Is Phenotypic Variability in Leaf Vein Density in Rice Associated with Grain Yield?
Abstract
Rice, Oryza sativa L. is the staple food of half of the world's population, mostly in Asia. Therefore, it is essential to increase rice yield to meet the rising food demand by the year 2050. This target is further challenged by rising atmospheric temperature, rainfall extreme events and limited land availability. The designing of C4 rice along with greater efficiencies of water, nitrogen and radiation use, has been identified as one of the best pathways to increase yield potential. Increased leaf vein density is an essential prerequisite for C4 photosynthesis. It facilitates rapid exchange of C4 carbon intermediates and photo-assimilates and has been identified as a new trait for genetic engineering of C4 photosynthesis into C3 crops. We hypothesised that a large genetic variability in terms of vein density may exist in unexploited Sri Lankan origin rice landraces where vein density can be associated with crop yield potential. To test this hypothesis, 292 rice landraces were studied under field condition and vein density was measured at six to eight weeks using a handheld digital microscope. At maturity, agronomic traits such as crop biomass, grain yield, harvest index and chlorophyll content were measured. A large genetic variation in vein density (4.1-7.5 veins/mm) was observed among the screened landraces. A high vein density of 7.5 veins/mm was recorded in landraces SL1, SL52, SL96, SL130 and SL143. The high vein density was positively correlated with the grain yield (r = 0.60; P = 0.0001) suggesting that the higher number of veins can be used in future breeding programs to boost the yield potential of rice. Further, identification of the presence of such high vein density candidates will lay the basis to genetically engineer C4 like rice to enhance crop yield and adapt to climate change.
Keywords
Vein density, C4 photosynthesis, Climate change, Food security, Rice
Introduction
Rice (Oryza sativa L.), is the staple food of more than half of the world's population and 90% of the total rice grown is consumed in Asia [1]. Ray, et al. have shown that global crop production needs to be increased twofold by the year 2050 to meet the projected demand for rice from the growing population, diet shifts and increased demand for biofuel [2]. It is quite challenging to enhance rice production with limited arable land availability and unpredictable climate change patterns. Yield projections under optimistic scenarios can only meet 59% of the increased rice production required at a rate of 1.4% per year [2]. However, under worst-case scenarios, with the usage of current rice production measures, it is predicted that global production could only increase by 21% at the rate of 0.5% per year by 2050 [2]. Therefore, new approaches are required to enhance yield improvement to address the rising food demands while better adapting to future climate change [3,4].
Radiation Use Efficiency (RUE) of C3 crops need to be increased via integration of C4 like traits to C3 crops [3,5] which may also improve the Nitrogen Use Efficiency (NUE) and Water Use Efficiency (WUE) [6]. One of the prerequisites for C4 biochemistry to function is recognized as Kranz anatomy together with high vein density [7-9]. In many C4 plants, veins are closely spaced at a 1:1 ratio of Bundle Sheath Cells (BS) to Mesophyll Cells (M) in an arrangement of (Vein-BS-M-M-BS-Vein) and are typically separated by 60-150 µm interveinal distance [7,10-12]. In C3 plants interveinal distance is generally higher than 200 µm and are distantly located (Vein-BS-M-M-M-M-M-M-M-M-BS-Vein) in C3 rice [7,10-13]. All C4 plants show reduced M: BS ratio which reduces the path length of photosynthesis and facilitates rapid intercellular diffusion of metabolites [7,13]. These findings suggest that high vein density plays an important role in photo-assimilate transfer and subsequent plant growth. The International C4 Rice Consortium was established in 2006 to develop C4 rice and estimated that 15 years of experiments will be required to establish a C4 pathway in C3 rice which is not possible to achieve through conventional breeding [5]. Many approaches have been used to develop C4 rice aiming to increase the yield potential from the 1990s. The resultant C4 rice will also need to possess an efficient CO2 concentrating mechanism along with high WUE, NUE and RUE when compared to C3 rice [14,15]. Despite concerted attempts, very limited progress has been made in improving photosynthesis through the engineering of a C4 biochemical pathway in rice. Failure to find high vein density candidates in screened landraces led scientists to attempt mutation breeding to induce variations in vein density and then use such mutants as candidates for C4 rice engineering [14].
