Effect of Sulfur on Rice Water Quality, Nutrient Uptake, and Yields Grown on Shallow Histosols
Abstract
High soil pH limits the availability of pH sensitive nutrients even though abundant levels are present in the soils. Elemental sulfur (S) application to lower soil pH and increase the nutrient availability to the crop is a possible and economically feasible solution. The objective of this study was to evaluate the effect of sulfur application on soil pH, rice water quality, nutrient uptake, and rice yield. An experiment was conducted to test the effect of different rates of sulfur application on rice grown in Everglades Agricultural Area (EAA) soil. The experiment was arranged in a completely randomized design with four replications of each treatment. Soil samples were evaluated for pre-and post-study and water samples were evaluated every week after flooding for up to 10 weeks. Plant tissues were evaluated at 44 days after planting. The parameters evaluated include pH total P, K, Ca, Mg, Fe, Al, Si, and B. Application of elemental sulfur at 68, 100, and 135 kg ha-1 had no significant effect on rice yield and plant tissue concentration. Surface water quality were not significantly affected by application of elemental S and 5.5 pH solution. Soil nutrient concentration decreased compared to pre-measurement except for Mg, which showed a significant increase after rice cultivation. Based on this result, a single application of sulfur at different rates as well as growing rice at 5.5 pH water was not effective in increasing the rice yield and nutrients uptake of alkaline organic soil of EAA.
Abbreviations
Al: Aluminum; B: Boron; Ca: Calcium; EAA: Everglades Agricultural Area; Fe: Iron; K: Potassium; Mg: Magnesium; P: Phosphorus; S: Sulfur; Si: Silicon
Introduction
Rice (Oryza sativa) is a worldwide nutritional staple and provides 20% of all human caloric needs [1]. As a result, rice production is an integral component of many economies. In 2019, 755.4 million tons of rice was produced globally, making it the 3rd largest agricultural crop [2]. Most rice is currently cultivated in eastern Asia because of its historic intertwinement with the region. Evidence shows that rice was first genetically domesticated in this area approximately 13,000-24,000 years ago [3]. Later, rice is said to have been brought to South Carolina from Madagascar in 1685 [4]. This event established the precedent for current day rice production in the United States.
Eventually, rice cultivation began in the Everglades Agricultural Area (EAA) of southern Florida in the 1950s. The production was small, at roughly 800 hectares (ha). The paddies were quickly struck by the white leaf virus (hoja blanca') and quarantined by the federal government [5]. Rice was reintroduced in south Florida in 1977 as a rotation crop with sugarcane [6]. During late spring and summer months, between sugarcane harvest and planting season, rice could be intermediately planted as a rotational crop for several agronomic benefits. First, it offers a source of additional income for growers and provides a food crop for the market [6]. Second, it keeps soils inundated with water to minimize soil oxidation [7]. Lastly, cultivating flooded rice on Histosols in the region has shown to benefit soil health [8], and reduce pest pressure [9]. Growers in the EAA are recognizing these benefits and are starting to grow rice in their fallow fields throughout summer as a Best Management Practice (BMP) in the region. Approximately 22,250 ha of farmland is fallow during summer within the EAA, of that, approximately 10,100 ha was used to cultivate flooded rice in 2018 [5].
Rice cultivation in the EAA region is considered a sustainable practice because of the unique nature of soils. These soils, which are historically referred to as "muck", fall under the histosol soil taxonomy classification developed in 1975 by the USDA [10]. These soils often contain more than 30% of organic material from decayed plant matter [11]. Through mass carbon sequestration and water inundation, these soils formed slowly over the last 4,400 years in the northern Everglades [12]. When land developers drained the EAA in the 1880s, they exposed these organic soils that had been atmospherically shielded by water inundation for many centuries. Consequently, they began to decompose through oxidation. This phenomenon is commonly known as soil subsidence [10]. Over the past century, subsidence has lowered the soil levels approximately by 2 meters [13]. In areas around the EAA, soil subsidence has become a looming threat to agriculture. In response, growers and others have searched for innovative and sustainable solutions to soil loss. Thus far, rotating rice cultivation with sugarcane production is one of the most promising solutions [5].
