Assessments of Tomato (Lycopersicum esculentum) Diseases and Agronomic Practices
Abstract
Tomato became the most profitable crop, providing small-scale farmers with a higher return than other vegetable crops in Western parts of Ethiopia. However, the national average tomato fruit output was frequently poor. Farmers, on the other hand, experience poorer yields due to biotic factors and fertilization that isn't up to snuff. Different diseases have been threatening tomato production and productivity in Western Ethiopia. The relative importance of each disease across locations, on the other hand, has not been analyzed or thoroughly characterized in order to develop an effective therapeutic approach. A survey study was conducted to determine the occurrence, distribution, tomato agronomic practices and status of tomato diseases in the Horo, Jima Genet, Bako Tibe, Boneya Boshe, Gudeya Bila, and Ilu Gelan special districts during the 2018 and 2019 cropping seasons. Because of its required crop and to save money, multipurpose stratified sampling methods were applied after identifying the true tomato growers. There were three zones, two Woredas, and two PA's (3 farms × 5 samples for each seedling and mature stage) × 2 = 360 samples. Seedling disorders infected 58.7% of seed beds, according to the findings. The far more infected seedlings (75 percent) Horo district came in second, followed by Jima Geneti, Bako Tibe, and Bonrya Boshe districts (64, 59.5 and 55.5 percent, respectively). Similarly, after transplanting, a survey revealed that 148.5 (97.6%) of samples were infected with at least one infection. According to the frequency of pathogen growth, 23.4 percent of the affiliated pathogens were type of bacteria spp. 19.5, (11.3%) were root knot nematode and virus spp., and 24.7, 20.7, and 23.6 percent were fungal genera late blight, early blight, and Fusarium spp. respectively. Farmers adopted 93.6% of recommended tomato agronomic practices during the off-season, 88.2% of fertilizer usage, 26.6, 31.8, and 41.85% of crop rotation on one year with potato, two years of with onion, and one year with maize, respectively. According to the current study, a complex of diseases exists at each stage of tomato growth, and the occurrence of these diseases is rising. To handle the region's complex diseases, a holistic and cumulative integrated approach is essential.
Keywords
Seedling infection, Disease complex, Incidence, Management practices
Introduction
Tomatoes are grown in Ethiopia's different regions, each with its particular agroecological circumstances. It grows between 700 to 2000 meters above sea level, where the climate is mild and dry during the day and chilly at night, allowing for optimal growth and development [1]. Since it became the most profitable crop, providing small-scale farmers with a higher return than other vegetable crops, total production of this crop has expanded dramatically in our country. However, Ethiopia's national average tomato fruit output was frequently poor (12.5 ton/ha), especially when compared to neighboring African countries such as Kenya (16.4 ton/ha) [2]. In Ethiopia, current farmer productivity is 90 q/ha, while yields of up to 400 q/ha have been observed on research plots. Farmers, on the other hand, experience poorer yields due to illnesses, pests, and other factors and fertilization that isn't up to snuff [3]. In various places, small-scale farmers, commercial growers, and state farm companies produce the crop for its fruits. The rift valley, particularly along the Awash River valley and the lakes region, has the most intensive production [4].
In the processing business, its production ranks first among vegetables. Because biotic stressors account for more than 42% of the world's potential agricultural yield (13 percent due to insects, 13 percent due to weeds, and 15% due to other pathogens), lowering this incidence will be one of the most essential ways to improve plant production [5]. These options were divided as follows by Saleem [6]: (I) plant material improvement (tolerance/resistance breeding); (ii) root health improvement (e.g., field rotation, soil tillage, management of soil-borne diseases); (iii) irrigation methods improvement (optimal water quality and availability); (iv) protection against airborne dangers (foliar diseases etc.,). In this context, the production of biotic stress-tolerant plants is an essential goal of plant breeding strategies, with ramifications for farmers as well as the seed and pesticide businesses. In fact, compared to the use of chemical pesticides or other parasite control approaches, genetic resistance has several clear advantages. Nominal genetic permanence, low cost once cultivars are produced, and great efficiency are among them [6].
Various biotic and abiotic factors influence tomato production. In most crops, biotic stressors are one of the leading reasons of yield decline on farmer's fields. Frequently, reports of losses of up to 100% of the production can be found. The most notable case of biotic stress in food insecurity occurred in Europe in 1845, particularly in Ireland and England, when Phytophthora infestans, the etiological agent for potato blight, destroyed roughly 80% of potato farms. More than 2 million people died of starvation as a result of the calamity, and many more moved to other areas [7]. Tomato pathogens have been identified as more than 200 pests and illnesses that impede output [8]. Many of them are common diseases caused by fungus, Oomycetes, bacteria, viruses, and nematodes all over the world [9]. The main biotic restrictions in Ethiopia are illnesses and insect pests that cause damage. In order to reduce the harm caused by illness and insect pests, much research has been performed in the country on both pathological and entomological issues.
