Receptor Kinases: A Sophisticate Protein Network to Maintain Plant life Continuity. An Update
Abstract
To govern the plant growth, defense from pathogen attack, to tolerate the harsh conditions and reproduction, plant employ very sophisticated and highly effective machinery in the form of receptor kinases (RKs) which make them adoptable in the diverse land environment.
Different classes of RKs including receptor like cytoplasmic kinases (RLCKs) unique in their functionality are of paramount importance in transmembrane signaling; and have emerged as a major class of signaling proteins, which regulate the normal plant life continuity.
For their central importance in vital functions of plants, RKs have brought overwhelming intention of researchers across the globe and have been considered as most flashpoint of research to investigate, by the plant biologists. We intended to provide an overview of current progress in understanding the associations of RKs with plant cellular responses to regulate the downstream signaling nodes to orchestrate a complex array of defense against microbial pathogens; to face any drought condition and how RKs are associated with signal peptides to coordinate the plant growth, pollen tube guidance and embryonic pattering. Following the rising attention of scientific community to explore the physiology and anatomy, these central players of plant life regulation in depth, we believe that this systematic review will provide an update on research about RKs and will be a source of blooming growth of research for beneficial knowledge to deal the diseases of plants.
Keywords
RKs, RLCKs, Cellular signaling, Plant immunity, Growth, Environmental stress, Reproduction, Future investigations
Abbreviations
TMRK: Transmembrane Receptor Kinase; RLPs: Receptor Like Proteins; LRR: Leucine Rich Repeat; EGF: Epidermal Growth Factor; USP: Universal Stress Protein Domains; GPI: Glycosylphosphatidylinositol; ABA: Abscisic Acid; MAPK: Mitogen Activated Protein Kinase; CDK: Cyclin Dependent Kinases; RLP42: Receptor Like Protein 42; PGs: Polygalacturonases; GRP :Glycine Rich Proteins; PRR: Pattern Recognition Receptors; PTI: Pattern Rrecognition Receptors-Triggered Immunity; MAMP: Pathogen-Associated Molecular Patterns; PAMP-TI: Pathogen Associated Molecular Patterns-Triggered Immunity
Introduction
To convey the external environmental stimuli received by receptors to central house of cell: nucleus, there is a huge traffic of cytoplasmic components in which special proteins named receptor like protein kinases (RLKs) are ones. Years of research have witnessed the critical role of RLKs in maintaining the normal plant cell functions such as replication, protein synthesis, defense signaling, growth and development and stress survival by specific protein signaling [1]. In plant biology, on the basis of their functions and presence around (inside or To convey the external environmental stimuli received by receptors to central house of cell: nucleus, there is a huge traffic of cytoplasmic components in which special proteins named receptor like protein kinases (RLKs) are ones. Years of research have witnessed the critical role of RLKs in maintaining the normal plant cell functions such as replication, protein synthesis, defense signaling, growth and development and stress survival by specific protein signaling [1]. In plant biology, on the basis of their functions and presence around (inside or
Interestingly, it is found that most of the RLK superfamily members don’t have any extracellular domain but contain endoplasmic domains to regulate vital functions of cell and categorized as distinct subfamily known as receptor like cytoplasmic kinases (RLCKs) [4, 6]. In the light of recent studies on RKs, it has been established that plants possess hundreds of these membrane localized receptor kinases which are involved in mediating cellular responses and to develop response against external stimuli [7]. Going through chemistry of RKs proteins, it has been found that they are rich in serine/threonine protein kinase that have short N and C terminals and several highly conserve subdomains in their peptidal chain [8]. Proteomics sequencing study reveals that about 70% of cytoplasmic domains of RLKs and RLCKs are different; however, there is 30% similarity in the Leucine rich repeat (LRR), epidermal growth factor (EGF and universal stress protein domains (USP) [9-11]. Translated from ribosomes, RLCK are predicted to localize in the plasma membrane after refined by the process of post-translational modifications. In these modifications, N-terminal myristoylation is the key step in which addition of myristic acid on the N terminal motif take place [12]. Later on myristic acid facilitates the anchoring of domains to membrane [13,14]. RLCKs have advantages of their localization on membrane by developing better interactions with other membrane proteins and RLKs to function in signalling in response to any extracellular cues perceived by RLKs [15,16]. Literature mining reveals that Arabidopsis, rice, soybean and corn have 147, 379, 267 and 175 RLCKs domains respectively [4,6].