Soils throughout the EAA are alkaline, with a pH of 6.5 to 7.5 [14]. Rice is fonder of acidic conditions with a pH of 5.5 to 6.5 [15]. To remedy the discrepancy, application of elemental sulfur (S) has been hypothesized to lower pH levels, releasing nutrients that would have been bound to the soils at high pH; thus, improve rice yields. In more biogeochemical detail, rice needs certain macro and micronutrients. It needs nitrogen (N), phosphorus (P), and potassium (K) in large quantities and silicon (Si), copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), and Boron (B) in lesser quantities. When soil pH is in the acidic range (5-6.5), the micronutrients such as Zn and Mn have been shown to become more bioavailable for rice plants [16]. These same micronutrients can often be lacking in flooded rice with Zn deficiencies as the most common disorder [17]. When soils are treated with S application, bacteria convert the elemental S into either hydrogen sulfide (H2S) under anaerobic conditions or sulfuric acid (H2SO4) under aerobic conditions. The influx of acid into the soil will change the hydrogen ion equilibrium and result in lowering the pH. Consequently, micronutrients will become more bioavailable for plant uptake, hence improving yields. Commercial rice farming in the region does not apply N, P, or K as fertilizers; it relies on the high organic matter contentment in the Histosols to provide the crop with essential nutrients. However, the high pH limits the bioavailability of these nutrients; hence hypothesized that the application of elemental S may facilitate some of the nutrients essential for rice growth. The goal of the study was to determine if there were linkages between theory and practical application of elemental S in flooded rice production systems grown on Histosols. Therefore, the objective of this study was to evaluate the effect of different rates of S application, and modes of delivery on soil pH, rice water quality, nutrient uptake, and rice yield.
Materials and Methods
To understand the consequences of S application on change in soil pH, nutrient availability, and rice yields an experiment was conducted at the University of Florida, Everglade Research and Education Center (EREC). A common variety of rice was planted (dry-seeding) in 70-gallon pots containing Histosol available in EAA areas. The treatment consists of three elemental S rates 68 kg ha-1,100 kg ha-1, 135 kg ha-1, untreated control (0 kg ha-1), and 5.5 pH water without elemental S. The treatments were arranged in a randomized completed block design with four replications of each treatment. Steady flooded conditions for rice growth were maintained using groundwater. The parameters evaluated include yield, plant tissue analysis, extractable nutrient in water, and extractable nutrient in the soil.
Soil samples were collected before planting rice (pre) and after harvest (post). All samples were oven-dried and sieved through a 2-mm screen. Soil samples were analyzed for pH, P, K, Ca, Mg, Fe, Al, Si, and B. pH was measured using 1.5 g of dried soil mixed with 7.5 mL of deionized water (1:2 soil: solution) and analyzed using an Accumet AB250 pH meter. This meter was calibrated before testing with 4, 7, and 10 pH standards. Available P, K, Ca, Mg, Fe, Al, Si, and B were measured using Mehlich -3 (M3) extractions. For soil nutrient analyses, 2 g of soil was weighed and transferred into a 50-mL extraction bottle. 20 mL of Mehlich-3 extracting solution was dispensed into each extracting bottle with a pipette. Samples were shaken for 5 minutes on a reciprocating shaker and then filtered through a Whatman No. 42 filter paper and acidified using 2% HNO3. The filtrates were analyzed for nutrients using inductively coupled plasma-optical emission spectrometer (ICP-OES), EPA method 200.7. Before testing, the system was calibrated using mixed calibration standard solutions and the calibration blank. This reduced error by establishing and calibrating the analytical curve. Once the samples were analyzed, the resulting values were used to calculate concentrations of the different elements present.
