Coronavirus (COVID-19) Pandemic – A Comprehensive Review of Demographics, Comorbidities, Vaccines, Therapeutic Development, Blood Type, and Long Covid
Abstract
The study summarizes the pandemic COVID-19's impact on symptoms, demographics, comorbidities, and vaccine and therapeutic development and demonstrates an association with cases and mortality for the past two years. There has been rapid scientific advancement over the past two years 2020-2022 in developing vaccines and therapeutics for combating the disease.
We chose three highly affected countries US, India, and China, to address the impact of demographics and comorbidities on COVID-19 using US Center for Disease Control and Prevention (CDC) data.
Based on the analysis of this data, we see that the infection rate is higher in females, while the percentage of death is higher in males than females (p < 0.0001), and the number of female cases among females has increased by 1.7% while the number of deaths among females has decreased by ~1%, within the last two years. The trend of getting affected by COVID-19 is similar during 2020-2022, i.e., Whites followed by Hispanics and Black people.
After a thorough review of many manuscripts, we concluded that diseases like cardiovascular disease (CVD), diabetes, hypertension, chronic pulmonary obstructive disease (COPD), and acute respiratory distress syndrome (ARDS) were the typical comorbidities leading to severe COVID-19 conditions. In addition, variants of COVID-19, current vaccine and therapeutic development efforts, and relation of COVID-19 with blood type are discussed.
Finally, to conclude that for designing vaccine trials, following FDA's guidance emphasizing stratification factors based on demographics and comorbidities should be considered while allocating treatment to patients.
Keywords
Pandemic, Comorbidities, Demographics, Vaccines, Clinical trials, COVID-19, SARS-CoV-2, Coronavirus, Drugs for COVID-19, Blood groups, Polynomial, Generalized additive model, and Generalized linear model
Introduction
In late December 2019, a series of pneumonia cases of unknown origin emerged in China, with clinical presentations resembling viral pneumonia. Deep sequencing analysis of lower respiratory tract samples indicated a novel coronavirus named 2019 (2019-nCoV), causing a worldwide concern [1]. In March 2020, the 'severe acute respiratory syndrome coronavirus 2' (SARS-CoV-2) virus was declared a global pandemic. COVID-19, officially named by World Health Organization (WHO), had a case count of over 21M (21,010,700), with 761,260 deaths as of August 14, 2020, and over 603M (603,336,130) total cases, and over 6M (6,491,951) deaths as of August 2022 according to Johns Hopkins Coronavirus Resource Center [2]. The United States had the highest number of cases at 5,280,315 and a death count of 167,828 as of August 2020, followed by Brazil, India, and Russia. As of August 30, 2022, US cases stand at 94.5 M and 1.04 M deaths, India at 44M cases and 528K deaths, Brazil has 34.5M cases and 684K deaths, and Russia with 19.3 M cases with 377K deaths. With the advent of the recent Omicron XE, South Korea has the highest number of cases, followed by Germany, Vietnam, France, the United Kingdom, and Italy. COVID-19 pandemic is the most significant global crisis since World War II that affected almost all the countries on Earth, according to the United Nations [3]. Within seven months, we have seen an exponential rise in the number of publications, a total of 11,452 publications on COVID-19 (November 2019 - May 2020), and google search shows more than 4 million (4,770,000) results as of August 2022 with a single search term of "COVID-19." Our primary focus here is to provide a narrative review covering the essential areas: a) The origin of COVID-19, b) Clinical features and symptoms, c) Variants of COVID-19, d) COVID-19 and comorbidities, e) blood type and COVID-19, e) Long COVID-19 and f) Vaccines and therapeutics developments.
Finally, we focus on understanding the impact of sex, race, and age on COVID-19 which affected US population.
We performed a comprehensive review of the published literature to help clinical researchers and health policymakers optimally allocate health care resources. From this review, health care providers and administrators can get a detailed overview of the ongoing COVID-19-related research and the progress of pharmaceutical and vaccine product development. We summarize the effect of demographics and comorbidities on COVID-19 and other vital items that can serve as an excellent resource and guide for managing current and future public health crises.
Material and Methods
Search strategy and eligibility criteria
Articles were searched across PubMed/Medline, including PubMed's COVID-19 literature collection labeled LitCovid (https://www.ncbi.nlm.nih.gov/research/coronavirus/) [4], and the Google Scholar database from December 2019 until August 2022. The studies were from different parts of the world published in English, with confirmed COVID-19 cases with sex, gender, and comorbidity information included. The search keys used for this study were: (coronavirus OR "coronavirus" OR covid19 OR "covid 19" OR sarscov2 OR 2019nCoV) AND ("clinical characteristics" AND "comorbidities"). Studies included epidemiological information, clinical characteristics, treatment, and a clear outcome description. In addition, explicit description of comorbidities including hypertension, diabetes, cardiovascular disease, malignancy, and the symptoms such as fever, cough, fatigue, and dyspnea and significantly impacting conditions such as acute respiratory distress syndrome (ARDS), malignancy, HIV, thyroid, diabetes, the incidence of severity, hospitalization, and mortality. The exclusion criteria were the absence of clinical characteristics, treatment outcomes, and clinical experience.
The databases were screened to find manuscripts that matched the study's objectives. After shortlisting the articles, the full-text articles were read thoroughly. Some manuscripts from the reference lists of other articles were also identified. Figure 1 shows word clouds using keywords and the names of the journals to understand which journals and keywords had the dominant frequency. We find that PLOS One and scientific reports journals have published a maximum number of times on COVID-19 or SARS-CoV-2.
Various studies have been published on epidemiology, clinical and prognostic-related features of COVID-19. The following review condenses COVID-19 information into an organized and comprehensible structure for the researchers working in the COVID-19 area.
Origin of COVID-19
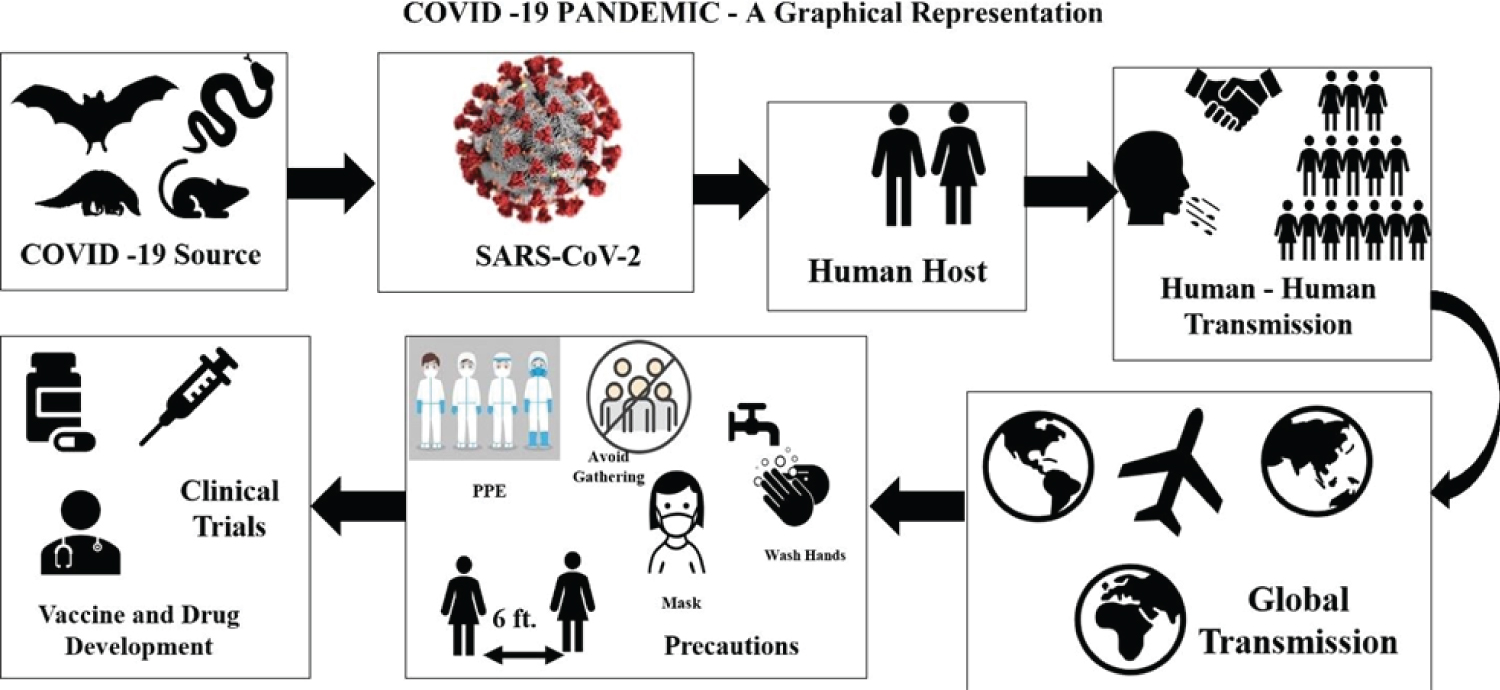
Coronaviruses are not new to human society. Coronavirus has occurred previously in China as a severe acute respiratory syndrome (SARS) virus and infected over 8000 people and caused 776 deaths in 2003 [5] and was named SARS-COV [6,7]. The Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was detected in Saudi Arabia in 2012 [8]. It infected more than 2603 individuals and caused 935 deaths across 27 countries as of December 2022, as reported by WHO. Initial Coronavirus 2019 (COVID-19) cases began at the Hunan wholesale seafood market in China that traded live exotic animals, and since then, it has severely affected humanity. The virus was identified by genome sequencing as a coronavirus with 96.2% homology (genetic identity) with bat coronavirus (CoV RaTG13) and 79.5% homology with SARS-CoV. Human transmission is through an unknown intermediate source, which scientists have suspected to be a bat, swine, snake, civet, pangolin, or mouse, among others [9-11]. Identifying a potential intermediate host of SARS-CoV-2 from the SARS-CoV-2 genome features can lead us to the proximal origin of the virus and spread from animal species boundaries to humans. At one point, it was mentioned not as a laboratory construct virus. [12]. Recent research finds SARS-CoV-2 bat sarbecovirus RaTG13 as the origin, claims of pangolin [13] being the intermediate host or the virus being a laboratory construct have been discarded [14]. Figure 2 gives an overview of the pandemic explaining its origin, transmission, and the recommended precautions. Several theories and myriad efforts have accumulated in the endless search for the source of the virus. The World Health Organization's (WHO's) Scientific Advisory Group for the Origins of Novel Pathogens (SAGO) will soon put out a report specifying studies that are urgently needed [15]. The 2022 report recently published mentions that all theories and hypotheses are on the table, and further studies and evidence are required to determine the origins of COVID-19 [17-19].