Approximately 13 diseases caused by various fungal, bacterial, and virus pathogens [10] and six diseases caused by different fungal, bacterial, and virus pathogens Late blight (Phytophthora infestans), early blight (Alternaria solani), septoria leaf spot (Septoria lycopersici), and viruses have all been identified as major commercially relevant tomato diseases in previous investigations. Some diseases that were once thought to be of small relevance have since risen to prominence. Powdery mildew (Leveillula taurica), root knot nematode (Meloidogyne spp.), and bacterial wilt are among these illnesses (Ralstonia solanacerum). There are also a number of factors that limit tomato output. These include a lack of better, high-performing cultivars, poor fruit set as a result of heavy rainfall and extremely high temperatures, as well as pests and illnesses. 100 percent yield losses are typical, especially when tomatoes are infected early in their growth [11]. The average global crop loss due to all illnesses was around 12.8 percent of potential production, but tomato alone suffered a loss of 21.8 percent [12].
In Ethiopia, the disease wiped out all crops on unimproved indigenous land on sensitive cultivars and 67.1% on vulnerable cultivars [10]. Tomatoes are a popular vegetable that contributes significantly to the country's economy. It is used as a raw material in the processing industry, as well as a valuable cash crop for farmers and a source of employment for both urban and rural residents. Tomato processing and export output, on the other hand, has been low, with national average yields of 0.4 t/ha [13] and dropping over time. The Oromiya Regional State contributes a substantial amount to the total tomato production in the country. The Western, Central, and Eastern sections of the region produce more tomatoes.
The crop's yield and production, however, are low in the region. This could be due to low- yielding varieties, drought, insect pests, illnesses, or inadequate cultural techniques, among other things [13]. The infections caused by various fungus, bacteria, and viruses are the most important ones [14]. Late blight and Bacterial wilt caused 60 to 100 percent losses of marketable fruit, whereas the virus caused 60 to 100 percent losses [15]. When substantial damage occurs on expanding fruits, bacterial spots induced by a seed-borne bacterial disease (Xanthomonas campestris pv. vesicatoria) can cause severe defoliation of plants, resulting in lower yield and loss of quality of harvested fruit [16].
Tomatoes are widely used due to factors like as high yield per plot area, simplicity of preparation for use, short maturing crop with low water requirements, and others [3]. However, the crop is more susceptible to biotic infection by a form of fungal disease that occurs after or before maturity. These illnesses have been found in various parts of Western Oromia, with unknown types/races and populations, posing a threat to farmers' target protection efforts. One sign or symptom is similar to the others, making it difficult for researchers to build resistant cultivars and making it difficult to take urgent chemical defenses. In the region, total crop failure owing to illnesses is widespread, and farmers are often forced to abandon their crops due to high infection pressure in the field [17]. Despite this, the identification and relative impact of each disease differs from one area to the next locations haven't really been profiled well. As a result, this research was started in order to assess farmers' agronomic practices, estimate the relative presence, distribution, and frequency of tomato infections, and document information for pest control strategies against tomato diseases in the study areas.
Materials and Procedures
The survey was carried out in the Western Ethiopian zones of East Wollega, West Showa, and Horro Guduru Wollega. All are found in Solanaceae Spp. growing places in the Oromia regional state's 18 zones. Located at various distances west of Addis Ababa. The Zone's annual rainfall ranges from 1800 to 2000 mm, while the Zone's mean minimum and maximum annual temperatures are 7℃-12℃ and 25℃-30℃, respectively [18]. The average annual rainfall is 1524 mm, according to 15 years of weather data received from the Zonal Agricultural Bureau. The three research Zones have an altitude range of 1387 to 2870 meters above sea level [19]. Eutric Vertisols, Humic Alfisols, and Humic Nitosols are the three most common soil types in the Zone. Nitosols are the most common, accounting for around 90% of the Zones. The examined zones were purposefully chosen to represent the region's key tomato-growing areas. The area's population is economically dependent on mixed farming.
Sampling methodology and sample size
Because of the required yield and to save money, the purposive stratified multiple stage" sampling approach was adopted after identifying the true tomato growers. Two woredas were chosen at random from the specified zone, and two PAs (peasant associations) were chosen from each district, with three farms per PA (5 samples per farm). Five disease-infected seedling samples and five mature plant samples were taken to the Wollega University Shambu campus Plant Science lab from each targeted farm. Three Zones, two Woredas, and two Pas (3 farms × 5 samples for each seedling and mature stage) × 2 = 360 samples were used in most cases. To identify the types and the more prevalent illness of tomato in the study area, all afflicted tomato plants by either disease were included. In the laboratory, disease isolation was carried out according to established techniques. Natural diseased plants of various parts (root, stem, leaf, and fruit) that showed suspected characteristic symptoms of several illnesses were gathered during the survey period. 360 samples were collected and transported to the Wollega University Shambu campus plant protection laboratories for pathogen isolation and identification. 360 samples were collected and transported to the Wollega University Shambu campus plant protection laboratories for pathogen isolation and identification.
Sample isolation
Each sample with probable illness symptoms was sliced into smaller pieces from the diseased part's edge and surface sterilized for 3 minutes in a 10% sodium hypochlorite solution before being rinsed three times with sterile water. Following Nelson, the sterilized pieces were placed in potato dextrose agar (PDA) and yeast potato sucrose agar (YPSA) media for fungal and bacterial pathogen isolation, respectively. Each pathogen was purified after a few days of growth by switching cultures to new media. The color of the mycelium and the morphology of the conidia were used to identify the species. Finally, each isolated pathogen was transferred to PDA and YPSA slant media, tagged, and stored at 4℃ for future research.