Being a tropical country with a wide array of micro climatic conditions, Sri Lanka harbours over 3000 landraces of rice including some traditional landraces well known for tolerance to prolonged drought, salinity and submergence [16]. However, no studies have been conducted to investigate phenotypic variability in photosynthesis related traits in this unique rice germplasm. In this study, we tested the hypothesis that alarge phenotypic variability in vein density remains within the Sri Lankan germplasm and this high vein density is associated with the yield potential of rice.
Materials and Methods
The field experiment was conducted at the Dodangolla Experimental Station (7° 33' 0" North, 80° 37' 0" East) Faculty of Agriculture, University of Peradeniya, Sri Lanka and the seeds were obtained from the Plant Genetic Resource Centre (PGRC), Gannoruwa, Sri Lanka. The examined population comprised of 292 traditional rice landraces and each landrace was replicated three times whereby each replicate consisted of 16 rice plants (hills).
Measurements of vein density and other leaf traits
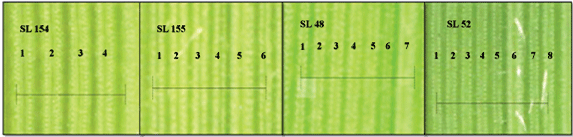
Vein density screening was carried out one and half to two months after planting. The widest part of the leaf surface of the fully expanded sunlit leaf was captured using a hand held microscope (Dino-Lite Digital Microscope AD7013MZT). An average of 10 leaves was measured per landrace. The number of veins in the captured imaged were counted off site. The number of veins in the widest part of the leaf was counted excluding the veins closer to the midrib and leaf margins. If a high vein density value of 7.5 veins/mm was recorded, and then a further vein density count was taken from the other side of the midrib. If two consistently high vein densities were recorded those plants were selected as potential candidates for further analysis. Other leaf traits such as leaf width were recorded using a digital Vernier calliper. Chlorophyll content was measured indirectly using a SPAD 502 Plus Chlorophyll meter. An average of 45 readings from both the adaxial and abaxial leaf surfaces of five plants was taken during the late vegetative stage.
Measurements of yield components
The average number of tillers of five plants was counted at the flowering stage. Number of panicles per plant, number of grains per panicle, number of filled grains and weight of 1000 filled grains were recorded. Filled grains of ten randomly selected panicles were separated using an aspirator and filled seeds were counted using a seed counter and empty seeds were counted manually. Three randomly selected plants were cut from the base of the shoot and dried in a forced air oven at 70 ℃ until constant weight was reached. Harvest index was calculated by dividing grain yield by shoot dry weight.
Statistical analysis
The association between vein density and other measured parameters (grain yield, shoot dry weight, SPAD, number of tillers, leaf width, number of panicles, number of grains per panicle, 1000 grain weight and Harvest index) were statistically analysed using Pearson correlation coefficients and analysis of variance test using SAS 9.4 [17].
Results
Measurements of vein density
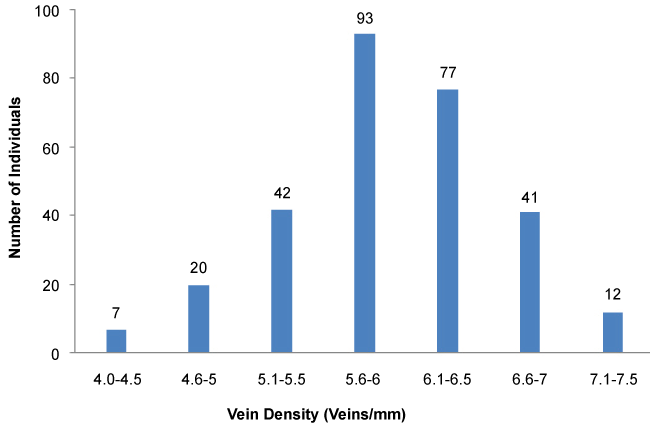
292 rice landraces were screened for phenotypic variation in vein density. A large variation in vein density ranging from 4.1-7.5 veins/mm (Figures 1 and Figure 2) was observed. Frequency distribution of vein densities in screened landraces is shown in (Figure 2). Based on the analysis, the screened population was categorized into 3 groups based on the number of veins; high (7.1 to 7.5 veins/mm), intermediate (5.1 to 7 veins/mm) and low (< 5 veins/mm) as shown in (Figure 2). The high vein density category, (7.1-7.5 veins/mm) was considered as a potential group for further studies. Accession, SL1, SL52, SL96, SL130 and SL143 showed the highest vein density of 7.5 veins/mm. However, these SL52, which had 7.5 veins/mm on both sides of the midrib was considered to be a potential high vein density candidate for further studies.