Clinical Features and Symptoms
The virus incubation period is 2-14 days; however, it is frequently 3-7 days [16-17]. The human-to-human spread of the virus occurs due to proximity to an infected person, or an individual exposed to coughing, sneezing, and respiratory aerosol droplets. These aerosols penetrate the human lung via inhalation through the mouth or nose. The symptoms include fever, cough, fatigue, sputum production, shortness of breath, sore throat, headache, diarrhea, nausea, and vomiting [18,19]. People over 65 years and those with underlying disorders (i.e., hypertension, chronic obstructive pulmonary disease, diabetes, cardiovascular disease) were more prone to rapidly develop acute respiratory distress syndrome, septic shock, metabolic acidosis, and coagulation dysfunction, often resulting in death [1]. Diarrhea and taste disorders were also noted in COVID-19 positive cases. There have been asymptomatic people with very few symptoms, such as runny nose, sore throat, or no symptoms [20]. Studies show that the need for invasive mechanical ventilation was common in patients with COVID-19 compared to influenza virus-affected individuals in the ICU [21]. Coronavirus S-protein binds with the human protein receptor ACE2, which is present in lungs, kidneys, and adipose tissue, and invades human tissues. Laboratory findings include lymphopenia, prolonged prothrombin time (PT), elevated lactate dehydrogenase (LDH), and elevated alanine aminotransferase (ALT), among others. COVID-19 mainly affects the lower lobes of the lungs, as seen on chest CT scans [22].
Variants of COVID-19
Viruses, in general, constantly mutate and thus producing several mild to dangerous variants that have harmed humankind. The SARS-CoV-2 genome is also prone to various mutations. Some of the identified variants are B.1.526 (Iota), B.1.525 (Eta), P.2 (Zeta), B.1.1.7(Alpha), B.1.351 (Beta), P.1(Gamma), B.1.617.2 (Delta) B.1.427, /B.1.429 (Epsilon) and B1.612, B.1.621.1 (Mu) The variant of concern has been B.1.1.529 (Omicron). Omicron's subvariants are B.A.4, BA.5, BA.2.12.1, and BA.2.75 [28] (Table 1).
COVID-19 and Comorbidities
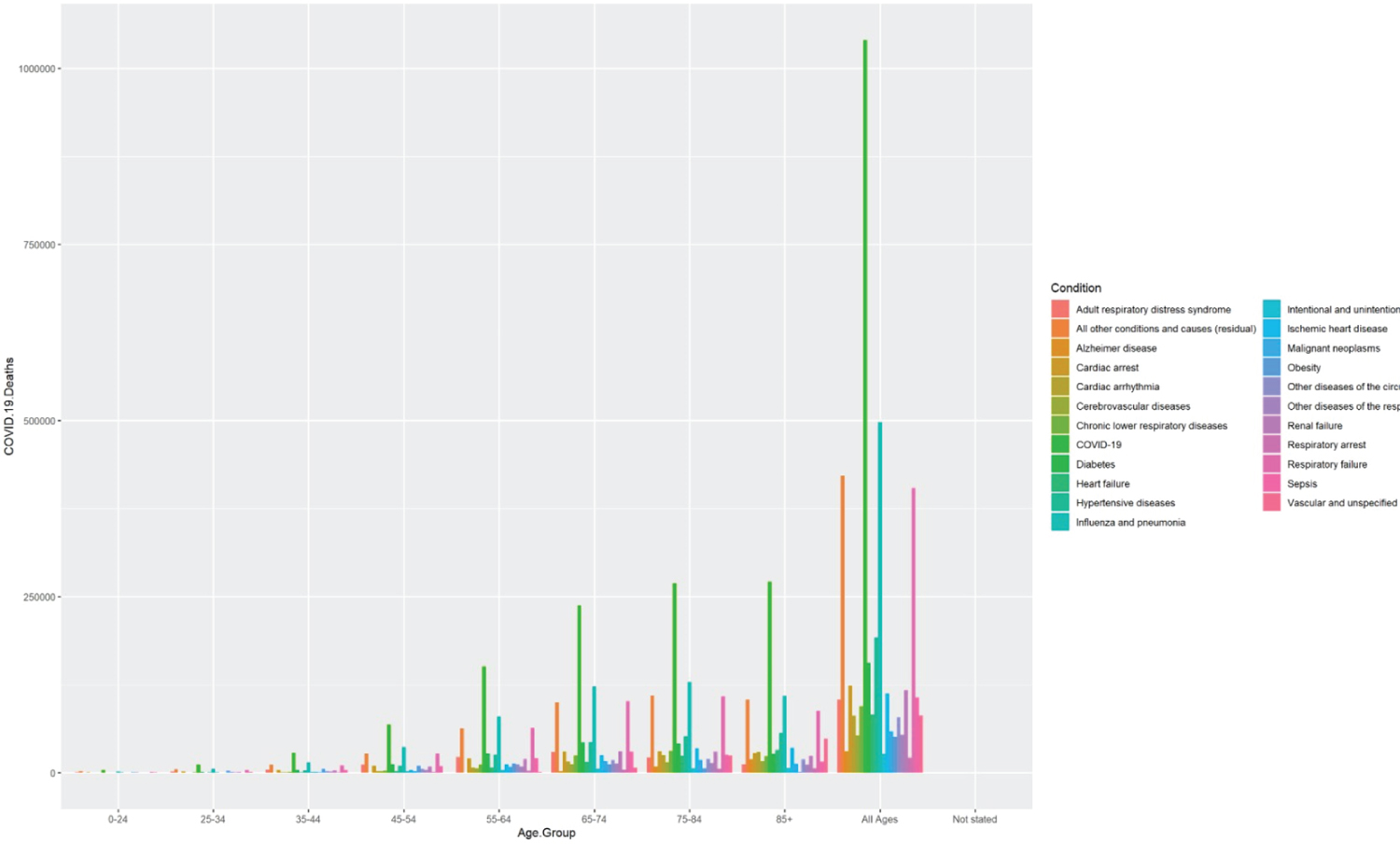
People with associated comorbidities and preexisting conditions, including heart failure, uncontrolled diabetes, COPD, asthma, and cancer, are at heightened risk and may require ventilator support or oxygen therapy during hospitalization. The comorbidities-associated negative effect of COVID-19 is described here. We have considered three countries US, China, and India, to understand the impact of the comorbidities on COVID-19 deaths as these top three countries were severely affected by the coronavirus. Using 2020-2022 data from CDC, we plotted the total number of COVID-19 deaths within the US.
