Posterior Reversible Leukpoencephaopthy Syndrome Associated with SARS-CoV-2 Antibodies
Keywords
COVID-19, SARS-CoV-2, Posterior Reversible Leukoencephalopathy Syndrome (PRES), PRES MRI, MR SPECT PRES
Ischemic stroke and encephalitis have been reported in patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1-3]. We report case of posterior reversible encephalopathy syndrome (PRES) in a patient with SARS-CoV-2 IgG antibodies.
A 60-year-old caucasian woman was transferred from an outside hospital with one week history of severe headache, intermittent nausea, vomiting, and a one day history of episodes of dizziness, ataxia, and falls. An initial non-contrast computed tomographic of head demonstrated diffuse cerebral edema prompting transfer to our institution for further evaluation and management. The patient did not recall any contact with known SARS-CoV-2 infected patient or any history of fever, respiratory symptoms or diarrhea. On initial evaluation, her blood pressure was 150/89 mmHg. The patient was alert and oriented, and exhibited slight dysmetria of the right upper extremity. Several abnormalities were noted on testing: Elevated leukocytes, fibrinogen, D-Dimer, creatinine phosphokinase, creatinine phosphokinase MB fraction, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, N-terminal pro-b-type natriuretic peptide, and C-reactive protein. Both absolute lymphocytes and eosinophils counts were low. A nasopharyngeal swab did not identify SARS-CoV-2 antigen by PCR testing. Cerebrospinal fluid (CSF) analysis demonstrated normal protein and glucose without the presence of leukocytes or erythrocytes. Serum specimen on day 2 of admission demonstrated presence of SARS-CoV-2 IgG antibodies, with elevated IgG index 1.66. Serum specimen also demonstrated presence of fluorescent anti-nuclear antibody (speckled with titer 1:1280), antineutrophil cytoplasmic antibodies (titer 1:160), and anti-myeloperoxidase (24.9 RLU).
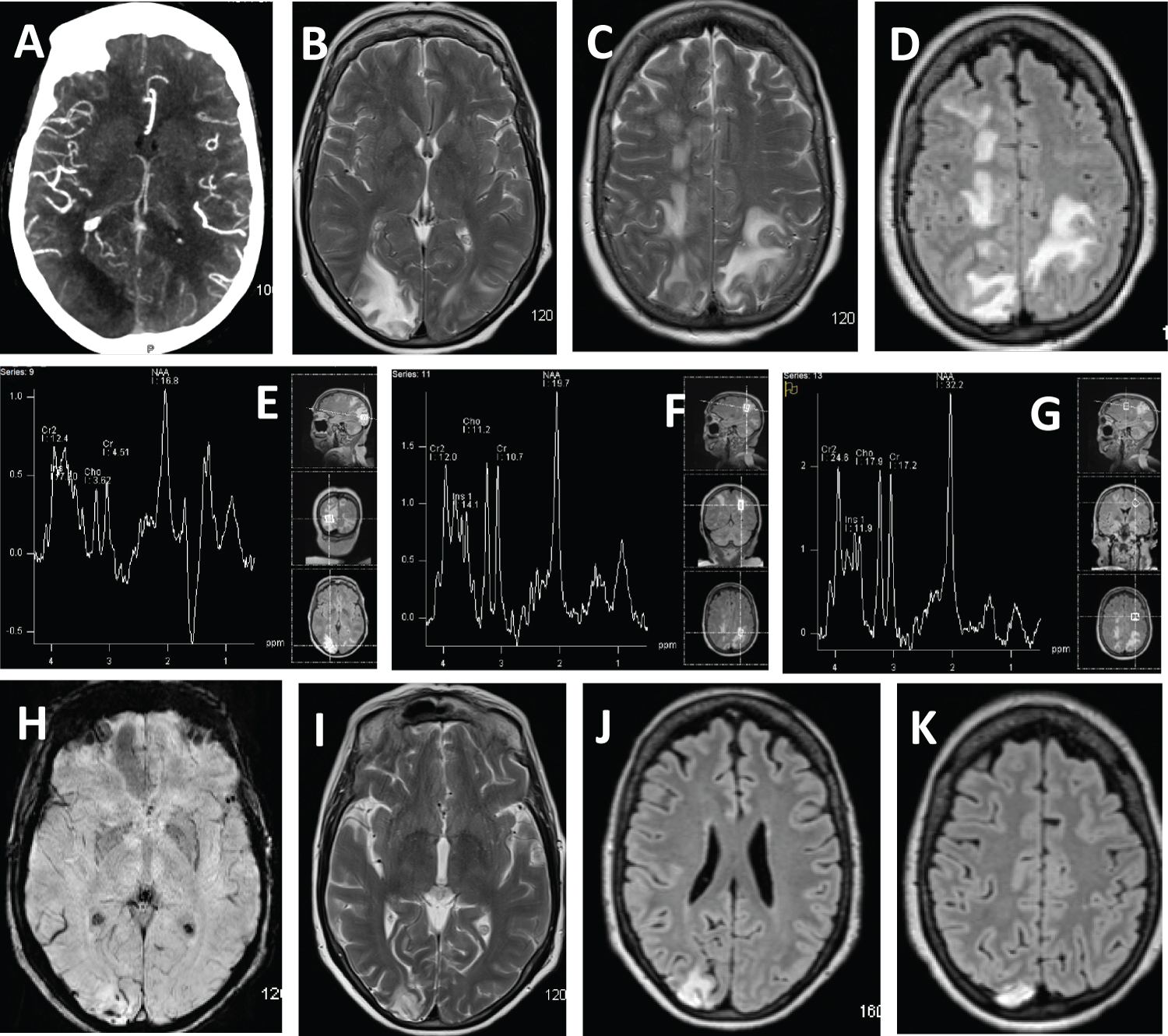
The CT angiogram head demonstrated vessel "pruing" in the right parieo-occipital lobes (Figure 1A). Initial MRI demonstrated multifocal T2-weighted and FLAIR hyperintense lesions predominantly involving bilateral parieto-occipital lobes with effacement of the sulci in these regions (Figure 1B, Figure 1C and Figure 1D). There were also multifocal small T2/FLAIR hyperintensities in bilateral centrum semiovale, left corona radiata, bilateral thalami, pons, and posteroinferior cerebellum bilaterally, with effacement of the cerebellar foliae (not picturized). On day 3, the patient underwent interrogation of the right occiptal and left parietal lobes with magnetic resonance spectrometry. The right occpital region revealed the presence of lactate along with attenuation of N-acetylaspartate, choline and creatine (Figure 1E) in comparison to normal tissue in the left frontal region (Figure 1G), suggestive of infarction. MRS in the left parietal lobe abnormal white matter revealed attenuation of N-acetylaspartate (Figure 1F) in comparison to normal tissue in the left frontal region (Figure 1G), suggestive of PRES.
