Sensitivity of Nasal and Oropharyngeal Swabs in Diagnosing COVID-19 in Hospitalised Patients
Abstract
Background: To evaluate the sensitivity of nasal and oropharyngeal swabs in diagnosing COVID-19 in hospitalised patients and comparing patient factors and admission investigations to the swab result.
Methods: Multicentre retrospective cohort study of all COVID-19 swab positive patients who were in-patients on 9th April 2020. Electronic case notes were analyzed for baseline characteristics including; age, gender, co-morbidities, admission blood tests and swab results. The results of each consecutive swab for COVID-19 was analysed for each patient. The number of swabs required to achieve a positive test was used to assess the sensitivity of the test.
Results: Of the 173 patients identified, 108 (62.4%) were males, mean age was 68.4 ± 14.7 years. Commonest co-morbidity was hypertension (50.9%). 152 (87.9%) patients had their first swab positive. Age over 71 years was positively associated (53.3% vs. 23.8%, p = 0.018) and age-adjusted Charlson Comorbidity Index ≤ 2 was negatively associated (25.0% vs. 52.4%, p = 0.009) with the first swab being positive. Admission blood tests and chest X-ray findings did not influence the swab results.
Conclusion: The sensitivity of swab for symptomatic and hospitalised patients was higher than previously thought and admission investigations do not predict the result of swab in COVID-19 positive patients.
Abbreviations
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19: Coronovirus Disease 2019; RT-PCR: Reverse Transcriptase Polymerase Chain Reaction; ACCI: Age Adjusted Charlson Comorbidity Index; SIMD: Scottish Index of Multiple Deprivation; CRP: C-reactive Protein; CXR: Chest X-Ray; BSTI: British Society of Thoracic Imaging.
Keywords
COVID-19, SARS-Cov-2, Pandemic, Sensitivity, RT-PCR, Nasal and Oropharyngeal swabs
Introduction
In December 2019 details of a novel coronavirus emerged from Wuhan, the capital city of Hubei province in China [1]. Just over one month later the World Health Organization declared the ensuing crisis a global pandemic. With an escalating death toll and the introduction of widespread stringent lock-down measures to halt the spread of the virus, the social and economic consequences will be lasting. Worldwide there is therefore an urgent need to expand of our knowledge of the virus and how to best manage it.
Coronaviruses are themselves well known, however it is the so called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that causes the disease, now termed COVID-19 [2,3]. While our understanding remains incomplete, knowledge of the transmission characteristics of the virus have since evolved. Person-to-person transmission is now well recognized. Like influenza virus, this is believed to be predominantly caused by droplet spread from the respiratory tract [4,5]. Patterns of transmission identified in specific clusters of cases across the world have strongly supported this theory, with household members identified as key contacts accounting for secondary infections [6]. There have also been some reports in the literature of the persistence of viruses on surfaces, bolstering the potential for SARS-CoV-2 to spread rapidly [7].
Fever has been shown to be one of the most common presenting complaints associated with COVID-19. In fact, one early study from Wuhan found that 98% of patients presenting with COVID-19 had a fever [8]. However, general fatigue and cough are also listed amongst the most common presenting symptoms [8]. The non-specific presentating symptoms of COVID-19 are therefore often virtually indistinguishable from other viral illnesses. Testing for the virus is recommended on the basis of clinical suspicion. However, with growing widespread reports of atypical presentations, maintaining a high index of suspicion is critical to this strategy [9].
With its transmission characteristics and often non-specific clinical presentation, accurate diagnostic testing is critical. This is essential, not only to increasing our understanding of the epidemiology of the virus, but also to managing the community case load. Early identification of positive cases is essential to enable rigorous infection control precautions to be implemented and limit transmission effectively.