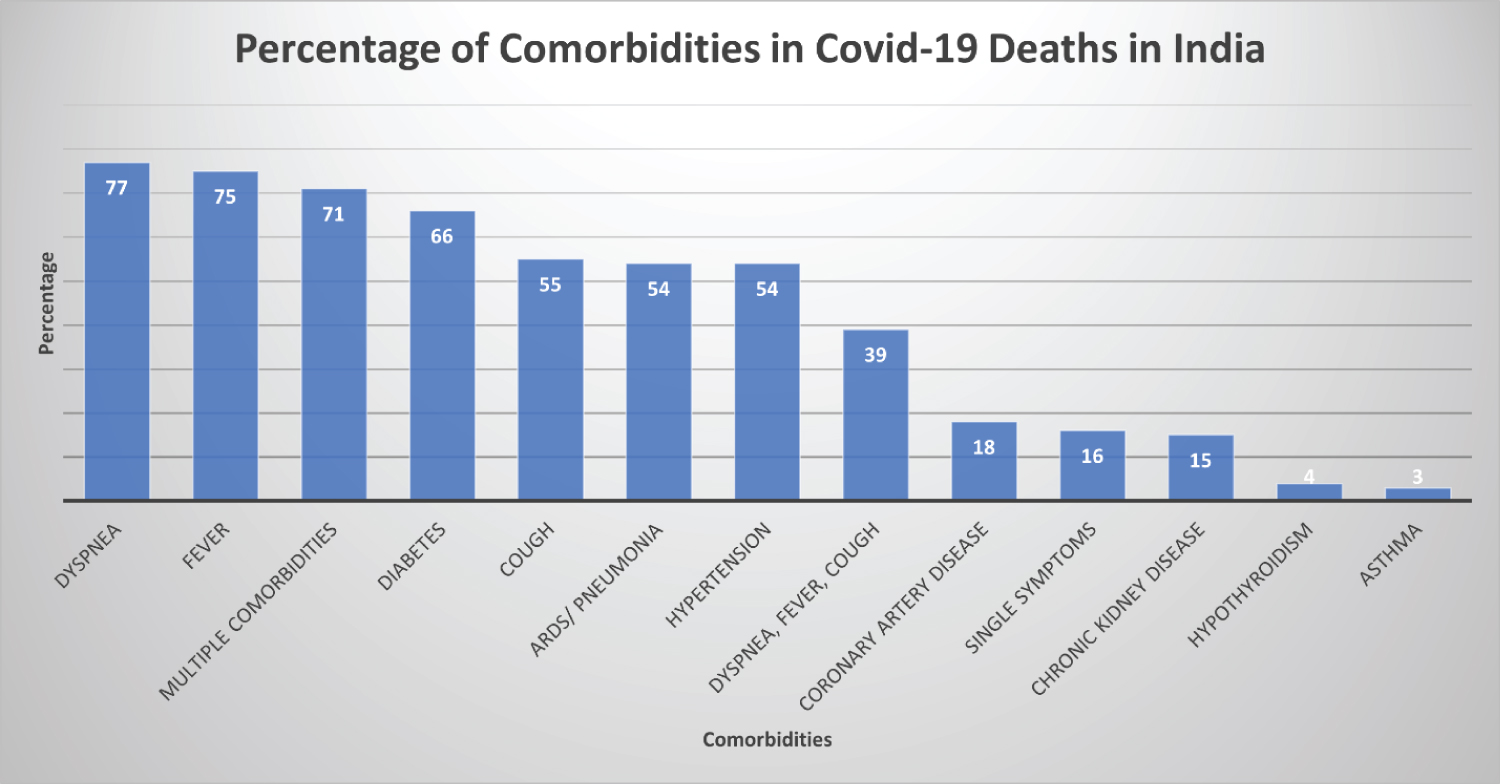
By age group and comorbidities [23] Based on the graph, we find, in all the age groups COVID-19 has been the primary cause of death, followed by influenza, pneumonia, hypertension, and heart failure. A recent article, where 2000 COVID-19 deaths in India were audited, showed the following comorbidities associated with fatalities (Figure 3, Figure 4 and Figure 5). The prevalence of comorbidity and concurrence of comorbidities increased with age; 60% of those aged 60 years or older had at least one comorbidity compared to 6% of those aged 40 years or younger (Χ2 = 61.9, p < 0.0001). The data of various combinations of comorbidities are as follows [24,25].
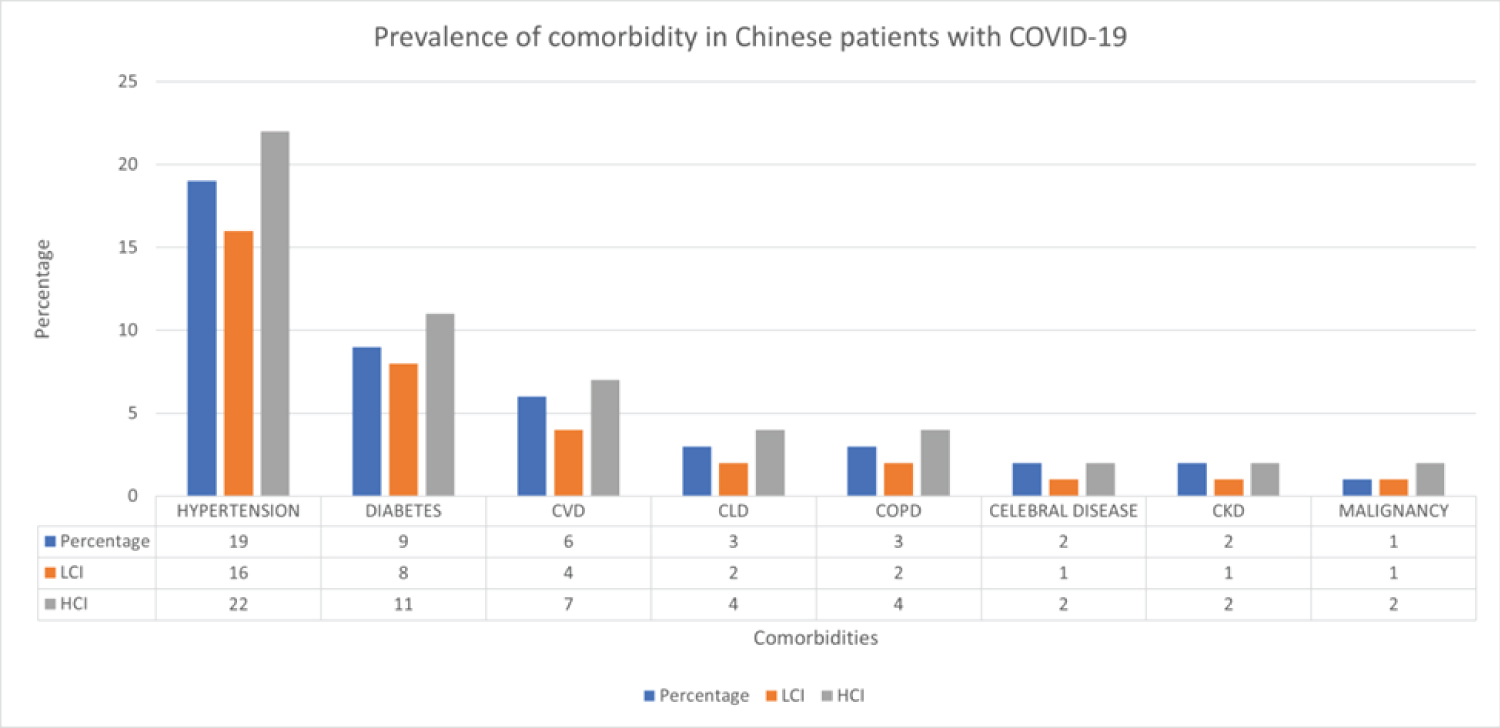
From comorbidities data in COVID-19 patients in China, we obtained the following figure to address the prevalence of comorbidities in Chinese patients with COVID-19 [26].
Pre-clinical and clinical studies indicate that ARDS is a primary manifestation of COVID-19 patients [27]. Among a large group of hospitalized patients (N = 14866) who died, three-fourths of them developed ARDS, and a significant of them required ICU and invasive ventilation [28]. The detailed prevalence of comorbidities shows that hypertension followed by diabetes and cardiovascular diseases were the most common comorbidities seen in COVID-19-positive patients across major epicenters worldwide, and these three morbidities led to a higher risk of COVID-19 mortality.
Diabetes and obesity
Patients with the pre-diabetic condition and severe metabolic complications are prone to an increased risk of severe outcomes from COVID-19, thus requiring closely monitored blood glucose control. Also, COVID-19 may trigger diabetes in healthy people resulting in a bidirectional relationship between the two diseases [29]. SARS-CoV-2 may cause pleiotropic alterations of glucose metabolism as it binds with ACE2 receptors expressed in essential metabolic organs, tissues, and kidneys that could complicate conditions of preexisting diabetes or lead to new mechanisms of disease [30]. Recent initiatives have been taken by an international group of leading diabetes researchers in the COVIDIAB project (covidiab.e-dendrite.com) to study the new-onset COVID-19-related diabetes, along with data collection on preexisting metabolic disturbances including diabetic ketoacidosis, and hyperosmolarity.
The scarcity of BMI data on COVID-19 patients may impact the understanding of the underlying relation of obesity to COVID-19 infection and its severity due to obesity-induced adipose tissue inflammation in type 2 diabetes, hypertension, and cardiovascular disease, but the relationship does exist [31]. Obesity can lead to decreased expiratory reserve volume, functional capacity, and respiratory system compliance. Also, previous hospitalizations due to the H1N1 virus were higher in obese individuals and prevalent among American Indians, Hispanics, and African Americans [32]. A review by Yang, et al. found that most of the younger COVID-19 hospitalized population suffered from obesity [33]. The presence of morbid obesity (BMI ≥ 35 kg/m2) aggravates respiratory disease and can result in a patient being in an intensive care unit (ICU) admission among COVID-19 patients [34].