The relationship between vein density and yield components
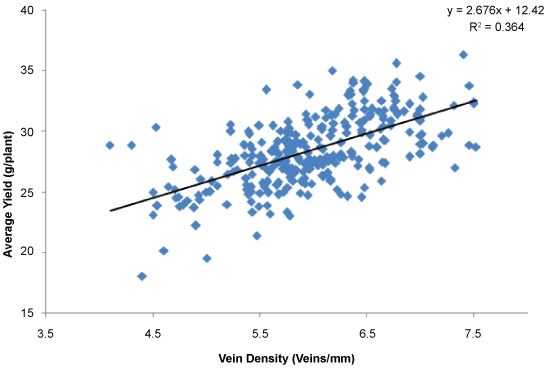
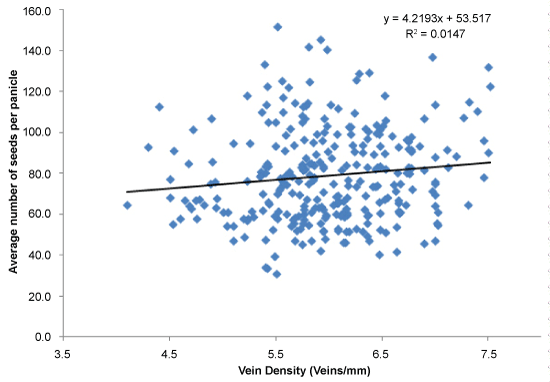
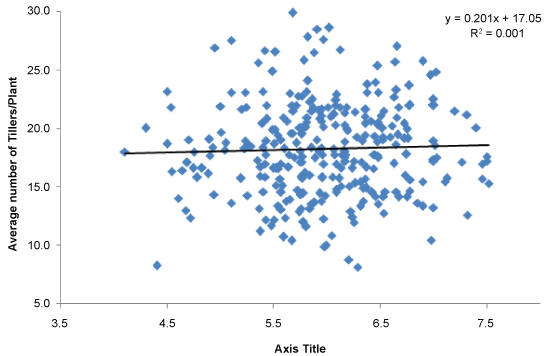
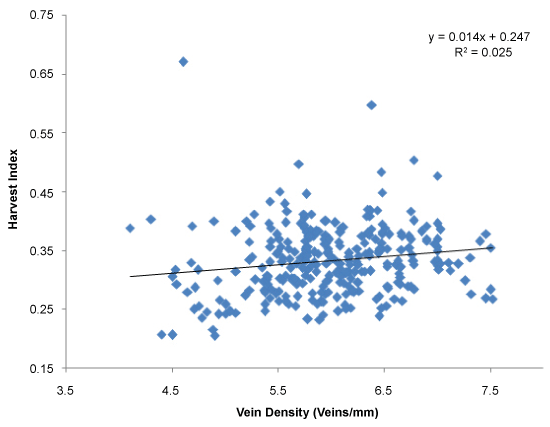
Grain yield and total dry mass were considered as indicator parameters of overall contribution of canopy photosynthesis at field level. The average yield of the screened population was around 28.4 g per plant (Figure 3). Further, the relationship between vein density and yield was established (Figure 3), and the yield is considered as a surrogate for photosynthesis. Results clearly demonstrated a positive correlation between grain yield and vein density (r = 0.60; P = 0.0001). The highest vein density candidate, SL52 had an average yield of 32.28 g of yield per plant (Figure 3). In this experiment, yield components of all accessions were measured including the number of panicles per hill, number of grains per panicle and 1000 grain weight. Most of the individuals in the population showed 15 to 20 panicles per plant (hill) and 80-100 grains per panicle (Figure 4) and 1000 grain weight of 22-26 g (Data not shown) in screened landraces. However, the number of panicles and 1000 grain weight was not associated with vein density with r value of 0.06 and 0.17 respectively. High vein density candidates showed lower panicle count ranging from 11.53 to 15.07. However, SL1 and SL130 produced a high number of seeds per panicle of 122.7 and 100.2 respectively while most of the other candidates produced average number of 78.7 seeds per panicle. Interestingly, the lowest vein density candidate produced a lower number of seeds of 64.6 seeds/panicle. However, SL52 produced an average of 74.3 seeds per panicle. It was observed that some of the high vein density candidates produced dense panicles with a high number of seeds and also produced less number of empty grains. There was no positive correlation between vein density and number of seeds per panicle as shown in (Figure 4) (r = -0.023; P = 0.68). High vein density candidates of SL1, SL52, SL96, SL130 and SL143 produced an average of 15.3, 17.2, 17.6, 16.9 and 15.7 tillers respectively. However, no correlation between tiller count and vein density was observed. (Figure 5). Most of the accessions showed a lower harvest index; conversely, some landraces showed an extremely high harvest index of 0.6 to 0.67 (Figure 6). However, no strong relationship was found between high vein density and harvest index (r = 0.15; P = 0.006) in the screened population (Figure 6). Harvest index of high vein density candidates was low and ranged from 0.26 to 0.37. This could partially be due to the fact that most of them were tall plants with a larger canopy and produced high shoot dry mass.