Disease Prevalence and Incidence
The disease's prevalence
Seed bed infections are common. In six seedling rising districts (Horo, Jima Genet, Bako Tibe, Boneya Boshe, Gudeya Bila, and Ilu Gelan), seedling disease was assessed. Farmers usually cultivate tomato by seedling preparation and transplantation in the main field. A total of 180 seed beds were evaluated (15 × 2 per district). The evaluation took place between February and June of this year. Each district's number of sick seed beds was recorded. Finally, disease prevalence was calculated by dividing the percentage of infected seed beds by the total number of investigated fields. Disease Prevalence and Incidence after Transplantation, the disease's prevalence is as follows: On a permanent field, 180 tomato farms were inspected before and after flowering. A 5-kilometer distance separated two nearby randomly examined pastures. Plant incidence was determined by measuring farms diagonally and estimating disease incidence using a 3 m × 3 m quadrant. In each quadrant, the number of infected plants and the total number of tomato plants were counted. The percentage of diseased plants in each field at each site was used to calculate disease incidence.
The farmer's production methods
Field inspection formats were created to collect additional data on farmers' agronomic practices (planting methods, planting timing, seed source, fertilizer application, pesticide use, and crop rotation system), and these data were collected during field inspections. To evaluate their current cultural practice in the selected disease, 72 tomato growers were questioned, and their fields were observed.
Analyze the data
The survey results were evaluated using descriptive statistical methods, which were dependent on the disease problem, farmer cultural practices, and the frequency of different tomato diseases across the site. The field sample was recognized in the laboratory, sorted by kind, and analyzed using descriptive statistics, such as frequency, percentage, and graph, using SPSS version 14 statistical processes.
Result and Discussions
Diseases of seedlings
In the current survey, 43.05% of the 180 seedbeds examined were contaminated with various illnesses (Table 1). Two pathogens that cause seedling illnesses, damping off and fusarium spp. as well as a combination of the two pathogens, were detected in seed beds, though data on their combined effect was not recorded. Damping off was responsible for 17.33% of the isolated pathogens, while fusarium spp. caused 15.5% of the field infection. This variation could be attributable to differences in the environment that favor disease development. Seedling illnesses are caused by a variety of pathogens, including fusarium oxysporum, damping off, Phytophthora spp. and others, according to Sharma [20]. Horo district has the greatest rate of tomato seedling disease (60%). It was followed by Jima Genet, Bako Tibe, and Gudeya Bila, who had disease prevalence rates of 49, 43.5, and 40 percent, respectively. The occurrence of seedling disease was lowest in Ilu Galan had the lowest percentage of seedling disease (31.5%) (Table 1). This variation could be attributable to differences in the environment that favor disease development.
After Transplantation, Prevalence and Incidence
Disease prevalence
Tomato diseases found after transplanting in the inspected area included bacterial speck (Pseudomonas syringae pv. tomato), bacterial wilt (Ralstonia solanacearum), fusarium wilt (Fusarium spp), Powdery mildew (Leveillula taurica), early blight (Alternaria solani spp), late blight (Phytophthora in Shankar, et al. [21] reported that, in addition to damping-off, tomato can be impacted by a number of diseases after transplantation. Tomato pathogens have been identified as more than 200 pests and illnesses that impede output [9]. Many of these, as stated in Foolad [9] are common diseases caused by fungi, oomycetes, bacteria, viruses, and nematodes. All pathogens had varying mean frequencies of each illness symptom across the investigated area. Bacterial wilt was found on 27.8% of the farms tested. Bacterial speck, Bacterial speck, Bacterial speck, late blight, fusarium wilt, and late blight were found in 27.7%, 26%, and 23% of the farms, respectively. Root knot nematodes, virus spp. and early blight of tomato-diseases all occurred at very low frequency of 20%, 13%, and 20.5%, respectively, when compared to other diseases (Table 2). At all growth stages of the crop, fusarium wilt and powdery mildew were found, whereas bacterial wilt, bacterial speck, and late blight illnesses were observed during and after blooming and fruiting. Variations in natural and artificial elements like as temperature, relative humidity, and soil moisture could explain their relative differences. Shankar, et al. reported similar findings [21].