Cancer
The significant risk to cancer patients was due to not getting necessary treatment for their cancer and medications as the healthcare providers concentrated on treating patients with COVID-19. It has been estimated that the delay in the completion of oncology clinical trials has been due to delayed or canceled enrollment appointments with approximately two-hundred international trials suspended [35], restrictions on hospital visits, and delayed surgeries due to the present infection [36,37]. Thus, cancer treatments have been modified to align with the situation, such as delaying screenings to decrease the risk of exposure, using personal protective equipment (PPE) in surgery rooms, and implementing the RADS (Remote visits, Avoid radiation, defer radiation, Shorten radiation) principle [38]. Cancer patients show more adverse outcomes after coronavirus infection than healthy individuals [39]. Kudere, et al. [40] concluded that among cancer patients with COVID-19, 30-day all-cause mortality was high with general and unique factors to patients with cancer. A recent study found a 74% increase in 30-day mortality among patients with COVID-19 and cancers as compared to COVID-19 without cancer [41]. Another study [42], found a 17% increase in 30-day mortality and a 10% increased risk of hospitalization among recently diagnosed cancer patients.
Immunocompromised patients
The immunocompromised patients are more likely to suffer severe consequences of SARS-CoV-2 infection [43,44]. In a recent study, seroconversion rates after two vaccine doses were 99% for people who were not immunocompromised vs. 92% for patients with solid cancer, 78% for patients with immune-mediated inflammatory disorders, 64% for patients with hematological cancer, and 27% for recipients of transplants. In another mRNA vaccine study of COVID-19-associated hospitalization of immunocompromised patients, the rate was 77% among immunocompromised adults than immunocompetent adults (90%) [42]. A survey by Pfizer investigators [45] which comprised data from 10 studies done in four countries between December 2020 and September 2021, showed vaccine effectiveness (VE) of 64% to 90% against SARS-CoV-2 infection, 73% to 84% against symptomatic illness, 70% to 100% against severe illness and 63% to 100% against COVID-19 related hospitalization among the fully vaccinated immune-compromised (IC) population. In an extensive study of 10 states covering a period of March 2020-February 2022, of 22345 adults hospitalized for COVID-19, 12.2% were immunocompromised. Among unvaccinated IC patients, aOR (adjusted odds ratio (AOR)) was 1.26, and in-hospital death, AOR = 1.34 than non-compromised patients. Chappell, et al. 2022 [46] did not find an increased risk of infection in children and young people with IC.
In summary, COVID-19 mRNA vaccines were effective against symptomatic COVID-19 among the immunocompromised patients but had lower efficacy than the controls [47].
Thyroid dysfunction
There have been instances of thyroid dysfunction during SARS, and risks of anti-thyroid drugs resulting in agranulocytosis remain, as the symptoms resulting from agranulocytosis and COVID-19 are similar, making it difficult for doctors to distinguish between the two [48]. A study in China found that the decrease in TSH and T3 levels positively correlated with the severity of the COVID-19 disease, with lower TSH levels present in more than half of the patients. The change in serum TSH and T3 levels should help to diagnose the course of COVID-19 [49]. There is evidence of some COVID-19 patients suffering from ear pain, which may indicate a sign of subacute thyroiditis along with tachyarrhythmia as one of the most common cardiovascular conditions, so monitoring of thyroid function in COVID-19 patients is essential [50]. Patients with thyroid eye disease and those undergoing treatment with immunosuppressive agents are at a very high risk of severe illness from coronavirus. Pregnant women undergoing treatment for hypothyroidism should continue to follow the advice to increase levothyroxine dose and regularly check their thyroid levels [51].
Smokers
Studies showed that smokers are 1.4 times more likely to have severe symptoms of COVID-19 and approximately 2.4 times more likely to be admitted to an ICU and need mechanical ventilation or die than non-smokers [52].
Preexisting COPD and ongoing smoking history likely worsen symptoms and treatment effectiveness for COVID-19 [53]. Compared to previous smokers and non-smokers, current smokers are more susceptible to severe complications, experience higher mortality rates, and require preventive measures [54].
Mental distress and anxiety
Anxiety and depression symptoms and self-reported stress have been common psychological reactions to the COVID-19 pandemic, as there was limited interaction with the outside world. Increased domestic abuse and family violence were reported due to confinement [55]. It is very likely that the healthcare workers may experience mental health problems due to their work under nerve-wracking pressure and the need to make decisions under unprecedented conditions of scant resources, life-support, and intensive care distribution to the ones who have a higher chance of survival over equally needy elderly patients [56]. Significant and growing financial burdens, conflicting messages and guidelines from authorities, and travel and visa restrictions all contributed to mental distress and anxiety and calls for immediate action [57,58]. It is suggested that health care providers, children, and residents of remote rural areas should be explored for mental distress and anxiety [59].
Giuntella, et al. [60] assessed mental health status among college students and found that at the onset of the pandemic, intermediate steps declined from 10,000 to 4,000 per day, sleep time increased by 25 to 30 minutes per night, time spent socializing reduced by over half to less than 30 min, and screen time more than doubled to over 5 hours per day. Among cohort consisted of 247249 individuals, 9979 (4.0%) of whom were diagnosed with COVID-19. In an average follow-up of 5.65 ± 4.26 months, participants had a higher prevalence of symptoms of depression (Prevalence ratio of 1.18), poor sleep quality (PR = 1.13) compared to individuals without a COVID-19 diagnosis [61].
According to a WHO news release on March 2, 2022, the prevalence of anxiety and depression increased by 25.0% in the first year of the pandemic. Ninety percent of the countries surveyed intended to include health and psychosocial support in their COVID-19 response. Even though the situation somewhat improved by the end of 2021 oved, many people needed help for preexisting and newly developed mental health issues.
HIV
In 2019, approximately 1.2 million people in the US were living with HIV [58]; this number was about thirty-eight million worldwide. The US, UK, South African studies and WHO platform trials showed the worst outcome for patients with HIV, i.e., increased mortality of COVID-19 patients with HIV. No clear evidence has emerged that any antiretroviral drugs can prevent SARS-CoV-2 infection. People with HIV and antiviral treatment (ART) do well on COVID-19 vaccines. Monoclonal antibodies (mAbs) tixagevimab plus cilgavimab is recommended for pre-exposure prophylaxis for advanced or untreated HIV patients. Two mAbs, bamlanvimab plus etesevimab and casirivimab plus imdevimab, have received FDA Emergency Authorization for post-exposure prophylaxis for HIV patients, but these drugs are not effective against Omicron variants. The management of patients with HIV is the same as those of the general population [63].
Blood Type and COVID-19
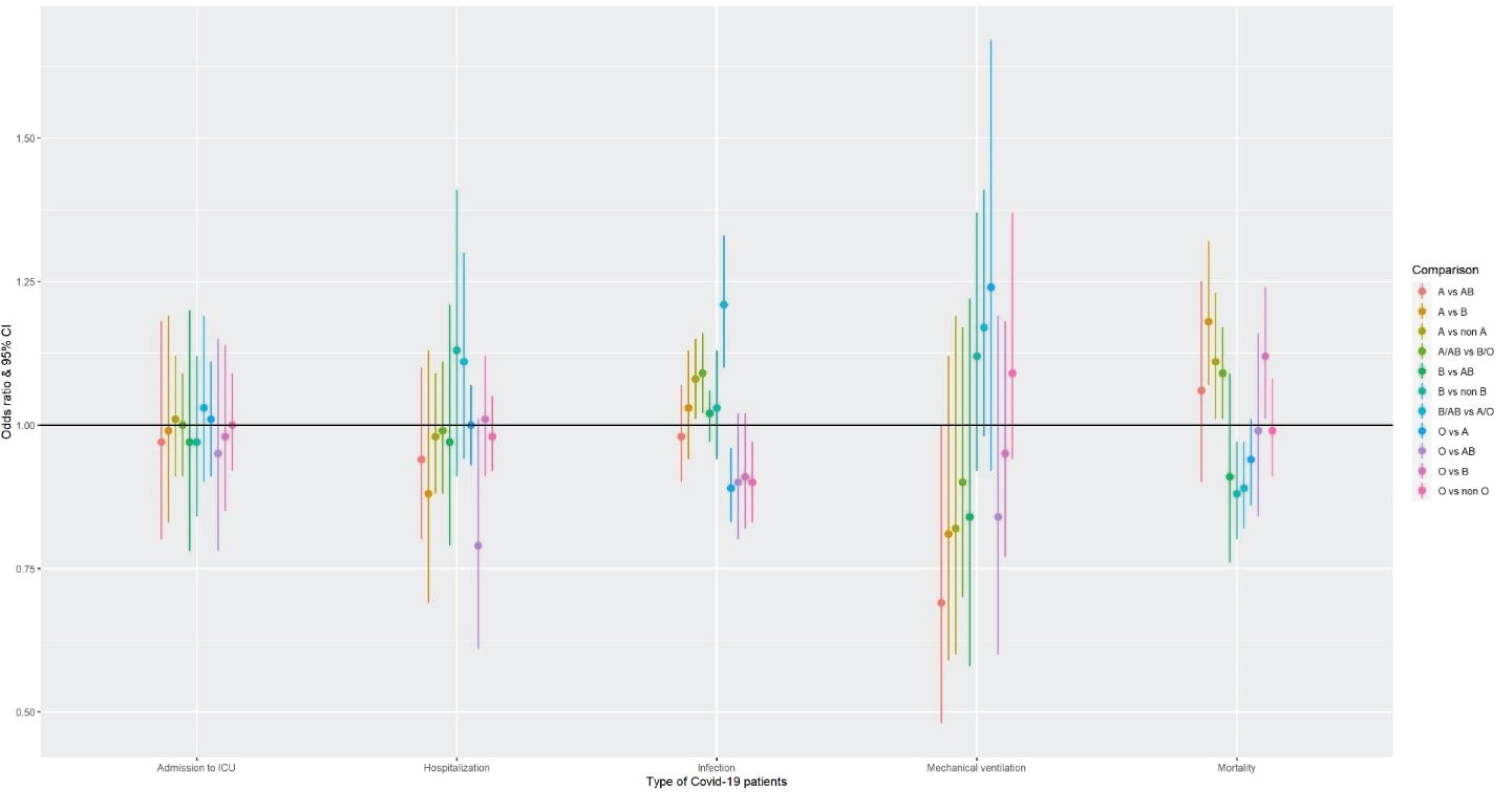
Several studies have identified that the blood group is a risk factor for COVID-19 infection. People with blood groups A, B, and AB have an increased risk of infection, while people with blood group O have a decreased risk of infection, possibly due to less blood clotting [61-64]. The odd's ratio comparison based on a meta- analysis to understand the susceptibility of COVID-19 infection associated with different blood group types with data from [65] is shown in Figure 6.