Water samples were collected weekly once flooding was initiated three weeks after planting for a total of 10 weeks. A 50 mL syringe was used to syphon surface water from the pots into 50 mL polypropylene vials. Samples were analyzed for pH, as well as total P, K, Ca, Mg, Fe, Al, Si, and B using an Agilent 5110 inductively coupled plasma optical emission spectrometry (ICP-OES, Santa Clara, CA), EPA method 200.7. For pH, approximately 20 mL of sample was poured into a disposable scintillation bottle for testing. The samples were then tested with an Accumet AB250 pH meter. The meter was calibrated beforehand with 4, 7, and 10 pH standards. For measuring total P, K, Ca, Mg, Fe, Al, Si, and B, the ICP-OES was first calibrated using mixed calibration standard solutions and the calibration blank. This reduced error by establishing and calibrating the analytical curve. If the samples were above the upper limit of quantification, they were diluted to a measurable concentration.
In addition to water and soil analyses, treatment effects were also assessed on rice leaf tissues and ultimately rice yields. The yields were estimated on a per pot basis and converted to kg ha-1. The Leaf tissue samples were collected at 45 days after planting and analyzed for total P, K, Ca, Mg, Fe, Al, Si, and B. For tissue analysis, 0.5 g of dried plant tissue sample was weighed in a 50-mL Folin digestion tube and 5 mL of concentrated nitric acid were added. The tube was then heated to 120-130 ℃ for 14-16 hours and then treated with hydrogen peroxide. After digestion, the sample was diluted to 50 mL with deionized water and analyzed using ICP-OES. Like water and soil samples, the spectrometer was first calibrated with standard solutions and a blank. In total, there were approximately 32 soil samples, 200 water samples, and 16 plant tissue samples throughout the experimentation.
Statistical Analysis
To evaluate the effects of elemental sulfur on soil chemistry, water chemistry, nutrient concentration of plant tissue, and rice yield data were analyzed through randomized complete block design with four replications using PROC MIXED procedure in Statistical Analysis of Variance (SAS version 9.4, SAS Institute Inc., Cary, NC, USA). Means were separated using Fisher's protected LSD when the F test was significant at p ≤ 0.05.
Results and Discussion
Visual observations and yield
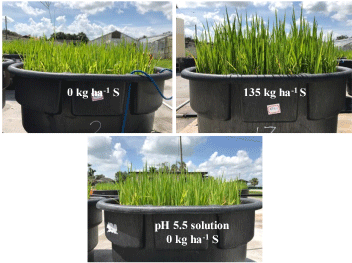
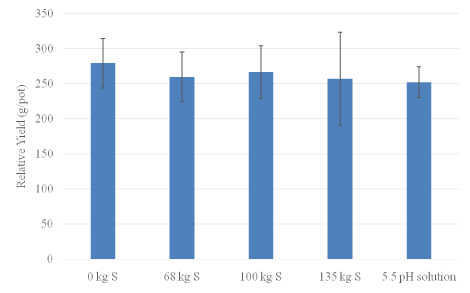
Initial observations were made visually to see if S had any effect on rice growth. At 44 days after planting, the pot that received 135 kg ha-1 S had a higher plant height compared to the control (Figure 1). However, at 65 days after planting no significant increase in leaf height was observed among treatments (Figure 2). Application of elemental S had no significant effect on rice yield. Each treatment produced between 250 g to 300 g per pot corresponding to ~ 5044 kg ha-1 wet weight, which is about the average rice yield we observe in Florida in EAA (Figure 3). This would indicate that the application of S has seemingly no negative impact on rice yield up to 135 kg ha-1 rate.
The increase in plant height in response to S at 44 days after planting is probably due to the enhanced availability of adequate nutrients which stimulated the plant growth [18] reported increased in plant height after S application in rice. Similarly, Chotchutima, et al. [19] also reported increase in height of Leucaena leucocephala following the addition of S. However, the short-term effect of S was likely due to strong buffering capacity of organic soils as reported by Kaler, et al. 2016 [20].