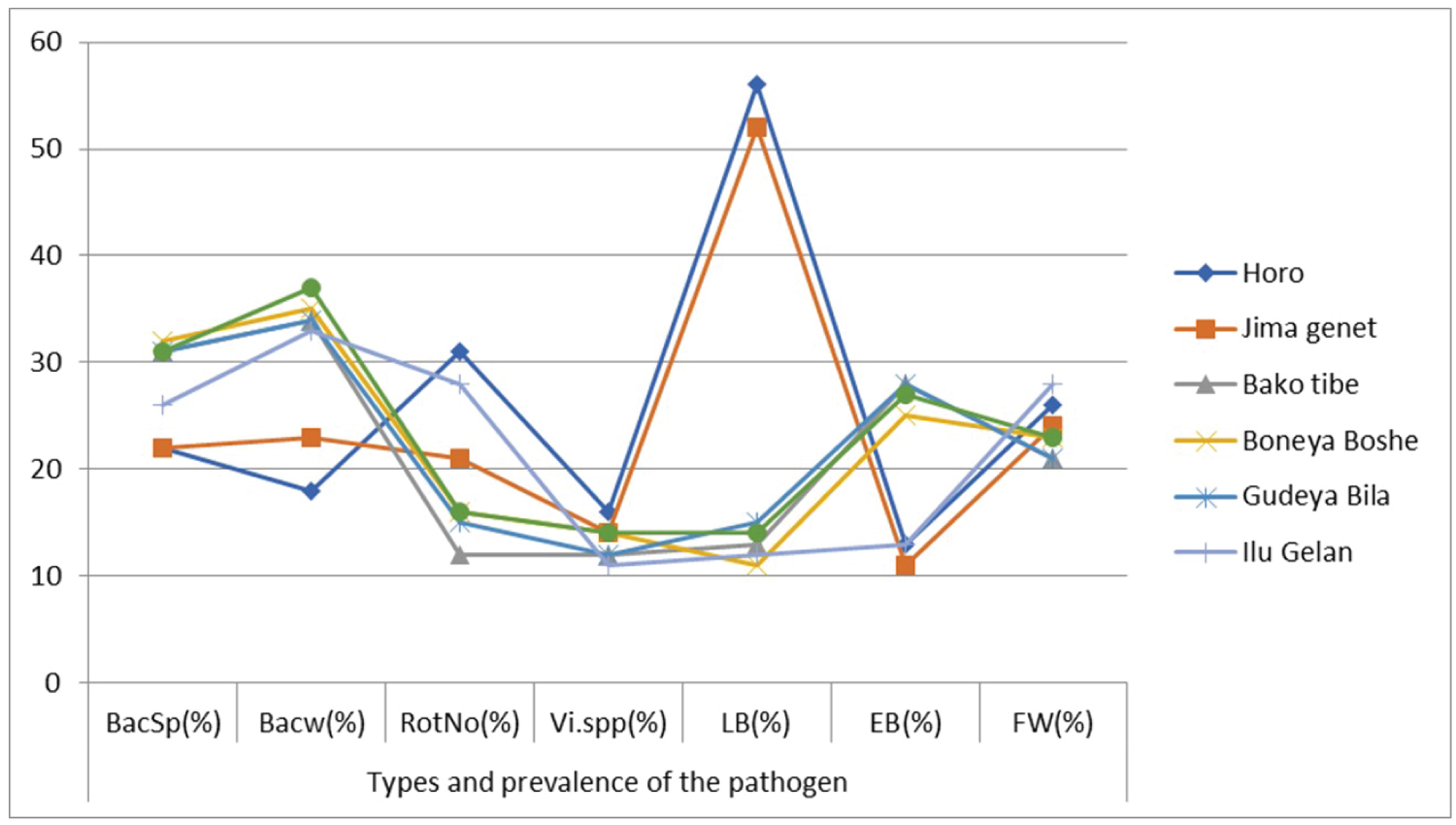
Each disease's relative prevalence differed between the surveyed districts. In the districts of Bonaya Boshe, Bako Tibe, Horo, and Ilu Gelan, high prevalence's of bacterial wilt (35%), bacterial speck (32 percent), late blight (56%), and Fusarium wilt (28%) were observed (Table 2). Horro had the highest incidence of late blight (56%), followed by Jima Genet (52%). Phytophthora Spp contaminated 11-15% of inspected farms in the remaining districts (Table 2). Fusarium wilt was most common in Ilu Gelan (28%), followed by Jima Geneti (24%), while root knot nematode was most common in Horo (31%), followed by Ilu Gelan (28 %) (Table 2).
Incidence of disease
Horo and Jima genet had the greatest mean late blight infection (56 and 52 percent, respectively). In Horo and Jima genet, Bako Tibe, Boneya Boshe, and Ilu Gelan, respectively, high infection by bacterial wilt (35%), bacterial spot of tomato (32%), and fusarium wilt (28%) (Figure 1). Viral infections and root knot nematode, on the other hand, were detected on the main field as minor diseases when compared to the others. Thus, Horo (16%) had the highest mean virus spp. score, while Horo (16%) had the highest mean field score of root knot nematode incidence (31%). The incidence was fewer in the remaining districts, ranging from 5% to 10%. In comparison to the other Zones, Horo Guduru Wollega Zone was the most affected with the disease investigated.
Tomato bacterial spot (Xanthomonas campestris pv. vesicatoria). This disease is one of the most common tomato illnesses in the regions studied. Infection was identified in practically all areas, with a rate of 22-31 percent. In this investigation, the maximum infestation was found in Gudeya Bila district (32%), while the lowest infection was found in Horro district (22%).
Bacterial spot can harm both seedlings and mature plants
Infections on seedlings can result in severe defoliation. Infections manifest as water-soaked spots on older plants, mainly on elder leaves. Leaf dots change color from yellow to bright green to black to dark brown over time. Older spots are black, somewhat elevated, and superficial, with a diameter of up to 0.3 inch (7.5 mm). Larger leaf blotches, especially on the leaf margins, are also possible [16]. On immature fruit, symptoms include a somewhat sunken appearance encircled by a water-soaked halo that quickly fades. Fruit stains get bigger, browner, and scabby. The bacterial spot bacterium can be found in crop debris, volunteer tomatoes, and weed hosts including nightshade and ground cherry from one season to the next. The bacterium is transferred via seeds and can be detected inside and on the surface of the seeds. Infection is aided by high relative humidity and free moisture on the plant.