Long COVID-19
After recovering from Covid-19, long-haul patients face severe symptoms of shortness of breath and chest pain. The most common symptoms are brain fog, fever, and headache, among others, irrespective of the variant of infection and age. Overall, 1 in 13 adults in the US (7.5%) have "Long COVID" symptoms, defined as symptoms lasting three or more months after first contracting the virus and that they didn't have before their COVID-19 infection [66]. Twenty-to-thirty per cent of the children experienced Long Covid symptoms. Concerns about permanent lung damage due to COVID-19-related symptoms of acute respiratory distress syndrome (ARDS) and Alzheimer's disease, possibly due to the most common brain fog symptom. Further research and attention to the needs of the patients with Long COVID through regular follow-ups and possibly by creating dedicated "post-Covid" clinics to understand Long COVID disease is needed.
Some research has indicated that the impact of nutrition and high-fat diets that lead to diabetes and obesity may also contribute to the long-term effects of COVID-19. More than 100 long-term symptoms have been reported in the scientific literature, of which the most common are: fatigue, headache, attention disorder, hair loss, and dyspnea. Subramanian, et al. 2022 [67] found 62 symptoms to be significantly associated with SARS-CoV-2 infection after 12 weeks.
Case reports have also suggested that children may experience similar Long Covid symptoms, of which females may be more affected [63-65]. The National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), and Royal College of General Practitioners (RCGP) characterized by post-COVID-19 syndrome (PCS; also called Long-COVID-19) symptoms lasting for over three months after the first COVID-19 symptom onset. A systematic review and meta-analysis showed that 63.2, 71.9 and 45.9% of the sample exhibited ≥ one post-COVID symptom 30, 60, or ≥ 90 days after onset or hospitalization, respectively. Fatigue and dyspnea were the most prevalent symptoms (pooled prevalence from 35% to 60%), depending on the follow-up period. The majority of each post-COVID symptom in isolation dropped off after 30 days (10-15%) but increased at 60 days or beyond (40-60%) [68-71]. Also, further research needs to be conducted on patients who have developed Covid-19 symptoms after vaccination to identify the frequency, timing, causes, severity, and levels of infectiousness [72].
Based on a study of 76422 participants, the authors summarized the following symptoms of long covid: chest pain, difficulties in breathing, pain when breathing, painful muscles, ageusia or anosmia (loss of sense of taste), tingling extremities, lump in the throat, feeling hot and cold alternately, heavy arms or legs, and general tiredness [73-74].
The participation of an international and interdisciplinary group of researchers with the availability of an extensive electronic health record (EHR) data set, such as the COVID-19 research database, would help a great deal in understanding the long-term effects of COVID-19. Multisite and multinational projects will help understand the consequences and characteristics of both local and global COVID-19-affected populations. This universal view from research studies, pooling data, and studies across different countries will help formulate international health policy, including opening long-term COVID-19 clinics wherever most needed.
Vaccines and Therapeutics Development for COVID-19
Multiple pharmaceutical companies have worked or are working towards developing effective COVID-19 vaccines using different platforms, such as Moderna Therapeutics, Inovio Pharmaceuticals, Novavax, Johnson & Johnson, Pfizer, Merck, Glaxo-Smith Kline, to mitigate the spread of this infectious disease and its variants. The chimpanzee adenovirus-vectored vaccine (ChAdOx1 nCoV-19) showed extremely promising results with an acceptable safety profile and homologous boosting increased antibody responses developed by the Oxford/AstraZeneca vaccine group [85]. WHO provided further updates on this vaccine on March 15, 2022 [85].
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1). Oxford/AstraZeneca vaccine has been approved in 141 countries. Pfizer/Biotech RNA vaccine has been approved in a maximum number of countries (146). Two recently published studies, one from Medicago, a Canadian company- funded trial (CoVLP+AS03) of a recombinant plant-based adjuvanted COVID-19 vaccine on 24,141 volunteers showed effectiveness in preventing COVID-19 caused by a spectrum of variants with efficacy ranging from 69.5% against symptomatic infections to 78.8% against moderate-to-sever disease [75]. In another phase III trial of an RBD-Dimer-Based COVID-19 vaccine ZF2001 conducted on 28,873 participants showed the vaccine to be safe and effective against symptomatic (75.0% efficacy) and severe-to-critical COVID-19 patients (87.6% efficacy) for at least six months after vaccination [76]. Based on the WHO data, 170 vaccine candidates are under development as of August 5, 2022, of which the highest number of candidates are based on protein subunit technology, followed by RNA-based platform (Table 2).
Based on VIPER (Vaccines, Infectious disease Prevention, and Epidemiology Research) Group COVID-19 Vaccine Development and Approvals Tracker Team, at least forty vaccine candidates have been approved in at least one country. Table 3 provides the details of the approvals.
For further information one can refer to the vaccine tracker [77].
The FDA approved Veklury (Remdesivir) for the treatment of hospitalized or not hospitalized COVID patients of 28 days or older and weighing at least 3 kgs with mild-to-moderate COVID-19 patients and are at high risk for progression to severe COVID-19 including hospitalization and death. The first covid positive case in the United States occurred on January 19, 2020. The patient's condition improved on remdesivir, an investigational broad-spectrum antiviral treatment at that time developed by Gilead Sciences, Inc. [78]. A randomized, controlled clinical trial evaluating the safety and efficacy of treatment with antiviral remdesivir for coronavirus disease 2019 (COVID-19) was initiated via Adaptive COVID-19 Treatment Trial (ACTT) [79] Statistically significant effect was shown in shortening the recovery period for those who received remdesivir compared to the placebo (median, 11 days, compared with 15 days for placebo [80]. The next trial, ACTT2 with remdesivir with baricitinib, a product licensed to Eli Lilly company by Incyte, if this addition can benefit patient recovery [81]. This trial did show the median time to recovery of 7 days with remdesivir with baricitinib as compared to a placebo of 8 days (p = 0.03) [76] Olumiant (Baricitinib) is approved for hospitalized adult patients requiring supplemental oxygen, non-invasive or invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO). The FDA has granted Emergency Use Authorization (EUA) to Lagevrio (Molnupiravir) for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults. With positive results and who are at high risk for progression to severe COVID-19, including hospitalization or death, Sotrovimab was granted for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death. FDA withdrew EUA on April 5 2022, 2022, due to increase in the proportion of COVID-19 cases in US regions caused by the Omicron BA.2 sub-variant and due to the ineffectiveness of Sotrovimab against this sub-variant. -`EUA has been granted by the FDA to combination agents like Paxlovid (Nirmatrelvir and Ritonavir) for mild-to-moderate COVID-19 in adults and children (12 years of age and older weighing at least 88 pounds [40 kg]) with a positive test for the virus that causes COVID-19, and who are at high risk for progression to severe COVID-19, including hospitalization or death. REGEN-COV (Casirivimab and Imdevimab) as post-exposure prophylaxis (prevention) for COVID-19 in adults and pediatric individuals (12 years of age and older weighing at least 40 kg) who are at high risk for progression to severe COVID-19, including hospitalization or death., and Tixagevimab co-packaged with cilgavimab) for pre-exposure prophylaxis for prevention of coronavirus disease 2019 (COVID-19) caused by the SARS-CoV-2 virus. In addition, FDA granted EUA to some immune modulators like Actemra (Tocilizumab) for hospitalized 2 years of age and older patients who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) and bamlanivimab and etesevimab for the treatment of mild to moderate COVID-19 in patients aged 12 and older who are at high risk for progressing to severe COVID-19 and/or hospitalization. A typical prescription for COVID-19 in India during the second wave of COVID-19 in April 2021 was azithromycin, doxycycline, ivermectin, hydroxychloroquine, vitamin C, vitamin D, zinc, acetylcysteine, and inhaled budesonide or dexamethasone [77].