Relationship between vein density and SPAD measurements
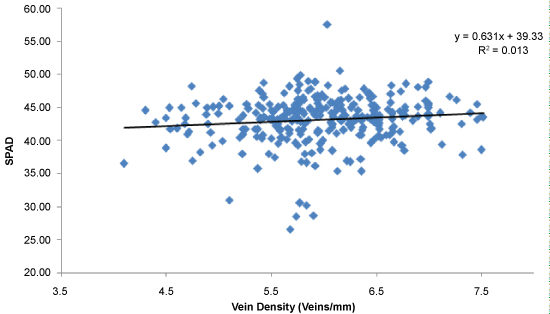
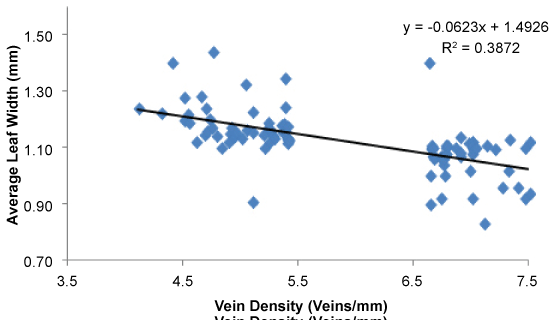
Leaf Nitrogen (Leaf N) status was assesed using a SPAD meter and the relationship between vein density and mean SPAD readings in the population is shown in the (Figure 7). Most of the individuals in the populations had high SPAD values. The average SPAD reading of the population was 43.1 (Figure 7). However, SPAD value was not associated with leaf vein density (r = 0.11, P = 0.05). The whole population was screened for leaf width and a negative correlation was observed between leaf width and vein density as shown in the (Figure 8). The results demonstrate that high vein density trait is associated with narrow leaf trait in the screened population (r = -0.62; P = 0.0001) (Figure 8). Though narrow leaf width was found to be correlated with high vein density, some of the high vein density candidates showed average leaf width. The selected candidates SL52 and SL130 showed an average leaf width of 1.12 cm and 1.10 respectively, whereas SL1, SL96 and SL143 showed narrow leaf widths of 0.91, 0.94 and 0.92 respectively. Similar observations were found in other vein categories as well.
Discussion
In general, rice is known to have a vein density of 5 veins/mm [18]. Interestingly, out of the 292 landraces used, 263 showed a vein density above 5 veins/mm. Not only that, 12 landraces showed a vein density above 7 veins/mm. This clearly indicates that landraces of tropical origin have a higher number of veins per leaf width. This could be an adaptation to maintain high water use efficiency under hot climatic condition to reduce transpiration and maintain higher rates of photosynthesis. C4 plants have evolved under arid and hot environments as an adaptation to balance photosynthesis by becoming radiation; water and nitrogen use efficiently [19-22]. Enhanced vein density is known to be an essential pre-requisite for the establishment of Kranz anatomy [7-9]. Previously, eight mutant rice lines with significantly high leaf vein densities, 7.5 veins/mm have been identified by Feldman, et al. to be used as genetic stock for the development of C4 rice by the Global C4 Rice Consortium [9]. Therefore, any plant that had a vein density of 7.5 veins/mm on both sides of the midrib was considered to be a potential high vein density candidate in this study. Out of the 5 identified high vein density candidates, only SL 52 was found to be a potential candidate for further studies on anatomical, biochemical and genetic regulations of vein density.