Internationally, it is the reverse transcriptase polymerase chain reaction (RT-PCR) that is most commonly used to confirm the diagnosis of COVID-19 [10]. This is typically in the form of a nasopharyngeal or oropharyngeal swab. However, since its introduction, some questions have been raised over the efficacy of this testing method. There have been reports of high rates of false negative results, generating the need for multiple tests to be carried out [10]. There has also been criticism regarding the instability of the test and time to process results. In a study of 51 hospitalized patients in China, 29% of the patients were incorrectly found to be COVID-19 negative on initial RT-PCR, requiring subsequent tests [11]. Variation in testing practices has been suggested to contribute to these findings. One study of 205 COVID-19 positive patients found the rate of RNA positive tests from the recommended oropharygeal swabs to be just 32% compared to broncheolar lavage which yielded 95% [12]. In the context of this virus, any inaccuracies can create critical uncertainty at a time when viral containment is key. It is also recognized that there is significant pressure on viral testing methods, both in terms of infrastructure for processing test and availability of testing kits. Ensuring appropriate use of tests is therefore of utmost importance.
The vague presenting symptoms of COVID-19 and virulence of the virus, in the absence of any vaccine or curative treatment necessitates the practice of rigorous testing methods. Knowledge of the sensitivity of the RT-PCR test is essential to guide this practice and formulate guidance for patient isolation and cohorting. This is of particular relevance in hospitals where nonsocomial transmission is likely to propagate infections if not rapidly contained.
The primary aim was to establish the sensitivity of RT-PCR swab in diagnosing COVID-19 in hospitalised patients. The secondary aim was to assess if patient factors or admission investigations have any impact on sensitivity of RT-PCR swab.
Methodology
A multicenter retrospective cohort study was carried out on all hospitalised patients with RT-PCR confirmed COVID-19. The study was carried out across three acute hospitals in a single NHS Trust on Friday the 9th April 2020. The hospitals included serve a population of 655,000 with over 1,660 in-patient's bed capacity. The COVID-19 positive patients were identified from the Trak Care Electronic Medical Record System. Patients with their first swab negative who went on to have further positive swabs were also included in the study. No patient with an RT-PCR swab positive was excluded from the study. At the time of the study the Trust's policy was to preform RT-PCR swabs only on symptomatic patients or patients suspected of having COVID-19. The electronic case notes were analyzed for baseline characteristics; age, gender and co-morbidities, admission blood test. The results of all consecutive RT-PCR tests were assessed for each patient, calculating the sensitivity as the number of tests taken to achieve a positive result.
Patients were divided into two groups based on if the first RT-PCR swab was positive or not and patient factors as well as the admission investigations were compared. For patients who were admitted with other pathologies and contracted COVID-19 infection in hospital (positive RT-PCR after 7 days of admission), the blood test results were taken from the date of positive RT-PCR. Age adjusted Charlson Comorbidity Index (ACCI) and Scottish Index of Multiple Deprivation (SIMD) were also calculated [13,14]. Chest radiographs were reported by consultant radiologist using BSTI grading who was blinded to the COVID-19 status [15]. For the age analysis comparison the patients were divided into two group; ≤ 70 years and ≥ 71 years.
Qualitative data were given as frequency and percentages while quantitative as mean ± S.D. Pearson uncorrected Chi-Square/Fisher's exact test was calculated in Stat pages to calculate p value and odds ratio with 95% confidence interval. P value of < 0.05 was considered to be statistically significant.
This study was registered with the NHS Lanark shire Clinical Quality Project, Project ID: 13104.
Results
A total of 173 patients were identified in the three acute hospitals on 9th April 2020, none were excluded. Patient characteristics are shown in Table 1. There were 108 (62.4%) male patients and the mean age was 68.4 ± 14.7 years. The commonest ethnicity was White Scottish, 123 (71.1%) and commonest co-morbidity was hypertension, 88 (50.9%).