Symptoms arise immediately at temperatures of 20℃ and above. Nighttime temperatures of 16℃ or lower, regardless of daytime temperatures, inhibit sickness growth. Some pathogen strains are equally virulent on tomatoes and peppers, whereas others are equally virulent on both [21]. The other pathogen that can damage tomatoes and other Solanaceae plants is tomato bacterial wilt (Ralstonia solanacearum). Plants that are mature and fruit-bearing are harmed in the middle of the summer. The first sign is the wilting of a few leaves. A lot of the time, this is neglected. The largest infestation (35%) was discovered in the Boneya Boshe district, while the lowest infection was discovered in the Horro district (18%) (Figure 1).
After that, the entire plant wilts and dies. Such extreme symptoms appear when the weather is hot (300℃-350℃), and the soil moisture is ample. In less favorable conditions, wilt and decline will occur more slowly, and many adventitious roots will sprout on the lower stems. Both cases have a brownish discoloration that starts in the vascular system and spreads to the pith and cortex in advanced cases. The roots will show varying degrees of degeneration. The findings of Roberto and Fritsche-inquiry Neto's [7] support up this conclusion reached under nearly comparable conditions. Other factors that affect pathogen survival in soil and water include soil type and structure, soil moisture content, and organic matter. pH and salt levels of the water, as well as the presence of antagonist microorganisms in the soil, may also support disease growth [21].
Early blight
Alternaria solani, the causal agent, is the cause of tobacco blight (early blight). It was more common in the hotter parts of the districts, such as Bako Tibe and Gudeya Bila, where average infestations of (28 percent, 28 percent, and 27 percent, respectively). Horo and Jima Genet, on the other hand, had the lowest rates of viral infection, with 11 percent and 13 percent, respectively (Figure 1). Environmental factors and soil types in a specific area may favor pathogen growth, resulting in variation in disease occurrence. Warm temperatures and extended periods of leaf wetness from frequent rain, overhead irrigation, or dews favor the disease, according to Nowicki, et al. [22], and early blight may be more widespread on old transplants, transplants lacking vigor, or transplants stressed by wilting.
Root knot
Meloidogyne spp. a type of root knot, is the source of this illness. It's one of the most common disorders that researchers come upon. However, its prevalence and mean infection were lower than those of the other pathogens tested. According to the survey's findings, the district of Horro had the highest mean infection rate (31%), while Bako Tibe had the lowest (12%) (Figure 1). The findings of [23], which state that Nematodes are active all year in warm, fertile, and wet soils, back this idea. Furthermore, this disease prevalence is available in all of the study areas, despite the fact that the percentages vary. Plants are assaulted at every step of their growth. Plants infected with Ralstonia solanacearum (bacterial wilt), Sclerotiumrolfsii (southern blight), Fusarium, Pythium, or Rhizoctonia are more susceptible to soil-borne diseases. This secondary infection carries the risk of being deadly. Internal stem and root tissue may become discolored, and the plant may die quickly as a result of the secondary infection. As a result, caution should be exercised in site selection and disease management procedures.
Tomato wilt caused by Fusarium
Fusarium Oxysporum. spp lycopersici is the causative agent. This pathogen is a serious problem in the districts investigated, which have high water holding capacity soil and poor drainage. Yellowing of the foliage is the first symptom, which appears on the lower leaves and progresses upward. The yellowing usually starts on one side of the vine. Later, infected leaves develop downward curling, browning, and drying. The top of the vine wilts during the day and recovers at night, but the wilting worsens over time until the entire vine is wilted permanently. Infected stems and big leaf petioles show vascular browning. The root systems of the plants that are affected are stunted. Fusarium wilt is the most common cause of tomato wilt in the United States. Ilu Gelan abut (28.5) had the greatest prevalence of fusarium wilt of tomato in the examined district, followed by Jima Genet (24%). The greatest incidence, on the other hand, was observed in Bako and Gudeya Bila, both with (21 percent) (Figure 1).
It is a pathogen with a narrow range of variation and a nearly uniform incidence across the examined districts. The findings are consistent with those of Ristaino [24], who found that significant rainfall and surface water accumulation are critical for fusarium wilt. This has far-reaching implications for disease control in the region. The pathogen is soil-borne and can live in the soil for many years without a host. The fungus associated with diseased tomato debris is the source of the majority of infections. Because of physiological changes in the root caused by root-knot nematode infection, Fusarium wilt resistant cultivars become more vulnerable to the fungus. Warm temperatures (for example, 27-30°F) encourage disease development 27-28℃, dry weather, and acidic soil (pH 5-5.6) are all factors. Tomato plants that grow quickly and are highly succulent and are fertilized with ammonium nitrate are particularly sensitive to the disease [16].
Blight in the late stages
This disease was discovered in all of the examined regions of Western Oromiya, where tomato cultivation is abundant even during the off-season. This finding is consistent with the findings of Getachew, et al. [25], who state that the illness is the most damaging and cost-effective disease on potato and tomato in Ethiopia. In many regions of the world, including Ethiopia, the disease's prevalence and severity have increased in recent decades, posing a severe danger to tomato and potato output [26]. Despite the fact that experts are working to lessen the disease's impact on fruit supply, the loss is still significant [25]. The borders of these patches are pale green or water soaked in severely affected tomatoes. The leaf spots may expand and become more noticeable. Rapidly consolidate until the entire leaflet is destroyed. A downy white mold growth forms along the leaf spot edges on the underside of the foliage when it is wet or humid. Lesions on the stem and petioles are dark brown and drenched in water, and they can sporulate.