Rice, in Sri Lanka, is known to have evolved over 1000 years through selection by farmers for their enhanced yield. The potential candidate, SL52 is well known among locals for its higher shoot production. The grain yield and total dry mass were considered an overall photosynthetic contribution at field level in this study because the developmental differences of leaf blades could offset positive changes in photosynthesis rates. Our results clearly demonstrates a positive correlation between grain yield and vein density (r = 0.60). We, for the first time, have demonstrated that vein density is a good indicator of rice yield improvement [23]. Padmaja, et al. and Karthikeyan, et al. have demarcated that the yield and yield components of rice such as the number of grains per panicle and 1000 seed weight have higher and broad sense heritability [24-25]. Therefore, this correlation can be a genotypic regulation rather than a phenotypic observation. Therefore, the heritability of yield and the environmental regulations of vein density should be re-tested to establish the relationship between vein density and plant yield in rice.
Most of the leaf N is invested in key photosynthesis-related proteins; among them, an average of 25% of leaf N is invested in Ribulose-1,5-Bisphosphate Carboxylase Oxygenase (RuBisCO) [26]. In rice, 20-30% of the total leaf nitrogen is invested in RuBisCO [26,27]. In C4 crops, strategically less N is invested in the photosynthetic machinery as compared to their C3 counterparts. Leaf SPAD measurements correlate well with leaf Nitrogen (N) and photosynthetic rates but the relationship substantially varies between plant species [26]. Since the resultant C4 rice is expected to show nitrogen use efficiency of 30% [28], SPAD and its correlation to high vein density was analysed. In this study, SPAD value was not correlated with leaf vein density. Bellasio and Lundgren reported less vein pigmentation in rice [29]. The lower SPAD reading could be a result of low amounts of chlorophyll pigments in the leaves as a result of the higher number of veins. Unpigmented BS have been identified as one of the key factors that maximise light penetration and therefore photosynthesis, by enhancing the availability of ATP through photophosporylation in C4 plants [29]. Lower SPAD readings may be a good indicator of efficient radiation use efficiency of the identified candidates. Bellasio & Griffiths showed that small BS size in rice restrict the regeneration of RuBisCO when compared to bigger BS in C4 plants [30]. There could be efficient light penetration due to less pigmentation and the inefficiency of RuBisCO regeneration could limit photosynthesis in the resultant C4 rice. Increasing BS to M ratio by decreasing the size and number of M, decreased leaf thickness, bigger size of BS or increased number of veins have been identified as primary requirements to establish C4 photosynthesis in C3 rice [31]. Smillie, et al. has shown that interveinal space is determined by the size of M rather than the number of M whereas, Chatterjee, et al. observed plants with lower interveinal spacing had less number of M [8,18]. Therefore, high vein density/lower interveinal spacing can be a result of deceased M cell size or number. Therefore, the presence of a high number of veins may offset the limitations imposed by BS cell size for RuBisCO regeneration and may possess the potential to compensate for the reduced rate of photosynthesis through enhanced transporation and diffusion of assimilates. Mc Kown & Dengler demonstrated that a reduced M: BS ratio is required to facilitate efficient photosynthesis by reducing the path length and rapid intercellular diffusion of C4 metabolites [7,13]. A detailed anatomical study of the identified candidates from this study is essential to underpin the causes of these observed phenotypic variations and its association with the measured morphological traits.