Researchers from Brazil established no distinguished improvement of hydroxychloroquine over placebo after 15 days from infection in a large-scale clinical trial consisting of 504 COVID-19 patients [78]. Protease inhibitors lopinavir and ritonavir used to treat infection with human immunodeficiency virus (HIV) [79] has been recommended by researchers to help in the recovery of MERS-CoV [80] and SARS-CoV [81]. Studies claim that after lopinavir/ritonavir (KALETRA®, AbbVie) was administered, β-coronavirus viral loads significantly decreased, and no or little coronavirus titers were observed in treating the index patient in Korea [82]. Recent clinical trials to identify the efficacy and safety of oral Lopinavir–Ritonavir for COVID-19 did not significantly benefit recovery, or in reducing mortality, or in diminishing throat viral RNA in critically ill COVID-19 patients [83]. Based on a recent meta-analysis, Patel, et al. [90] found that there is no additional benefit of Lopinavir-Ritonavir for COVID-19 patients [84]. The traditional Chinese medicine such as Radix astragali (Huangqi), Radix glycyrrhiza (Gancao), Radix saposhnikoviae (Fangfeng) [82,83] showed positive effect towards H1N1 influenza virus and SARS virus prevention does provide an alternative for preventing COVID-19 in a high-risk population [85]. Clinical trial researchers may want to investigate innovative statistical design for Phase II/III clinical trials to evaluate therapeutic interventions for COVID-19 [86], test-negative design [87,88,93].
Significance of Demographic Variables: Sex, Race, and Age – US
Experience
Based on data from the CDC website relating to demographical characteristics the tables show both data from August 2020 and as well as of August 2022 number of deaths and cases in the US are listed below (Table 3, Table 4 and Table 5), we tested the hypothesis of the significance of gender, race, and age and their impact on the death and case rates by the binomial proportion test. Statistically significant differences between males and females in infection rates and deaths were found. Table 3 provides the number of cases and deaths in 2020 and the same for 2022 in parentheses. Note that the infection rate is higher in females, while the death percentage is higher in males than females (p < 0.0001). In addition, we see that number of female cases has increased by 1.7%, and the number of deaths has decreased by ~ 1% within the last two years.
The trend of getting affected by COVID-19 is similar from 2020-2022, where Whites, followed by Hispanics and Black people, are the most affected. In Table 6, there is a hike in the number of cases and deaths in children (0-4) in 2022. More children and teens are affected by the disease, which may be due to the opening of the schools and relaxing the masking restrictions. The death rates have risen in the 18-64 groups by 2.4%, and in the elderly population (75+), both cases and deaths have declined.
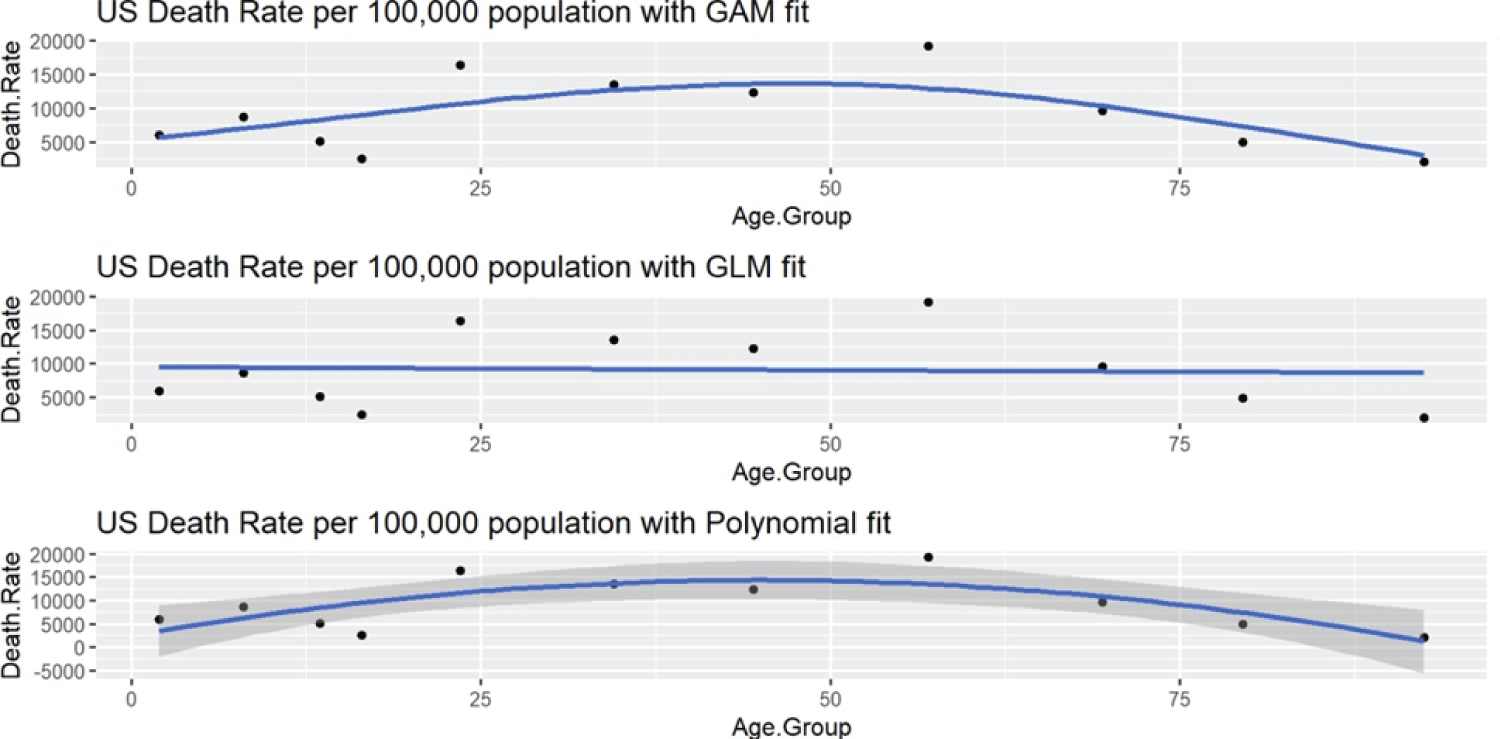
A clear demonstration of mortality rates by age will help health policy planners design an appropriate health insurance plans and health structure. Note that, when we test for the group differences in Table 4, Table 5 and Table 6, the p-values are much smaller (p < 0.0001) than 0.05 due to the large sample size in each of the three groups, indicating that all three factors (race, gender, and age) are significant. The changing demographics of COVID-19 are captured here [89]. The data on cases and mortality in the most affected countries and case fatality ratios are provided on the Johns Hopkins (Univ. of Medicine) website [90]. Figure 7 shows the US death rate as of Aug 2022, fitted with a polynomial, a generalized additive (GAM), and a generalized linear model (GLM), and the line of best fit seems to be the polynomial.
Conclusion
COVID-19 did spread rapidly since its initial identification in Wuhan, China. Literature and information on this newly emerged coronavirus are being continually published at a pandemic speed.
Early quarantining, diagnosis, and immediate treatment collectively contributed to better patient outcomes. Mass testing [91], complete isolation of infected patients, and the use of face masks [92] were crucial to interrupt the spread of the virus, flatten the curve, and prevent a rapid economic decline [93]. The effect and impact of COVID-19 are vast with different facets and it is challenging to encapsulate various aspects of the pandemic within a single literature review; however, our study is broad and generic to be helpful to the researchers and the public alike. COVID-19 proved to be one of the greatest threats to peoples health and safety due to its highly contagious nature. Unfortunately, the virus has spread to more than 197 (215 including disputed lands) countries, leading to national emergencies, the shutdown of airports, travel bans, and complete lockdown in several large and small countries.