According to the findings of the current study, the highest severity of the disease was observed in Horo abut (56%) and Jima Genet (52%) districts between mid-June and the first week of July. This conclusion agrees with Shankar, et al. [21], who found that the disease affects all Solanum species and requires extended periods of leaf wetness from regular rain or dew production, as well as low to moderate temperatures (13-20℃). The development of disease is slowed by hot, dry weather. The sickness was particularly severe in two of the surveyed locations' damp districts, Horo and Jima Genet (Figure 1). On the other hand, the severity of late blight was found to be mild in Boneya Boshe (11.5%) and Ilu Gelan (13%) where the climate is hot, dry, and windy (Figure 1). Shankar, et al. [21] concurred, reporting that late blight incidence and severity could be low under hot and friable air flow conditions.
Spp. Viral illnesses
On the field, symptoms of virus infection included potato virus Y of tomato, tomato bushy stunt, tomato fern leaf, tomato mosaic virus, and fruit deformities [27]. Incidences of virus illness were documented in all districts. Gudeya Bila had the highest virus incidence (16%), followed by Boneya Boshe and Jima genet, each having a mean average of (14%) apiece. The virus is transmitted through seeds. This finding is supported by Hiskias, et al. [23], who found that the incidence, distribution, and relative importance of viruses infecting hot pepper and tomato in Ethiopia's major growing areas might be enhanced unless scientific management interferes with producers' operations [28]. Infected tomato seeds can be a source of infection as well as a mechanism for the virus to spread across long distances. During ordinary horticultural operations, the virus can be disseminated by horticulture workers on contaminated hands, clothing, and tools transplanting, tying, pruning, grafting, pollinating, cultivating, spraying, watering, and picking are just a few examples. ToMV is a tobacco mosaic virus strain that is closely related to ToMV (TMV). Under harsh environmental conditions, the virus can survive for two years in plant debris in dry soil, one month in moist soil, and 22 months in root debris in fallow soil [21]. As a result, caution should be exercised when exchanging tomato seed and seedlings from areas with a high viral incidence.
Farmers' reactions to tomato-related cultural practices
The majority of disease found on tomato farms can be controlled by following suggested cultural practices such as using recommended fertilizer, planting at the proper time, using resistance types, spacing, and disease-free seed, crop rotation, and secondary host management. Farmers' cultural practices were varied in the investigated districts, with only a few farmers employing the recommended cultural practice. For example, in majority of the selected districts, seeding occurs during the off-season because on-season tomato cultivation is challenging because the prevalence of tomato disease and infestation increases with high wetness. Furthermore, the land used for off-season growth is confined to plots of land that are close to local irrigation infrastructure. This could possibly be why farmers aren't using the proper crop rotation for their field. As a result, the majority of Tomato growers produce 93.6 percent of their tomatoes during the off season, while the remaining 6.45 percent use intense cultivation to produce tomatoes during the rainy season (Table 3).
Regardless of the variety, the recommended inter and intra-row spacing for tomatoes is 30 cm and 70 cm, respectively, while farmers in the studied districts were using 15 to 25 and 35 to 50 in ranges. This suggests that farmers in the area still don't understand how to reduce disease incidence by spacing their crops. Fertilizer was used by the majority of farmers in the inspected region during planting, however the amount and types of fertilizer used varied from farmer to farmer and district to district. In terms of fungicide application, only 1.2 percent of farmers utilized chemical spray following a disease outbreak on the field, and no one used seed treatment prior to sowing. Seed treatments, use of specified seed rate, spreading seeds in rows, and planting in rows have all been found to improve nursery management. Similarly, staking has demonstrated better outcome in boosting the yield and productivity of tomato [29].
After producing tomatoes, all of the farmers interviewed practiced rotation; however, the year interval and types of crops used for rotation varied from farmer to farmer. With potato, 26.6 percent use a one-year rotation, 31.85% use a two-year rotation, and 41.8 percent use a one-year rotation with maize (Table 3). However, most fungal and bacterial pathogens that cause tomato disease can live in the soil or in crop residues for one to three years after they first appeared in the area. Rotating with the same crop family, which may act as an alternate host by itself, is not recommended since, for example, rotating potato with tomato can facilitate disease in the Solanaceae family, such as late blight, in the debris. As a result, the area's farmers require agronomic extension services for the future.