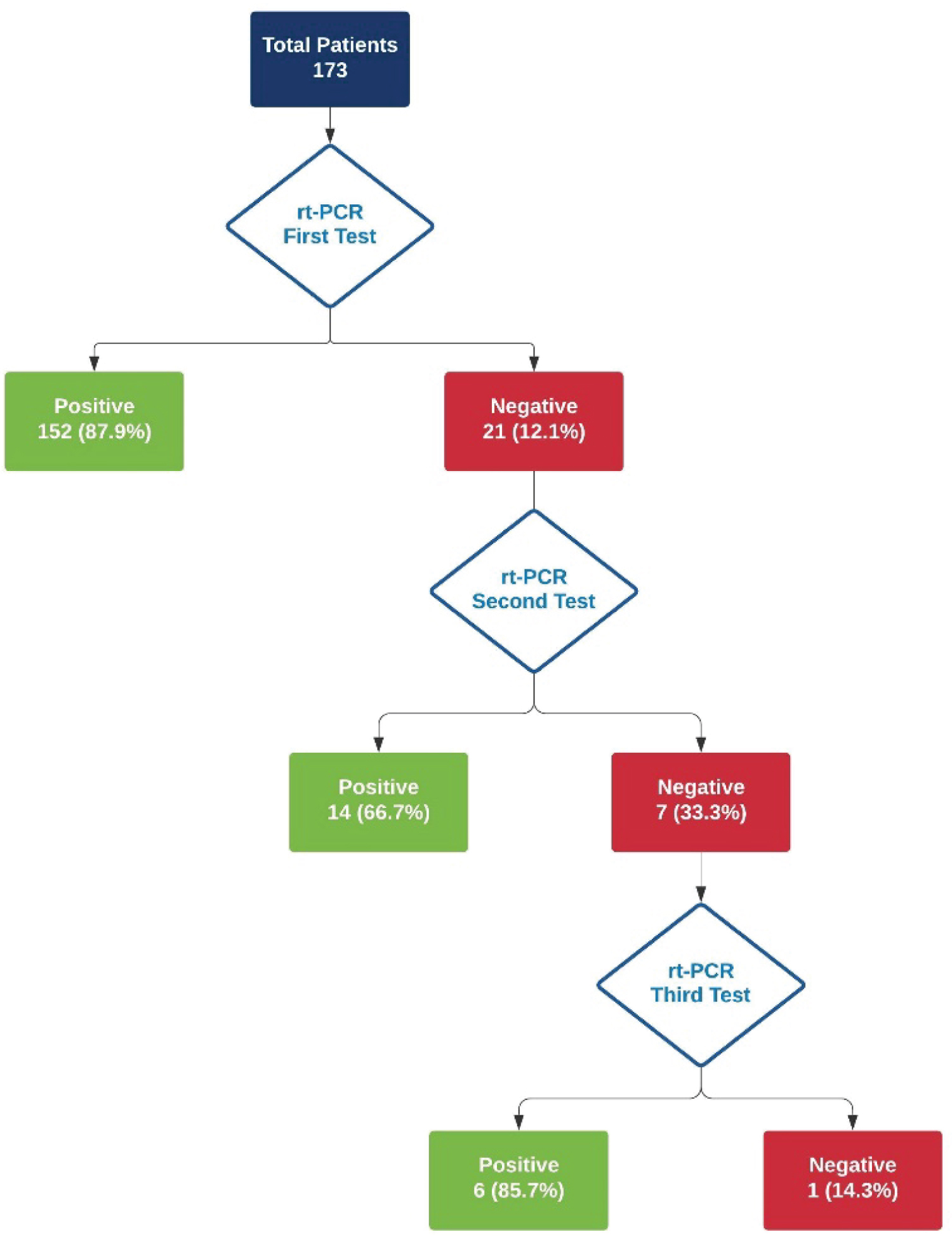
RT-PCR swab results is shown this the flowchart in Figure 1. One hundred fifty two (87.9%) patients had their first RT-PCR swab test positive. Twenty one (12.1%) patients who were tested negative had second swab taken, of these 14 (66.7%) were positive on the second swab. Seven (33.3%) patients had second swab negative who received are third swab. Of these 6 (85.7%) became positive after a third swab. One patient required 4 swabs before becoming positive for COVID-19.