Leaf tissue concentration
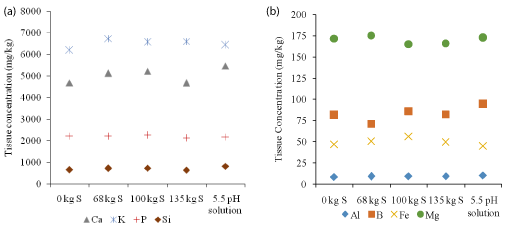
Applications of varying levels of S and rice grown on 5.5 pH water had no significant effect on leaf tissue concentration (Al, B, Fe, MG, Ca, K, P, and Si) compared to untreated control (Figure 4a and Figure 4b).Results indicate elemental S application in organic soil did not preferentially increase nutrient uptake in rice. In contrast to our result Oo, et al. 2007 [21] and Singh, et al. 2012 [20] reported increased in P and K concentration in grain and straw of aromatic rice. No significant effect of S on leaf tissue concentration was likely to be regulated by environmental factors such as soil, climate and growth stage of plant. Despite increased in Ca concentration in soil after the application of elemental S, the Ca concentration in maize plant tissue decreased [22]. The decreased in tissue Ca may be related to lower availability of Ca for plant uptake and antagonistic effects of another nutrient such as Mn and Zn with Ca uptake [22]. The accumulation of Fe in rice plants was reported to depend on S levels and the rice tissue itself [23]. The application of S at 60 mg S kg-1 increased the accumulation of Fe in brown rice stem and leaves, whereas excessive S application inhibits the accumulation of Fe in rice plant [23].
Changes in soil nutrient content
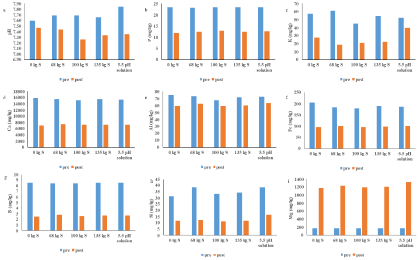
The reduction of soil pH was observed after rice cultivation irrespective of treatment (Figure 5a). This would indicate that the process of rice cultivation could lower soil pH. However, the rate of reduction was higher for S treated and a plot that received 5.5 pH water compared to control. The soil pH was slightly basic (above 7.2) even after applying S and grown under acidic water. Higher soil pH even after adding S and acidic water may be due to the presence of higher buffering capacity of soil against the acidification of S oxidation [24,25]. The pH range for this soil prior to S application was 7.6. The high pH before application of S indicates the presence of high carbonate and bicarbonates contents in the soil which resist changes in soil pH [26].
The reduction in soil pH due to oxidation of elemental S may be temporary depending on the soils buffering capacity [27]. Ye, et al. 2010 [25] did not observe a significant reduction of soil pH after application of S at 440 kg ha-1 in calcareous soils. Ye, et al. [25] observed a slight dropped in soil pH two months after the addition of S at 448 kg ha-1 and an increase thereafter from 6.0 to 6.4 at the end of the growing season. A decrease in soil pH is possible because the buffering effects often take place more slowly than the formation of sulfuric acid [25]. Some authors reported a limited reduction in soil pH of calcareous soils after S application [28]. Regardless of the mode of delivery of S (granular elemental or liquid in the form pH 5.5) seem to have facilitated in lowering soil pH at the end of the study.
Soil P, K, Ca, Al, Fe, B, and Si decreased after rice cultivation irrespective of S application (5 b-h). However, Mg increases after rice cultivation irrespective of S application (5 i). The reduction of soil nutrients after rice planting was likely due to the plant uptake and leaching loss. Reduction of soil pH after rice planting might be due to the uptake of certain nutrients like NH4+ and nitrification which reduce soil pH in the rhizosphere [29,30].
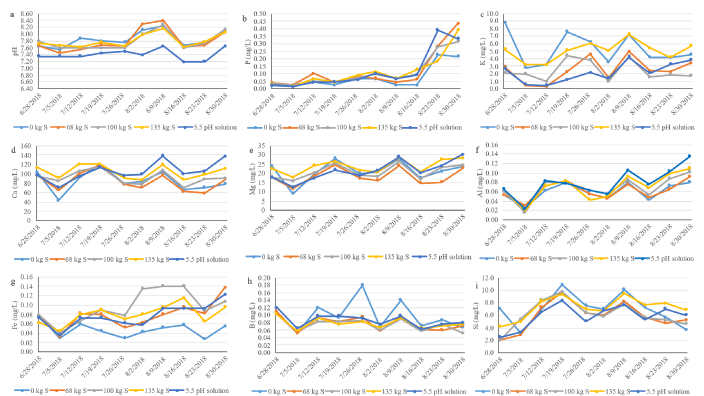
Changes in surface water concentration
The result from surface water concentration in the rice plots explains several important trends (Figure 6a, Figure 6b, Figure 6c, Figure 6d, Figure 6e, Figure 6f, Figure 6g, Figure 6h and Figure 6i). We observed fluctuation in the nutrient concentration throughout the 10-weeks measurement which might be associated with the growth and development of rice plants. A study conducted by Wu, et al. [23] observed the lowest Fe content in the soil solution during the rice tillering period, whereas the greatest Fe content during the rice bolting period. Our finding is also in agreement with this finding as we observed a decrease in Fe content in the second week of measurement which is tillering stage and Fe was maximum at later measurement which is the bolting stage (Figure 6h). However, S level had no effect on Fe contents during the rice growing period.