Our comprehensive review of COVID-19 pandemic will help healthcare researchers, healthcare providers, health policy executives, and health insurance companies to have a broader picture of the pandemic and help implement prevention and treatment strategies to mitigate the COVID-19 pandemic effect on health, psychological, economic, and medical consequences. Most affected people were preexisting health conditions such as cardiovascular disease, hypertension, metabolic disease, COPD, ARDS, and obesity. Our research finds that race, age, sex, and comorbidities have influenced the outcome of COVID-19 patients. It is recommended that these factors be considered in designing future COVID-19 clinical trials to be clinically relevant while developing new therapeutics and vaccines. The patient population can be stratified on these three factors, i.e., race, age, and sex, since a large number of infected patients can be enrolled in a short period. If this is not possible in some situations, then dynamic methods of randomization can be explored to expedite enrollment. A recent finding showed the statistical significance of sex and gender in COVID-19 infection and deaths across 75 countries worldwide. Clinical researchers and health insurance planners can follow the suggested approach to target specific populations to have effective future vaccination and immunization strategies. Although everyone should follow the CDC guidelines of prevention, it is recommended that females should be more vigilant in following the CDC guidelines due to a higher rate of Coronavirus infection among them. Also, the effect of age is clearly delineated in our review to help vaccine administrators to determine vaccination priority [94]. Further research is required to develop more accurate estimates of COVID-19 mortality [95], newer and different prediction models for forecasting new cases and deaths [96]. To understand the health care needs of the Long Covid population, health resources in terms of dedicated clinics and hospital beds, are needed to help millions recovering from this disease. Multidisciplinary research teams need to be organized to study the effect of COVID-19 on an ongoing basis [97-104].
Declarations
Availability of data and materials
The dataset used and analyzed during the current study is available from the corresponding author upon reasonable request. The dataset generated and/or analyzed for the USA during the current study is available from https://covid.cdc.gov/covid-data-tracker/#demographics.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was supported by the NIH grant P42 ES023716 (PI: Dr. S Srivastava), 1P50 AA024337 and 1P20 GM113226 (PI: Dr. Craig McClain), and 1P30ES030283 (PI: Dr. C States). Dr. Rai, Wendell Cherry Chair in Clinical Trial Research, has also partially supported the research. The funders except Dr. Rai had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Author's contribution
AB has contributed to data curation, formal analysis, conducting the study, methodology, and writing the article, AS has provided valuable inputs conducting the study, reviewing, and editing the article. SNR has contributed to reviewing the article with constructive inputs in writing and analysis.
Acknowledgments
The authors would like to acknowledge Dr. Craig McClain, Dr. S. Srivastava, Dr. C States for the funding to conduct the research.
References
- Huang C, Wang Y, Li X, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395: 497-506.
- (2020) COVID-19 Map- Johns Hopkins Coronavirus Resource Center. University of medicine, John Hopkins, USA.
- Guterres A (2017) This is a time for science and solidarity. United Nations.
- Chen Q, Allot A, Lu Z (2020) Keep up with the latest coronavirus research. Nature 579: 193.
- Shereen MA, Khan S, Kazmi A, et al. (2020) COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res 24: 91-98.
- Peiris JSM, Yuen KY, Stöhr K, et al. (2003) The Severe Acute Respiratory Syndrome. N Engl J Med 349: 2431-2441.
- Van Der Hoek L, Pyrc K, Jebbink MF, et al. (2004) Identification of a new human coronavirus. Nat Med 10: 368-373.
- Zumla A, Hui DS, Perlman S (2015) Middle East respiratory syndrome. The Lancet 386: 995-1007.
- Guo YR, Cao QD, Hong ZS, et al. (2020) The origin, transmission, and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- An update on the status. Mil Med Res 7: 1-10.
- Tiwari R, Dhama K, Sharun K, et al. (2020) COVID-19: Animals, veterinary and zoonotic links. Vet Q 40: 169-182.
- Wang C, Horby PW, Hayden FG, et al. (2020) A novel coronavirus outbreak of global health concern. The Lancet 395: 470-473.
- Andersen KG, Rambaut A, Lipkin WI, et al. (2020) The proximal origin of SARS-CoV-2. Nat Med 26: 450-452.
- Liu P, Jiang JZ, Wan XF, et al. (2020) Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2) PLoS Pathog 14: e1008421.
- Boni MF, Lemey P, Jiang X, et al. (2020) Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat Microbiol 5: 1408-1417.
- Maxmen A (2022) Scientists struggle to probe COVID’s origins amid sparse data from China. Nature 603: 773-775.
- Jin YH, Cai L, Cheng ZS, et al. (2020) A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 7: 1-23.
- Lauer SA, Grantz KH, Bi Q, et al. (2020) The incubation period of coronavirus disease 2019 (CoVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med 172: 577-582.
- (2020) CDC, Symptoms of Coronavirus, COVID 19, USA.
- Guan W, Ni Z, Hu Y, et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708-1720.
- Vetter P, Vu DL, L’Huillier AG, et al. (2020) Clinical features of covid-19, BMJ.
- Cobb NL, Sathe NA, Duan KI, et al. (2021) Comparison of clinical features and outcomes in critically Ill patients hospitalized with COVID-19 versus influenza. Ann Am Thorac Soc 18: 632-640.
- Hasel S, Khalili N, Bakhshayeshkaram M, et al. (2020) Lobar distribution of COVID-19 pneumonia based on chest computed tomography findings: A retrospective study. Arch Acad Emerg Med 8: e55.
- Conditions Contributing to COVID-19 Deaths, by State and Age, Provisional 2020-2022 | Data|Centers for Disease Control and Prevention.
- Pandey R, Rai D, Tahir MW, et al. (2022) Prevalence of comorbidities and symptoms stratified by severity of illness amongst adult patients with COVID-19: A systematic review. Arch Med Sci Atheroscler Dis 7: 5-23.
- Koya SF, Ebrahim SH, Bhat LD, et al. (2021) COVID-19 and comorbidities: Audit of 2,000 COVID-19 deaths in India. J Epidemiol Glob Health 11: 230-232.
- Yin T, Li Y, Ying Y, et al. (2021) Prevalence of comorbidity in Chinese patients with COVID-19: Systematic review and meta-analysis of risk factors. BMC Infect Dis 21: 1-13.
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. (2020) Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 34: 101623.
- Potere N, Valeriani E, Candeloro M, et al. (2020) Acute complications and mortality in hospitalized patients with coronavirus disease 2019: A systematic review and meta-analysis. Crit Care 24: 389.
- Dimitrov GV, Lateva ZC, Dimitrova NA (1990) Effects of changes in asymmetry, duration and propagation velocity of the intracellular potential on the power spectrum of extracellular potentials produced by an excitable fiber. Electromyogr Clin Neurophysiol 28: 93-100.
- Rubino F, Amiel SA, Zimmet P, et al. (2020) New-Onset Diabetes in Covid-19. N Engl J Med 383: 789-790.
- Kassir R (2020) Risk of COVID-19 for patients with obesity. Obes Rev 21: e13034.
- Dietz W, Santos-Burgoa C (2020) Obesity, and its Implications for COVID-19 Mortality. Obesity (Silver Spring) 28: 1005.
- Yang J, Hu J, Zhu C (2020) Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol 93: 257-261.
- Kalligeros M, Shehadeh F, Mylona EK, et al. (2020) Association of obesity with disease severity among patients with Coronavirus disease 2019. Obesity 28: 1200-1204.
- Upadhaya S, Yu JX, Oliva C, et al. (2020) Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov 19: 376-377.
- Landman A, Feetham L, Stuckey D (2020) Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 21: 335-337.
- Wang H, Zhang L (2020) Risk of COVID-19 for patients with cancer. Lancet Oncol 21.
- Akula SM, Abrams SL, Steelman LS, et al. (2020) Cancer therapy and treatments during COVID-19 era. Adv Biol Regul 77: 100739.
- Zhang L, Zhu F, Xie L, et al. (2020) Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol 31: 894-901.
- Kuderer NM, Choueiri TK, Shah DP, et al. (2020) Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. The Lancet 395: 1907-1918.
- Chavez-Macgregor M, Lei X, Zhao H, et al. (2022) Evaluation of COVID-19 mortality and adverse outcomes in US Patients with or without cancer. JAMA Oncol 8: 69-78.
- Marks KJ, Whitaker M, Agathis NT, et al. (2022) Hospitalization of infants and children aged 0-4 years with laboratory-confirmed COVID-19 - COVID-NET, 14 States, March 2020-February 2022. MMWR Morb Mortal Wkly Rep 71: 429-436.
- Parker EPK, Desai S, Marti M, et al. (2022) Response to additional COVID-19 vaccine doses in people who are immunocompromised: A rapid review. Lancet Glob Health 10: 326-328.
- Ferdinands JM, Rao S, Dixon BE, et al. (2022) Waning 2-Dose 657 and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency 658 Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of 659 Delta and Omicron Variant Predominance -VISION Network, 10 States, August 2021-January 660 2022. Morb Mortal Wkly Rep 71: 255-263.
- di Fusco M, Marczell K, Deger KA, et al. (2022) Public health impact of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) in the first year of rollout in the United States. J Med Econ 25: 605-617.
- Tenforde MW, Patel MM, Gaglani M, et al. (2022) Effectiveness of a third dose of Pfizer-BioNTech and Moderna Vaccines in preventing COVID-19 hospitalization among immunocompetent and immunocompromised adults - United States, August-December 2021. MMWR Morb Mortal Wkly Rep 71: 118-124.