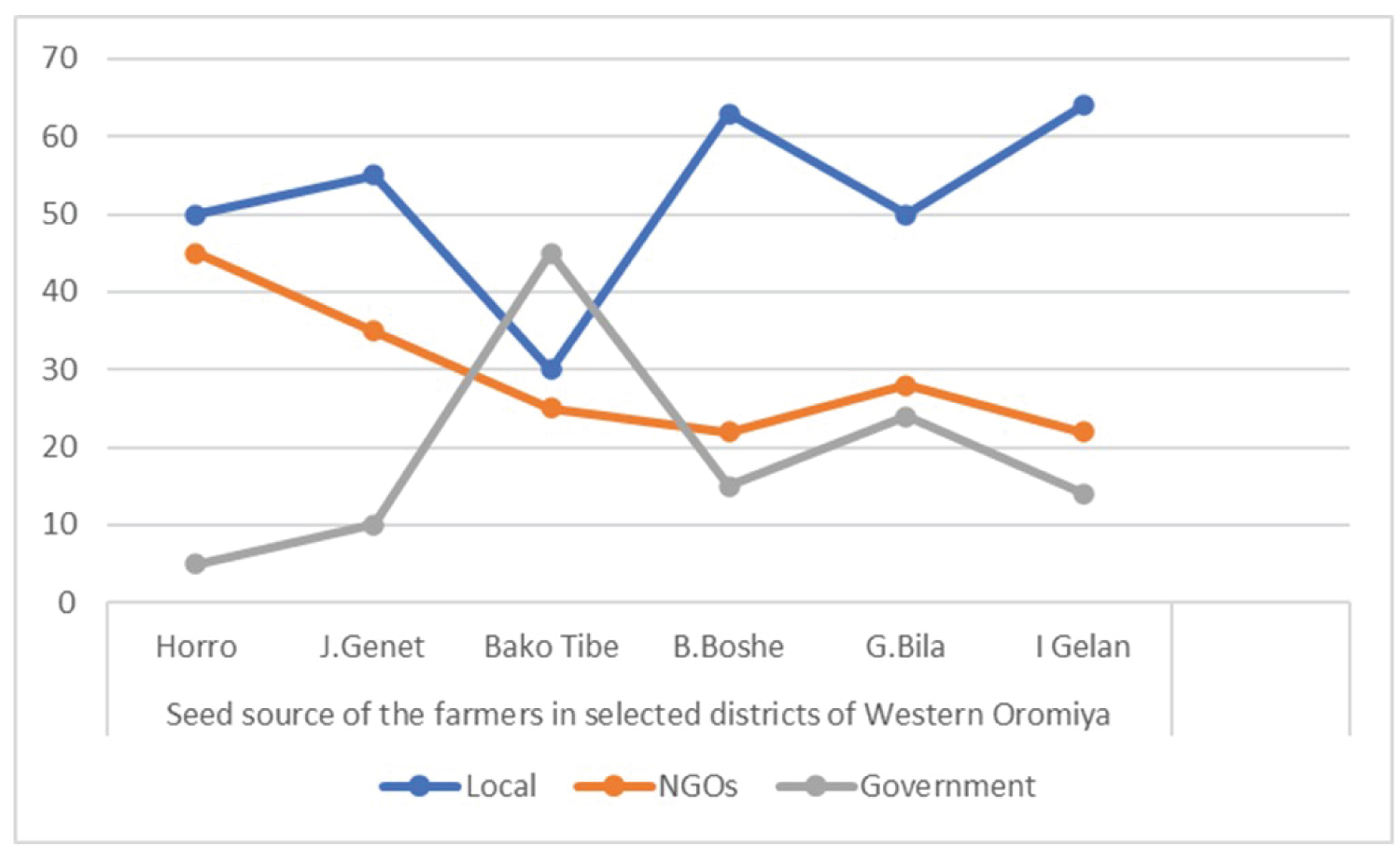
Tomato seed is obtained from a variety of sources by farmers in the study area. 52.5 percent of the farmers polled got their supplies from a local shop, 29% from certain NGOs, and the rest got their supplies from the government, agricultural offices, research centers, and other organizations (Figure 2). Only a small percentage of farmers use local extraction to prepare their own seed. However, it is well recognized that purchasing seeds from a local market and conventional extraction methods are unreliable and more likely to be infected by seed borne pathogens, and hence have a role in disease transmission, particularly for seed borne bacterial and fungal pathogens. Contaminated seeds or soils have been described as sources of inoculums that impact seedlings before or after emergence. Sharma [20] claimed that cultural control procedures in the field are crucial components for disease control, but it all started with seed care in order to avoid such difficulties.
Conclusion
Despite the fact that yield loss owing to each pathogen is not well understood or quantified in our country, this investigation revealed the existence of a complicated disease in tomato seedlings and later growth stages. Across the examined areas, more than eight distinct disease kinds that are seriously harming tomato production and productivity were discovered. The most common diseases in tomato-producing areas of Western Oromia are fungal disease (late blight, fusarium wilt, and early blight), bacterial disease (bacterial spot and bacterial wilt of tomato), viral illness, and worms. Other fungal spp. such as damping off, are also common diseases that damage tomato seedlings on seed beds.
According to a farmers' adoption survey, the region's implementation of recommended cultural methods on tomato production is quite low. Farmers in the area continue to use poor tomato cultural methods. Farmers' practices analysis of suggested tomato cultural practices revealed 93.6 percent planting off season, 88.2 percent fertilizer use, 26.6, 31.8, and 41.85 percent crop rotation on one year with potato, two years with onion, and one year with maize, respectively. Other simple but crucial agronomic cultural techniques that farmers can readily perform were not noticed on the fields of the farmers in the selected districts.
As a result, efforts should be made to integrate various disease control approaches. These include the development of resistance varieties, the implementation of improved agronomic cultural practices such as spacing, stalking, and recommended crop rotation, and the creation of awareness of the disease and its management among farmers and experts from site selection to post-harvest handling. To handle the complex diseases that have emerged in the region, a holistic cumulative integrated approach is necessary urgently.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This research was funded by Wollega University, Ethiopia. We express our sincere gratitude to the Melkasa Agriculture Research center for providing us with improved barley variety seeds.