No change in Mehlich 3 extractable P, Ca, Mg, K, Fe, Zn, Mn, Si, and Cu due to the application of elemental Sat 0, 90, 244, and 448 kg ha-1 in EAA soil was also reported by Kaler, et al. [30].The effect of elemental S on soil pH and nutrient availability is not clear in the literature [31-33]. The positive effect of S on a significant change in soil chemical properties and nutrient solubility is well documented for certainsoils [34-38] while no effect of elemental S as a soil amendment on soil chemical properties and plant nutrient availability is reported by others [33,39-41].
Change in water nutrient concentration over time was observed (Table 1). Silicon and K were not affected by both S and low pH treatments over time, whereas pH decreased by pH 5.5 treatment compared to S treatment and untreated control. Aluminum concentration was higher at pH 5.5 treatment compared to untreated control and S at 68 kg ha-1. Phosphorus, Fe, and Ca concentration in the water increased over time, whereas B concentration had decreased over time compared to control. This would indicate that the application of S at different rates impacted the solubility of P, Fe, Al, Ca, and B. Soil acidity favors the solubilization of mineral cations in soil with high Ca and Mg carbonate content. Studies conducted by Wu, et al. [23] indicated that S was important for Fe accumulation in rice in low S soils. However, Fe was reported to decrease with S application when cultivated on soils with high S content. Heydarnezhad, et al. [42] reported S application significantly increases the solubility of P, Fe, and Zn in calcareous soils. The solubility and availability of nutrients improve crop yield. Calcium regulates plant growth and development, plays a key role in a number of metabolic pathways, promotes the functioning of enzymes and proteins [22] as well as interacts with other nutrients [43]. These results indicate that while the application of S in fact favors solubilization of certain nutrients, this does not translate into uptake by the rice grown on shallow Histosols.
Conclusion
The application of elemental S up to 135 kg ha-1on shallow Histosols within the EAA did not improve the yield and nutrient use efficiency of rice. Growing rice using a pre-buffered 5.5 pH water also did not significantly affect the rice yield. Soil Mehlich 3 nutrient (P, K, Ca, Fe, Al, Si, and B) concentration was significantly reduced after rice cultivation across all treatments. While Mg concentration was significantly increased, probably due to precipitation from the groundwater being used in the study. Soil pH was not affected by different rates of elemental S application up to 135 kg ha-1, implying that these shallow Histocols have a very high pH buffering capacity, and would potentially require higher inputs of S to lower soil pH. The limited effect of elemental S on soil pH in this study undermined the hypothesis that by adding elemental S, would lower soil pH, facilitate nutrient uptake, and increase rice yield.
Conflicts of Interest
Authors declares no conflicts of interest.
Acknowledgements
This study was funded by the Florida Rice Growers Inc. The authors would like to thank Dr. Raju Khatiwada, Salvador Galindo and Leandra Gonzalez for their assistance with field and lab work.
References
- (2004) Food and Agricultural Organization of the United Nations.
- (2020) Agriculture Organization Corporate Statistical Database. Crops. Food and Agriculture Organization of the United Nations. Rome.
- Choi JY, Platts AE, Fuller DQ, et al. (2017) The rice paradox: Multiple origins but single domestication in asian rice. Molecular Biology and Evolution 34: 969-979.
