normal controls. These authors also demonstrated a correlation between the severity of SDB and PSG movement measurements and a normalization of these after adenotonsillectomy. Additionally, some found children with OSA showed sleep patterns with consolidation of movement and sleep runs and suggested that movement distribution may be a more sensitive marker for sleep fragmentation, rather than frequency of movement alone [11,16]. Using actigraphy, Suratt, et al. [26] investigated the relationship between nocturnal movements and neurocognitive performance in children with suspected SDB. They reported that a higher frequency of sleep-related movements correlated with slower reaction times, while a consolidation of movements correlated with reduction of performance on vocabulary and memory tasks.
The most prominent limitation for our study is the lack of utilization of PSG to confirm and classify the severity of SDB in our target population. Although there is a significant difference in SRDB scores between our target and control populations, indicating that the grouping likely reflected the underlying disease state, inclusion of PSG data might have clarified the relationship between Fitbit sleep-related movements and degree of SDB as measured by parameters such as the apnea-hypopnea index. While the prevalence of restless leg syndrome (RLS) and periodic leg movements (PLM) are not well established in children, it is generally not considered high in this population. However, another limitation of our study is that children with these or other movement disorders were not excluded via polysomnography. Our study is also limited by a small sample size and a sample of convenience. Lastly, our analysis of data did not include a complete examination of distribution of movements during the sleep period and in light of the findings of others, this information would be critical to reveal further differences between our groups.
Irrespective of these shortcomings, the results of our study reveal that children with SDB move more during sleep and this movement may be an important indicator of sleep fragmentation. Coupled with the results from others, our findings emphasize the possibility that accelerometer measured nocturnal movements might be used as a means to evaluate for sleep disturbance. Current guidelines regarding milder forms of SDB and PS are lacking. Our knowledge that these children, despite lack of intermittent hypoxia, can suffer adverse consequences on their behavioral, cognitive and emotional health suggests that diagnosis of those at risk is still imperative. The limitations of PSG, including its cost, intrusiveness, inability to measure variations in sleep patterns over time and insensitivity in detecting sleep fragmentation may diminish its predictive value for these children. Thus, there is a substantial need to identify other methods of evaluating sleep quality in this population.
Conclusion
The increasing availability and sophistication of low-cost health technologies make them an attractive option for gaining additional information on children's sleep. Our findings verify that children with SDB have more sleep-related movements and highlight the possibility that movement may be a useful marker for sleep fragmentation. The ability of the Fitbit to obtain several nights of data regarding sleep patterns and movement within a child's natural environment combined with its cost-effectiveness and ease of use make the Fitbit an attractive, ecologically valid instrument. Further studies are needed to determine the potential use of Fitbit as a screening device for identifying children with SDB at risk for sleep disturbance.
References
- Brockmann PE, Urschitz MS, Schlaud M, et al. (2012) Poets, Primary snoring in school children: Prevalence and neurocognitive impairments. Sleep Breath 16: 23-29.
- Brockmann PE, Bertrand P, Pardo T, et al. (2012) Prevalence of habitual snoring and associated neurocognitive consequences among Chilean school aged children. Int J Pediatr Otorhinolaryngol 76: 1327-1331.
- Blunden S, Lushington K, Kennedy D, et al. (2000) Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. J Clin Exp Neuropsychol 22: 554-568.
- Arman AR, Ersu R, Save D, et al. (2005) Symptoms of inattention and hyperactivity in children with habitual snoring: Evidence from a community-based study in Istanbul. Child Care Health Dev 31: 707-717.
- Walter LM, Nixon GM, Davey MJ, et al. (2012) Differential effects of sleep disordered breathing on polysomnographic characteristics in preschool and school aged children. Sleep Med 13: 810-815.
- Spruyt K, Gozal D (2012) REM and NREM sleep-state distribution of respiratory events in habitually snoring school-aged community children. Sleep Med 13: 178-184.