- Marra AR, Kobayashi T, Suzuki H, et al. (2022) The long-term effectiveness of coronavirus disease 2019 (COVID-19) vaccines: A systematic literature review and meta-analysis. Antimicrob Steward Healthc Epidemiol 2: e22.
- Dworakowska D, Grossman AB (2020) Thyroid disease in the time of COVID-19. Endocrine 68: 471-474.
- Chen M, Zhou W, Xu W (2020) Thyroid function analysis in 50 patients with COVID-19: A retrospective study. Thyroid 31: 1-12.
- Bellastella G, Maiorino MI, Esposito K (2020) Endocrine complications of COVID-19: What happens to the thyroid and adrenal glands? J Endocrinol Invest 43: 1169-1170.
- Boelaert K, Visser WE, Taylor PN, et al. (2020) Endocrinology in the time of COVID-19: Management of hyper- and hypo- thyroidism. Eur J Endocrinol 183: 33-39.
- Vardavas CI, Nikitara K (2020) COVID-19 and smoking: A systematic review of the evidence. Tob Induc Dis 18: 20.
- Zhao Q, Meng M, Kumar R, et al. (2020) The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J Med Virol 92: 1915-1921.
- Alqahtani JS, Oyelade T, Aldhahir AM, et al. (2020) Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PLoS One 15: e0233147.
- Usher K, Bhullar N, Durkin J, et al. (2020) Family violence and COVID-19: Increased vulnerability and reduced options for support. Int J Ment Health Nurs 29: 549-552.
- Greenberg N, Docherty M, Gnanapragasam S, et al. (2020) Managing mental health challenges faced by healthcare workers during covid-19 pandemic. BMJ 368: m1211.
- Pfefferbaum B, North CS (2020) Mental Health and the Covid-19 Pandemic. N Engl J Med 383: 510-512.
- Holmes EA, O’Connor RC, Perry VH, et al. (2020) Multidisciplinary research priorities for the COVID-19 pandemic: A call for action for mental health science. Lancet Psychiatry 7: 547-560.
- Rajkumar RP (2020) COVID-19 and mental health: A review of the existing literature. Asian J Psychiatr 52: 102066.
- Giuntella O, Hyde K, Saccardo S, et al. (2021) Lifestyle and mental health disruptions during COVID-19. Proc Natl Acad Sci USA 118: e2016632118.
- Magnúsdóttir I, Lovik A, Unnarsdóttir AB, et al. (2022) Acute COVID-19 severity, and mental health morbidity trajectories in patient populations of six nations: An observational study. Lancet Public Health 7: 406-416.
- (2022) Global Statistics.HIV.gov.
- NIH (2022) Special Considerations in People With HIV, COVID-19 Treatment Guidelines.
- Barnkob MB, Pottegård A, Støvring H, et al. (2020) Reduced prevalence of SARS-CoV-2 infection in ABO blood group O. Blood Adv 4: 4990-4993.
- Gutiérrez-VM, Leache L, Librero J, et al. (2022) ABO blood group and risk of COVID-19 infection and complications: A systematic review and meta-analysis. Transfusion (Paris) 62: 493-505.
- CDC (2022) Nearly One in Five American Adults Who Have Had COVID-19 Still Have “Long COVID.” National Center for Health Statistics.
- Su Y, Yuan D, Chen DG, et al. (2022) Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185: 881-895.
- Callard F, Perego E (2021) How and why patients made Long Covid. Soc Sci Med 268: 113426.
- Raveendran A, Jayadevan R, Sashidharan S (2021) Long COVID: An overview. Diabetes Metab Syndr 15: 869-875.
- Crook H, Raza S, Nowell J, et al. (2021) Long covid-Mechanisms, risk factors, and management. BMJ 374: n1648.
- Fernández PC, Palacios CD, Gómez MV, et al. (2021) Prevalence of post-COVID-19 symptoms in hospitalized and non- hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur J Intern Med 92: 55-70.
- Lipsitch M, Krammer F, Regev-Yochay G, et al. (2021) SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nature Reviews Immunology 22: 57-65.
- Ballering A, van Zon SKR, Olde Hartman TC, et al. (2022) Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: An observational cohort study. Lancet 400: 452-461.
- Folegatti PM, Ewer KJ, Aley PK, et al. (2020) Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396: 467-478.
- Tenforde MW, Patel MM, Gaglani M, et al. (2022) Effectiveness of a third dose of Pfizer-BioNTech and Moderna Vaccines in preventing COVID-19 hospitalization among immunocompetent and immunocompromised adults - United States, August-December 2021. MMWR Morb Mortal Wkly Rep 71: 118-124.
- Zhao X, Li D, Ruan W, et al. (2022) Effects of a prolonged booster interval on neutralization of omicron variant. N Engl J Med 386: 894-896.
- (2022) Vaccines - COVID19 Vaccine Tracker. Approved Vaccines.
- Holshue ML, DeBolt C, Lindquist S, et al. (2020) First case of 2019 novel coronavirus in the United States. N Engl J Med 382: 929-936.
- NIH (2020) Adaptive COVID-19 Treatment Trial (ACTT).
- Beigel JH, Tomashek KM, Dodd LE, et al. (2020) Remdesivir for the treatment of Covid-19 - Preliminary report. N Engl J Med 383: 1813-1826.
- NIH (2020) NIH Clinical trial testing antiviral remdesivir plus anti-inflammatory drug baricitinib for COVID-19 begins. NIH: National Institute of Allergy and Infectious Diseases.
- Kalil AC, Patterson TF, Mehta AK, et al. (2021) Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med 384: 795-807.
- Bhaumik S, John O, Jha V (2021) Low-value medical care in the pandemic-is this what the doctor ordered? Lancet Glob Health 9: 1203-1204.
- Cavalcanti AB, Zampieri FG, Rosa RG, et al. (2020) Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med 383: 2041-2052.
- Cvetkovic RS, Goa KL (2003) Lopinavir/ritonavir: A review of its use in the management of HIV infection. Drugs 63: 769-802.
- Arabi YM, Asiri AY, Assiri AM, et al. (2020) Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): Statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials 21: 8.
- Chu CM, Cheng VCC, Hung IFN, et al. (2004) Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 59: 252-256.
- Lim J, Jeon S, Shin HY, et al. (2020) Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: The application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J Korean Med Sci 35: e79.
- Cao B, Wang Y, Wen D, et al. (2020) A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382: 1787-1799.
- Patel TK, Patel PB, Barvaliya M, et al. (2021) Efficacy and safety of lopinavir-ritonavir in COVID-19: A systematic review of randomized controlled trials. J Infect Public Health 14: 740-748.
- Alrashed AA, Khan TM, Alhusseini NK, et al. (2021) Severity of COVID-19 infection in ACEI/ARB users in specialty hospitals: A retrospective cohort study. J Infect Public Health 14: 726-733.
- Wang Z, Chen X, Lu Y, et al. (2020) Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends 14: 64-68.
- Rai SN, Qian C, Pan J, et al. (2020) Statistical design of Phase II/III clinical trials for testing therapeutic interventions in COVID-19 patients. BMC Med Res Methodol 20: 220.
- Singh H, Dahiya N, Yadav M, et al. (2022) Emergence of SARS-CoV-2 new variants and their clinical significance. Can J Infect Dis Med Microbiol 2022: 1-8.
- Dean NE, Hogan JW, Schnitzer ME (2021) Covid-19 Vaccine effectiveness and the test-negative design. N Engl J Med 385: 1431-1433.
- Venkatesan P (2020) The changing demographics of COVID-19. Lancet Respir Med 8: E95.
- Johns Hopkins (2023) Mortality Analyses - Johns Hopkins Coronavirus Resource Center.
- Peto J (2020) Covid-19 mass testing facilities could end the epidemic rapidly. BMJ 368: m1163.
- Feng S, Shen C, Xia N, et al. (2020) Rational use of face masks in the COVID- 19 pandemic. Lancet Respir Med 8: 434-436.
- Wise J (2020) Covid-19: Risk of second wave is very real, say researchers. BMJ 369: m2294.
- Srivastava N, Bhattacharyya A, Seth A, et al. (2020) Does Nature Have a Systematic Sex Bias: Prevalence, Mortality, and Trend of COVID-19. 129: 129-135.
- Baud D, Qi X, Nielsen-Saines K, et al. (2020) Real estimates of mortality following COVID-19 infection. Lancet Infect Dis 20: 773.
- Bhattacharyya A, Chakraborty T, Rai SN (2022) Stochastic forecasting of COVID-19 daily new cases across countries with a novel hybrid time series model. Nonlinear Dyn 107: 3025-3040.
- del Rio C, Collins LF, Malani P (2020) Long-term Health Consequences of COVID-19. JAMA 324: 1723-1724.
Corresponding Author
Shesh N Rai, Department of Bioinformatics and Biostatistics, University of Louisville, Louisville, KY, USA
Copyright
© 2023 Bhattacharyya A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.