Patient factors and admission investigations are compared in Table 2 for first swab positive verses negative patients. Patients over the age of 71 years were more likely to have their first swab positive (53.3% vs. 23.8%, p = 0.018). Patients with ACCI of 0-2 were less likely to have their first swab positive (25.0% vs. 52.4%, p = 0.009). Admission blood tests result and chest X-rays findings did not have any influence on the result of the swab result.
Disscussion
Without a definitive treatment or the availability of a vaccine at present, detection is vital to contain the ongoing rapid spread of COVID-19. This study found the RT-PCR to have a sensitivity of 87.9% for COVID-19 in hospitalised patients. This figure suggests that test results may be more reliable than previously thought, enabling more decisive clinical decision making. By contrast, the existing literature suggests the sensitivity of the nasal and oropharyngeal RT-PCR to be much lower and extremely variable, ranging from 30 to 60% [16,17]. One explanation for the discrepancy between our study and those previously relates to the impact of viral load upon symptom severity. Research has shown that the duration of time from symptom onset significantly influences the sensitivity of the RT-PCR. It has been shown to be highest in the first seven days from symptom onset, 63-73%, only remaining elevated from 8-14 days in the presence of severe disease [17]. This is significant with respect to the findings in this study, with hospitalised patients by definition most likely to have severe disease in comparison to those within the community. In addition, due to the limited resource of testing kits the current global practice is selective testing. In our study RT-PCR tests were carried out on symptomatic patients; those with fever, persistent cough or those with a high clinical index of suspicion of COVID-19. Mass testing is likely to reduce the sensitivity, as highlighted by the literature. It is also important to highlight that the majority of previous studies are from the very early stages of the crisis. Since then our procedures and understanding have evolved. Awareness of the limitations of the test have influenced clinicians and likely reinforced good practice in achieving samples.
There is also a suggestion that different viral strains and virus characteristics have recently emerged, which is likely to impact upon the sensitivity of the test. The variable pattern of hospitalization of patients with COVID-19 across the world supports this theory. Early studies from the epicenter of the virus in Wuhan suggested that patients were most likely to be admitted two to four days from symptoms onset [18]. However, later analysis has suggested a lengthening of the time from onset of symptoms to presentation with dyspnoea, with a median closer to seven days [19,20]. This finding is also relevant because the sensitivity of the RT-PCR is suggested to vary with time from symptom onset [17]. This study therefore suggests that RT-PCR testing for COVID-19 is highly sensitive in the hospitalised population, enabling more effective infection control and isolation practices to be implemented.
In the study, elderly patients (over the age of 70 years) were in particular found to be more likely to test positive from their first swab. It has been shown that elderly patients are more likely to progress to severe disease [21]. Severity itself has been shown to be associated with the increased sensitivity of the RT-PCR swab. With age, the ability of the immune system to develop an immune response also is impaired, which may enable viral load to increase more markedly [22]. Moreover, the test itself, a nasal and oropharyngeal swab may be better tolerated in elderly patients, allowing a more representative sample to be taken. While in this study the sensitivity of the RT-PCR swab was found to be greater in elderly patients, this is likely to relate to the severity of the disease and impact of viral load.
Although a number of biochemical markers have been associated with the diagnosis of SARS-CoV-2, in this study, admission blood tests did not predict the likelihood of swab being positive. Lymphopenia, in particular has been consistently associated with the virus [23]. Specifically it has been suggested to correlate with severity of the disease. In our study the presence of lymphopenia on admission did yield more positive first swabs (57.2% vs. 66.7%, p = 0.411) however, it did not reach statistical significance. This is consistent with the previously described association between higher viral load and disease severity and increased sensitivity of the RT-PCR.
This study has a number of particular strengths. Firstly, the patients within this cohort are closely comparable to those previously described in terms of demographics and co-morbidities [24]. In addition, much of the published research to date is from the early phases of the epidemic. Crucially, this research reflects current understanding and the rapid evolution of practice since the pandemic began. This is important for developing relevant strategies for containing the spread of the virus. With social lock-down measures, containment of the virus within the hospital setting is also of heightened importance, to limit spread to vulnerable patients and staff.