- Zhu Y, Au CT, Lam HS, et al. (2014) Sleep architecture in school-aged children with primary snoring. Sleep Med 15: 303-308.
- O'Brien LM, Mervis CB, Holbrook CR, et al. (2004) Neurobehavioral implications of habitual snoring in children. Pediatrics 114: 44-49.
- Fuentes-Pradera MA, Botebol G, Sánchez-Armengol Á, et al. (2003) Effect of snoring and obstructive respiratory events on sleep architecture in adolescents. Arch Pediatr Adolesc Med 157: 649-654.
- Goh DY, Galster P, Marcus CL (2000) Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med 162: 682-686.
- Lamprecht ML, Bradley AP, Williams G, et al. (2016) Temporal associations between arousal and body/limb movement in children with suspected obstructed sleep apnoea. Physiol Meas 37: 115-127.
- Marcus CL, Lutz J, Carroll JL, et al. (1985) Arousal and ventilatory responses during sleep in children with obstructive sleep apnea. J Appl Physiol 84: 1926-1936.
- Fukumoto M, Mochizuki N, Takeishi M, et al. (1981) Studies of body movements during night sleep in infancy. Brain Dev 3: 37-43.
- Fukumizu M, Kohyama J (2004) Central respiratory pauses, sighs, and gross body movements during sleep in children. Physiol Behav 82: 721-726.
- Gori S, Ficca G, Giganti F (2004) Body movements during night sleep in healthy elderly subjects and their relationships with sleep stages. Brain Res Bull 63: 393-397.
- Coussens S, Baumert M, Kohler M, et al. (2014) Movement distribution: A new measure of sleep fragmentation in children with upper airway obstruction. Sleep 37: 2025-2034.
- Bélanger MÈ, Bernier A, Paquet J, et al. (2013) Validating actigraphy as a measure of sleep for preschool children. J Clin Sleep Med 9: 701-706.
- Chervin RD, Hedger K, Dillon JE, et al. (2000) Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med 1: 21-32.
- Chervin RD, Weatherly RA, Garetz SL, et al. (2007) Pediatric sleep questionnaire: Prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 133: 216-222.
- Kim SJ, Lee YJ, Cho SJ, et al. (2011) Relationship between weekend catch-up sleep and poor performance on attention tasks in Korean adolescents. Arch Pediatr Adolesc Med 165: 806-812.
- Meltzer LJ, Hiruma LS, Avis K, et al. (2015) Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 38: 1323-1330.
- Kolla BP, Mansukhani S, Mansukhani MP (2016) Consumer sleep tracking devices: A review of mechanisms, validity and utility. Expert Rev Med Devices 13: 497-506.
- Mantua J, Gravel N, Spencer RMC (2016) Reliability of sleep measures from four personal health monitoring devices compared to research-based actigraphy and polysomnography. Sensors (Basel) 16.
- Cook JD, Prairie ML, Plante DT (2017) Utility of the Fitbit Flex to evaluate sleep in major depressive disorder: A comparison against polysomnography and wrist-worn actigraphy. J Affect Disord 217: 299-305.
- Osterbauer B, Koempel JA, Davidson Ward SL, et al. (2016) A Comparison study of the fitbit activity monitor and PSG for assessing sleep patterns and movement in children. J Otolaryngol Adv 1: 24-35.
- Suratt PM, Diamond R, Barth JT, et al. (2011) Movements during sleep correlate with impaired attention and verbal and memory skills in children with adenotonsillar hypertrophy suspected of having obstructive sleep disordered breathing. Sleep Med 12: 322-328.
Corresponding Author
Debra Don, MD, Division of Otolaryngology - Head and Neck Surgery, Children's Hospital Los Angeles, 4650 Sunset Blvd Mailstop #58, Los Angeles, CA 90027, USA, Tel: (323)361-2145.
Copyright
© 2018 Don D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.