- West J (2007) Rice and slavery: A fatal gold seeder.
- Bhadha JH, Khatiwada R, Tootoonchi M, et al. (2019) Interpreting redox potential (Eh) and diffusive fluxes of phosphorus (P) and nitrate (NO3-) from commercial rice grown on histosols. Paddy and Water Environment 18: 167-177.
- Alvarez J, Kidder G, Snyder GH (1979) The economic potential for growing rice and sugarcane in rotation in the Everglades. In Proceedings Soil and Crop Science Society of Florida 38: 12-15.
- Bhadha JH, Wright AL, Snyder GH (2020) Everglades agricultural area soil subsidence and sustainability. University of Florida IFAS EDIS Publication, SL311.
- Bhadha J, Khatiwada R, Galindo S, et al. (2018) Evidence of soil health benefits of flooded rice compared to fallow practice. Sustainable Agriculture Research 7: 31-41.
- Cherry R, Tootoonchi M, Bhadha J, et al. (2015) Effect of flood depth on rice water weevil (Coleoptera: Curculionidae) populations in florida rice fields. Journal of Entomological Science 50: 311-317.
- Izuno F, Bottcher A (1994) Everglades Agricultural Area (EAA): Water, soil, crop and environmental management. University Press of Florida, Gainesville, USA.
- McCollum SH (1978) Soil survey of palm beach county area, florida. National Cooperative Soil Survey. USA.
- McDowell L, Stephens J, Stewart E (1969) Radiocarbon chronology of the florida everglades peat 1. Soil Science Society of America Journal 33: 743-745.
- Aich S, McVoy CW, Dreschel TW, et al. (2013) Estimating soil subsidence and carbon loss in the everglades agricultural Area, florida using geospatial techniques. Agriculture, ecosystems & environment 171: 124-133.
- Wright AL, Hanlon EA, Sui D, et al. (2009) Soil pH effects on nutrient availability in the Everglades Agricultural Area. EDIS, USA.
- Bhadha JH, Trotta L, VanWeelden MT (2016) Trends in rice production and varieties in the Everglades Agricultural Area. 4-4.
- Moro E, Crusciol CAC, Nascente AS, et al. (2017) Nitrate reductase, micronutrients and upland rice development as influenced by soil pH and nitrogen sources. Communications in Soil Science and Plant Analysis 48: 2642-2651.
- Das SK (2014) Role of micronutrient in rice cultivation and management strategy in organic agriculture-A reappraisal. Agricultural Sciences 5: 765-769.
- Singh AK, Meena MK, Upadhyaya A (2012) Effect of sulphur and zinc on rice performance and nutrient dynamics in plants and soil of Indo Gangetic plains. Journal of Agricultural Science 4: 162.
- Chotchutima S, Tudsri S, Kangvansaichol K, et al. (2016) Effects of sulfur and phosphorus application on the growth, biomass yield and fuel properties of leucaena (Leucaena leucocephala (Lam.) de Wit.) as bioenergy crop on sandy infertile soil. Agriculture and Natural Resources 50: 54-59.
- Kaler AS, McCray JM, Wright AL, et al. (2016) Nutrient availability response to sulfur amendment in histosols having variable calcium carbonates. Communications in Soil Science and Plant Analysis 47: 2178-2188.
- Oo NML, Shivay YS, Kumar D (2007) Effect of nitrogen and sulphur fertilization on yield attributes, productivity and nutrient uptake of aromatic rice (Oryza sativa). Indian Journal of Agricultural Sciences 77: 772-775.
- Karimi M, Yazdani-Biouki R, Hussin A, et al. (2020) Effect of elemental sulphur on calcium uptake and translocation in maize grown in a high pH soil of malaysia. Journal of Plant Productions (Agronomy, Breeding and Horticulture) 43: 497-506.
- Wu CYH, Lu J, Zheng YH (2014) Influence of sulfur supply on the iron accumulation in rice plants. Communications in Soil Science and plant Analysis 45: 1149-1161.
- Jaggi RC, Aulakh MS, Sharma R (2005) Impacts of elemental S applied under various temperature and moisture regimes on pH and available P in acidic, neutral and alkaline soils. Biology and Fertility of Soils 41: 52-58.