There are however some limitations of this study. There is likely to be selection bias, as hospitalised patients are more likely to be have severe disease and therefore return a positive tests. This study also did not correlate the RT-PCR result to the duration of onset of symptoms. There remain some unanswered questions with respect to the RT-PCR that would merit further study. In particular, this study reflects the sensitivity of the test in hospitalized patients, the value of its use in the wider population with milder disease not requiring hospitalization remains uncertain.
In conclusion, the sensitivity of COVID-19 RT-PCR swab for symptomatic and hospitalised patients is better than previously thought. Older age is positively associated first RT-PCR swab being positive. If the first swab is negative and there is strong clinical suspicion then further swabs show be taken.
Conflict of Interest
Authors declared no conflict of interest.
Funding
None.
Acknowledgment
Not applicable.
References
- Wang C, Horby PW, Hayden FG, et al. (2020) A novel coronavirus outbreak of global health concern. The Lancet 395: 470-473.
- Jin Y, Yang H, Ji W, et al. (2020) Virology, epidemiology, pathogenesis, and control of covid-19. Viruses 12: 372.
- Wu F, Zhao S, Yu B, et al. (2020) A new coronavirus associated with human respiratory disease in China. Nature 579: 265-269.
- Li Q, Guan X, Wu P, et al. (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. The New England Journal of Medicine 382: 1199-1207.
- Riou J, Althaus CL (2020) Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill 25: 2000058.
- Chan JFW, Yuan S, Kok KH, et al. (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. The Lancet 395: 514-523.
- van Doremalen N, Bushmaker T, Morris DH, et al. (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine 382: 1564-1567.
- Huang C, Wang Y, Li X, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395: 497-506.
- Hamming I, Timens W, Bulthuis M, et al. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology 203: 631-63
- Beeching NJ, Fletcher TE, Beadsworth MBJ (2020) Covid-19: Testing times. BMJ 369: 1403.
- Fang Y, Zhang H, Xie J, et al. (2020) Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology 296: e115-e117.
- Wang W, Xu Y, Gao R, et al. (2020) Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323: 1843-1844.
- Charlson ME, Pompei P, Ales KL, et al. (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373-383.
- (2020) The Scottish index of multiple deprivation (SIMD). The Scottish Government.
- (2020) Imaging BS of T. BSTI COVID-19 CXR Report Proforma.
- Ai T, Yang Z, Hou H, et al. (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 296: e41-e45.
- Yang Y, Yang M, Shen C, et al. (2020) Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections.
- Zhang Y, Ma ZF (2020) Impact of the COVID-19 pandemic on mental health and quality of life among local residents in Liaoning Province, China: A cross-sectional study. Int J Environ Res Public Health 17: 2381.
- García-Basteiro AL, Chaccour C, Guinovart C, et al. (2020) Monitoring the COVID-19 epidemic in the context of widespread local transmission. The Lancet Respir Med 8: 440-442.
- Zhou F, Yu T, Du R, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. The Lancet 395: 1054-1062.
- Liu K, Chen Y, Lin R, et al. (2020) Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect 80: e14-e16.
- Pinti M, Appay V, Campisi J, et al. (2020) Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol 46: 2286-2301.
- Liu Y, Yang Y, Zhang C, et al. (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63: 364-374.
- Verity R, Okell LC, Dorigatti I, et al. (2020) Estimates of the severity of coronavirus disease 2019: A model-based analysis. The Lancet Infectious Diseases 20: 669-677.
Corresponding Author
Dr. Khurram Shahzad Khan, Surgical Registrar, Department of General Surgery, University Hospital Hairmyres, East Kilbride, G75 8RG, Scotland, UK, Tel: 00-44-7533-537292
Copyright
© 2020 Khan KS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.