The pateint was discharged on prescriptions of aspirin 81 mg and atorvastatin 40 mg. She required minimal assistance with ambulation at discharge, with modified Rankin scale of 3. At one-month follow up, the patient reported marked recovery with intermittent positional vertigo, and short-term memory deficits, the latter of which was longstanding. She reported no recollection of the first two days of admission, but was now back to living interpedently. Her blood pressure was 130/78 mmHg. She had no neurological deficits on exam, and a Montreal Cognitive Assessment a score of 26/30. Her disability was graded as modified Rankin of 1. The abnormalities previously noted on laboratory testing had completely resolved or improved. Serum specimen did not demonstrate presence of SARS-CoV-2 IgG antibodies. Serum specimen demonstrated presence of fluorescent anti-nuclear antibody (speckled with titer 1:1280), antineutrophil cytoplasmic antibodies (titer 1:320), and anti-myeloperoxidase (27.2 RLU). The Suspectbility Weight Image demonstrated intravascular thrombus, persistent hyperdense signal in the right occipital region consisted with infarction (Figure 1H) and interval resolution of previously noted T2 and FLAIR hyperintense signal in the right semiovale and left parietal region consistent with PRES (Figure 1I, Figure 1G and Figure 1K). Abnormal signal in the right cerebellum was completed resolved (not picturized).
The diagnosis of PRES was based on resolution of clinical improvement, MRI changes, MR spectroscopy findings, and absence of inflammatory changes in the CSF. PRES has been previously reported in patients with infection, sepsis or shock [4]. T-cell, endothelial cell activation and inflammatory cytokine production including TNF-α, IL-1, IFN-γ and IL-6 have been implicated in PRES in the settings of infection and sepsis [5]. A similar overproduction of immune cells and their signaling molecules have been identified in patients infected with SARS-CoV-2 [6]. A previous report found elevated IL-6, IL-8 and TNF-α in four children with SARS-CoV-2 IgG antibodies without SARS-CoV-2 antigen detection who presented with Kawasaki Disease and Toxic Shock Syndrome [7]. Humanized monoclonal antibody (mAb) Tocilizumab, targeting IL-6 has been successful in stabilizing the alveolar capillary membrane, reducing alveolar wall edema, and preventing/reversing acute respiratory distress syndrome in some patients [8] and may be useful in patients with PRES associated with SARS-CoV-2 infection.
References
- Johan Virhammar, Eva Kumlien, David Fällmar, et al. (2020) Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology 95: 445-449.
- Stéphane Kremer, François Lersy, Mathieu Anheim, et al. (2020) Neurologic and neuroimaging findings in COVID-19 patients: A retrospective multicenter study. Neurology 95: e1868-e1882.
- Carlos Manuel Romero-Sánchez, Inmaculada Díaz-Maroto, Eva Fernández-Díaz, et al. (2020) Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 95: e1060-e1070.
- Bartynski WS, Boardman JF, Zeigler ZR, et al. (2006) Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol 27: 2179-2190.
- Bartynski WS (2008) Posterior reversible encephalopathy syndrome, part 2: Controversies surrounding pathophysiology of vasogenic edema. American Journal of Neuroradiology 29: 1043-1049.
- Feldstein LR, Rose EB, Horwitz SM, et al. (2020) Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 383: 334-346.
- T Waltuch, P Gill, LE Zinns, et al. (2020) Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med.
- Prete M, Favoino E, Catacchio G, et al. (2020) SARS-CoV-2 inflammatory syndrome. clinical features and rationale for immunological treatment. Int J Mol Sci 21: 3377.
Corresponding Author
Dr. Navpreet K Bains, DO, MBA, Departments of Neurology, University of Missouri Columbia Medical Center, One Hospital Drive CS & E Room 540, Columbia, MO 65212, USA
Copyright
© 2020 Qureshi AI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.