References
- Almeida RPP, Nunney L (2015) How do plant diseases caused by Xylella fastidiosa emerge? Plant Dis 99: 1457-1467.
- Kader AA, Rolle RS (2004) FAO: The role of postharvest management in assuring the quality and safety of horticultural produce. Agricultural Organization of the United Nations, Rome 96: 4-17.
- FAO (2019) Food and agricultural organization annual report. Statistical Bulletin, Rome, Italy 150: 1-2.
- Desalegn NS, Gomathi N, Netsanet B, et al. (2020) Estimation of general and specific combining ability effect for yield and quality characters in tomato (Solanum lycopersicum Mill. L.). Afr J Agric Res 17: 321-328.
- Akhtar KP, Saleem MY, Iqbal Q, et al. (2016) Evaluation of tomato genotypes for late blight resistance using low tunnel assay. J Plant Pathol 98: 421-428.
- Saleem MY, Akhtar KP, Iqbal Q, et al. (2016) Development of tomato hybrids with multiple disease tolerance. Pak J Bot 48: 771-778.
- Fritsche-Neto R, Borém A (2012) Plant breeding for biotic stress resistance. Springer-Verlag Berlin Heidelberg. ISBN 978-3-642-33087-2 (eBook).
- Foolad MR, Sullenberger MT, Ohlson EW, et al. (2014) Response of accessions within tomato wild species, Solanum pimpinellifolium to late blight. Plant Breed 133: 401-411.
- Foolad MR (2015) Genome mapping and molecular breeding of tomato. Int J Plant Genomics 2007: 1-52.
- Worku M, Sahela S (2018) Review on disease management practice of tomato wilt caused fusarium oxysporum in case of Ethiopia. J Plant Pathol Microbiol 9: 1-4.
- Cohen S, Antignus Y (2014) Tomato yellow leaf curl virus: A whitefly borne geminivirus of tomatoes. Adv Dis Vect Res 10: 259-288.
- James CW (2011) Estimated losses of crops from plant pathogens. In: Handbook of pest management in agriculture 1: 79-94.
- Fekadu M, Dandena G (2016) Status of vegetable crops in Ethiopia. Uganda J Agric Sci 12: 26-30.
- Green SK, Kim JS (2018) Characteristics and control of viruses infecting peppers: A literature review asian vegetable research and development centre. Technical Bulletin No. 18: 1-60.
- Jones MD, Smith SE (2004) Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? Canad J Bot 82: 1089-1109.
- Sun X, Nielsen MC, Miller JW (2012) Bacterial spot of tomato and pepper. Plant Pathology Circular No. 129 (Revised).
- Temam Hussien (2016) Disease of vegetable crops and their importance in hararghe, eastern, Ethiopia. paper presented at the inaugural conference and 3rd national horticultural workshop: 27-30.
- ARDO (2008) Agriculture and Rural Development Office of the Regions climatic report.
- IPMS (2007) Analysis of production costs, market opportunities and competitiveness of Desi and Kabuli chickpeas in Ethiopia.
- Sharma PD (2011) (edn). Plant Pathology a textbook for university students. Rastogi Publications, India: 224-263.
- Shankar R, Harsha S, Bhandary R (2014) A practical guide to identification and control of tomato diseases. Tropica Seeds.
- Nowicki M, Foolad MR, Nowakowska M, et al. (2013) Potato and tomato late blight caused by Phytophthora infestans: An overview of pathology and resistance breeding. Plant Dis 96: 4-17.
- Hiskias Y, Lesemann DE, Vetten HJ (2008) Occurrence, distribution and relative importance of viruses infecting hot pepper and tomato in the major growing areas of Ethiopia. J Phytopathol 147: 5-11.
- Ristaino JB (2011) Influence of rainfall, drip irrigation, and inoculum density and the development of Phytophthora root and crown rot epidemic disease and yield in bell pepper. Psychopathology 81: 132-137.
- Gudero G, Hussien T, Dejene M, et al. (2018) Integrated management of tomato late blight [Phytophthora infestans (Mont.) de bary] through host plant resistance and reduced frequency of fungicide in arbaminch areas, Southern Ethiopia. J Biol Agriculture and healthcare 8: 94-109.
- Bakonyi J, Heremans B, Jamart G (2012) Characterization of phytophthora infestans isolates collected from potato in flanders, Belgium. J Phytopathol 150: 512-516.
- Abraham Tadesse (2009) Increasing crop production through improved plant protection, Plant Protection Society of Ethiopia (PPSE), PPSE and EIAR, Ethiopia.
- Central Statistics Agency (2016) Statistics agency agricultural sample survey 2015/2016. Statistical Bulletin. Addis Ababa, Ethiopia 578: 131.
- Girma Tegegne, Yusof Keder (2009) Enhancing and demonstrating appropriate vegetable production practices in the central rift valley of Ethiopia. 8: 131.
Corresponding Author
Desalegn Negasa Soresa, Department of Plant Science Plant breeding section, Faculty of Agriculture, Wollega University, Ethiopia, Tel: 0913394181
Copyright
© 2022 Soresa DN. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.