- Ye R, Wright AL, Orem WH, et al. (2010) Sulfur distribution and transformations in everglades agricultural area soil as influenced by sulfur amendment. Soil science 175: 263-269.
- Rogovska NP, Blackmer AM, Mallarino AP (2007) Relationships between soybean yield, soil pH, and soil carbonate concentration. Soil Science Society of America Journal 71: 1251-1256.
- Bloom PR (2000) Soil pH and pH buffering. In: Handbook of Soil Science. M Summer, CRC Press, Boca Raton, FL, USA, B333-B352.
- Hassan N, Olson RA (1966) Influence of applied sulfur on availability of soil nutrients for corn (Zea mays L.) nutrition. Soil Science Society of America Journal 30: 284-286.
- Bolan NS, Hedley MJ, White RE (1991) Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant and soil 134: 53-63.
- Kaler AS, McCray JM, Wright AL, et al. (2016) Nutrient availability response to sulfur amendment in histosols having variable calcium carbonates. Communications in Soil Science and Plant Analysis 47: 2178-2188.
- Klikocka H (2011) The effect of sulphur kind and dose on content and uptake of micro-nutrients by potato tubers (Solanum tubersosum L.). Acta Scientiarum Polonorum Hortorum Cultus 10: 137-151.
- Safaa MM, Khaled SM, Hanan SS (2013) Effect of elemental sulphur on solubility of soil nutrients and soil heavy metals and their uptake by maize plants. The Journal of American Science 9: 19-24.
- Skwierawska M, Zawartka L, Skwierawski A, et al. (2012) The effect of different sulfur doses and forms on changes of soil heavy metals. Plant, Soil and Environment 58: 135-140.
- Chien SH, Gearhart MM, Villagarcía S (2011) Comparison of ammonium sulfate with other nitrogen and sulfur fertilizers in increasing crop production and minimizing environmental impact: A review. Soil Science 176: 327-335.
- Cui Y, Dong Y, Li H, et al. (2004) Effect of elemental sulphur on solubility of soil heavy metals and their uptake by maize. Environment International 30: 323-328.
- Karimizarchi M, Aminuddin H, Khanif MY, et al. (2014). Elemental sulphur application effects on nutrient availability and sweet maize (Zea mays L.) response in a high pH soil of Malaysia. Malaysian Journal of Soil Science 18: 75-86.
- Vidyalakshmi R, Paranthaman R, Bhakyaraj R (2009) Sulphur oxidizing bacteria and pulse nutrition- A review. World Journal of Agricultural Sciences 5: 270-278.
- Wang AS, Angle JS, Chaney RL, et al. (2006) Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant and soil 281: 325-337.
- De la Fuente C, Clemente R, Bernal MP (2008) Changes in metal speciation and pH in olive processing waste and sulphur-treated contaminated soil. Ecotoxicology and Environmental Safety 70: 207-215.
- Sameni AM, Kasraian A (2004) Effect of agricultural sulfur on characteristics of different calcareous soils from dry regions of Iran. I. Disintegration rate of agricultural sulfur and its effects on chemical properties of the soils. Communications in soil science and plant analysis 35: 1219-1234.
- Shenker M, Chen Y (2005) Increasing iron availability to crops: Fertilizers, organo-fertilizers, and biological approaches. Soil Science & Plant Nutrition 51: 1-17.
- Heydarnezhad F, Shahinrokhsha P, Vahed HS, et al. (2012) Influence of elemental sulfur and sulfur oxidizing bacteria on some nutrient deficiency in calcareous soils. International Journal of Agriculture and Crop Sciences 4: 735-739.
- Meriño-Gergichevich C, Alberdi M, Ivanov AG, et al. (2010) Al 3+-Ca2+ interaction in plants growing in acid soils: Al-phytotoxicity response to calcareous amendments. Journal of soil science and plant nutrition 10: 217-243.
Corresponding Author
Naba R Amgain, Postdoctoral Associate, Everglades Research and Education Center, University of Florida, Belle Glade, Florida, USA.
Copyright
© 2021 Amgain NR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.