Leaf morphology plays an important role in determining leaf photosynthetic rates. This can be seen in both leaf surface area and resistance to gas exchange measurements [8]. It is possible that increases in grain yield and shoot biomass in high vein density candidates could be associated with improved N, water and metabolite transfer efficiency. Our results demonstrate that high vein density is associated with narrow leaf width in the screened population with r value of -0.62. It has been reported that narrow leaf blades show a higher likelihood of increased vein density [8-9]. Our results also showed that screening for narrow leaf trait showed potential to be used as one of the primary traits for large-scale screening to reduce the time required for screening. As shown in (Figure 6), some high vein density candidates showed wider leaf width while low vein density candidates showed narrow leaf width. A similar relationship between interveinal spacing/high vein density and leaf width was reported elsewhere [18]. This variation could be caused due to an increase in leaf width and the plant may require more veins per unit area to maintain efficient transfer of metabolites. On the other hand, some plants may have the ability to adapt and maintain a high rate of metabolite transfer by adjusting the size or number of the mesophyll cells between veins and this could cause variations in vein density. Smillie, et al. have found that interveinal spacing is determined by cell expansion rather than cell division whereas Chatterjee, et al. observed plants with lower interveinal spacing had lower number of MC [8,18]. It is likely that a close relationship exists between mesophyll cell size, number and vein density, and this trait could be used as a screening tool to increase crop productivity in the future. The authors will carry out a detailed anatomical study of the selected landraces to find the underpinning causes of the observed variations.
Though the yield and leaf width showed positive correlation with vein density, these traits are mainly governed by genotype and environmental factors, especially light and temperature which are known to influence variations in the above measured traits. Therefore, the environmental effect on the above traits will be separately addressed in subsequent experiments. Since Kranz anatomy together with high vein density have been identified as essential prerequisites for the establishment of C4 biochemistry, identification of such high vein density candidates will be a major contribution towards the improvement of photosynthesis in rice. This will pave the foundation for the manipulation of photosynthesis through conventional breeding approaches since the redesigned C3 rice will have the potential to increase photosynthesis under elevated CO2 levels rather than C4 rice due to suppressed rates of photorespiration. Studying the under pining causes of genetic regulation will faciliate the production of C4 rice through transgenic approach, mutational breeding or genome editing using molecular tools.
Conclusion
Oryza sativa landraces of tropical origin have evolved under a wide range of microclimatic conditions as an adaptation to balance photosynthesis under hot environmental conditions by increasing vein density which positively correlated with crop yield. These findings suggest that crop yield can be improved by incorporating vein density traits into current plant breeding programs. The screening of narrow leaf width can be used as a basic parameter in the initial screening to select potential candidates for vein density. Further, if high vein density trait is associated with high photosynthesis rates and other biochemical parameters such as the higher activity of RuBisCO, it will pave the foundation for the next green revolution and open up avenues to breed high yielding rice cultivars with other desirable traits.
Acknowledgment
The authors acknowledge Plant Genetic Resource Centre of Sri Lanka for the provision of seeds, staff at the Department of Crop Science, Faculty of Agriculture, University of Peradeniya, Sri Lanka for their assistance and provision of facilities, Mr Prasanna Amarasekera and the staff of Dodangolla Experimental Station, Sri Lanka for their assistance, Mr B.G.P.S.V. Senavirathna for assistance in statistical analysis and the Australian Commonwealth Government for support through an Australian Government Research Training Program Scholarship, The University of Melbourne and the University of Peradeniya for the provision of financial support to conduct the research.
References
- Sheehy J, Ferrer A, Mitchell P, et al. (2007) How the rice crop works and why it needs a new engine. How the 280 rice crop works and why it needs a new engine. In: Sheehy, JE, Mitchell PL, Hardy B, Charting new pathways to C4 rice. Int Rice Res Inst, Phillipines, 3-26.
- Ray DK, Mueller ND, West PC, et al. (2013) Yield trends are insufficient to double global crop production by 2050. PLoS One 8: e66428.
- Hibberd JM, Sheehy JE, Langdale JA, et al. (2008) Using C4 photosynthesis to increase the yield of rice-rationale and feasibility. Curr Opin Plant Biol 11: 228-231.
- Furbank RT (2013) Photosynthesis research and its application to yield potential. In: Gready J, Dwyer SA, Evans JR, Applying photosynthesis research to improvement of food crops. ACIAR, Australia, 14-19.
- Mitchell PL, Sheehy JE (2007) Surveying the possible pathways to C4 rice. In: Sheehy JE, Mitchell PL, Hardy B, Charting New Pathways to C4 rice. International Rice Research Institute, Phillipines, 399-412.
- Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161: 341-370.
- McKown AD, Dengler NG (2007) Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). Am J Bot 94: 382-399.
- Smillie I, Pyke K, Murchie E, et al. (2012) Variation in vein density and mesophyll cell architecture in a rice deletion mutant population. J Exp Bot 63: 4563-4570.
- Feldman AB, Murchie EH, Leung H, et al. (2014) Increasing leaf vein density by mutagenesis: laying the foundations for C 4 rice. PLoS One 9: e94947.
- Langdale JA (2011) C4 cycles: past, present, and future research on C4 photosynthesis. Plant Cell 23: 3879-3892.
- Dengler NG, Dengler RE, Donnelly PM, et al. (1994) Quantitative leaf anatomy of C3 and C4 grasses (Poaceae): bundle sheath and mesophyll surface area relationships. Ann Bot 73: 241-255.
- Dengler NG, Nelson T (1999) Leaf structure and development in C4 plants. In: Sage RF, Monson RK, C4 plant biology. Academic Press, USA, 133-172.
- McKown AD, Dengler NG (2010) Vein patterning and evolution in C4 plants. Botany 88: 775-786.
- Rizal G, Karki S, Thakur V, et al. (2012) Towards a C4 rice. Asian Journal of Cell Biology 7: 13-31.
- Von Caemmerer S, Quick WP, Furbank RT, et al. (2012) The development of C4 rice: current progress and future challenges. Science 336: 1671-1672.
- Plant Genetic Resource Centre of Sri Lanka (1999) PGR Catalogue - Passport Information. (1st edn), PGRC, Sri Lanka.
- SAS 9.4, SAS Institute Inc. Cary, USA.
- Chatterjee J, Dionora J, Elmido-Mabilangan A, et al. (2016) The Evolutionary Basis of Naturally Diverse Rice Leaves Anatomy. PLoS One 11: e0164532.
- Taylor HM, Jordan WR, Sinclair TR, et al. (1983) Limitations to efficient water use in crop production. American Society of Agronomy Madison, WI.
- Schulze ED, Ellis R, Schulze W, et al. (1996) Diversity, metabolic types and δ13C carbon isotope ratios in 315 the grass flora of Namibia in relation to growth form, precipitation and habitat conditions. Oecologia 106: 352-369.
- Lara MV, Andreo CS (2011) C4 plants adaptation to high levels of CO2 and to drought environments. Abiotic Stress in Plants-Mechanisms and Adaptations.
- Christin PA, Osborne CP (2014) The evolutionary ecology of C4 plants. New Phytol 204: 765-781.
- Nawarathna RN, Dassanayake KB, Nissanka SP, et al. (2015) Are vein density candidates laying a foundation for C4 rice? in Postgraduate symposium of Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Australia.
- Padmaja D, Radhika K, SubbaRao LV, et al. (2008) Advance for Quantitative Characters in Rice (Oryza 325 sativa L.). Indian J Plant Gen Res 21: 3-326.
- Karthikeyan P, Anbuselvam Y, Elangaimannan R, et al. (2010) Variability and heritability studies in rice (Oryzasativa L.) under coastal salinity. Elec J Plant Breeding.
- Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9-19.
- Makino A, Osmond B (1991) Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol 96: 355-362.
- Center CR Science of C4 Rice.
- Bellasio C, Lundgren MR (2016) Anatomical constraints to C4 evolution: light harvesting capacity in the bundle sheath. New Phytol 212: 485-496.
- Bellasio C, Griffiths H (2013) The operation of two decarboxylases, transamination and partitioning of C4 metabolic processes between mesophylll and bundle sheath cells allows light capture to be balanced for the maize C4 pathway. Plant Physiol 164: 466-480.
- Lundgren MR, Osborne CP, Christin PA, et al. (2014) Deconstructing Kranz anatomy to understand C4 evolution. J Exp Bot 65: 3357-3369.
Corresponding Author
Ruwanthi Nawarathna, Department of Agriculture and Food systems, Faculty of Veterinary and Agriculture Sciences, The University of Melbourne, Parkville, VIC 3010, Australia, Tel: +61420887983.
Copyright
© 2017 Nawarathna RN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.