In term of secondary literature, the aim of present review is to summarize the outcomes of recent research on the key role of RLCKs in regulating the diverse process in plants. Considering the essential functions of RLCKs in maintaining normal plant life continuity, this review highlights the unexplored directions in RLCKs functioning and will provide a source blooming growth of research to explore further in-depth about RLCKs physiology, mechanisms of action and role in immunity, stress and resistance.
Method and Literature Mining Strategy
Relevant articles were selected against the specific keywords as per outlines of study by using the different search databases: PubMed, Google, Google Scholar and Research Gate. In addition to the relevancy of the title and abstracts, articles were selected for inclusion based on the year of publication, which was mainly between 2016 and 2021. However, little older publications and data reports were also cited to strengthen the background of the subject. All selected articles are cited accordingly.
RKs and Plant Immune Response
Although plants lack specialized immune system, however they show strong immunological responses against any pathogen attack as like animal innate immune system and it relies on both cell surface and cytoplasmic immune receptor proteins [17]. Pathogens exposure to a plant life is deadly detrimental to its vegetation and reproduction. Different pathogens attack on plants and try to dominate the whole plant defense system by secreting different types of poisonous chemicals: alkaloids, steroids, hormones and enzymes. These secretory chemicals destroy and digest plant cells and structures and help pathogen to penetrate the plant [18]. This pathogen exposure leads to activation of plant defense system in which plant immune system starts to eliminate the pathogens by disposing off their poisonous attacking chemicals [19]. In this defense response process, there are several components of plant immune system, which interact with each other and generate collective response at cellular level to organismic level. In cellular response, plant cell rich in several types of receptors, RLCKs contribute mainly and respond to any malfunction of cellular activities in a plant cell. These receptors are unique and super specific to convey the signal like pathogen attack to inside of cells to release counter response molecules for defense [20,21].
An increasing number of RKs and RLPs are found to be function as PRRs and monitor the immunological response patterns initiated by the entry of any foreign pathogen and release of defensive chemicals from the host [22-25]. These PRRs actions lead to trigger the immune system signaling that is characterized by transient calcium influx, radioactive oxide species (ROS) production and activation of mitogen activated protein kinases (MAPK), cyclin dependent kinases (CDKs) and transcriptional programming to stop the pathogen progression [26,27]. For example, receptor like protein 42 (RLP42) in Arabidopsis thaliana, senses the attack of fungal endo-polygalacturonases (PGs) pathogens by its pg9 (At) conserved fragment of 9-amino-acids; and starts instituting a complex defense immunological response unit with other associated co-receptors such as suppressor of BIR1(SOBIR1) and somatic embryonic receptor-like kinase (SERK) [28]. Similarly, specific receptors in resistant tomato plant cells sense the entry of Cuscuta and Crip21 pathogens by their short peptide epitope of glycine rich protein (GRP) on cell wall [29]. Importance of those pattern recognition receptors (PRRs) mediated immune receptors of RLCK in sunflower plant was seen in gene HaOr7 encoding a LRR receptor like kinases. It confers resistance against Orobanche Cumana which is a parasite plant living on roots of sunflower. It has been observed that in susceptible lines, the protein LRR is without transmembrane and kinase domains [30]. Considering importance and physiological functions of these cytoplasmic kinases, RLCKs are consider as flash point of research for better understanding about these pattern recognition immunological responses and the signaling pathways were carried out from different researchers.
In different transcriptomic analysis on Arabidopsis to observe the amplitude and timing of early immune responses, Wan observed that RLP23, a receptor of the NLP (Necrosis-and ethylene-inducing-like protein peptides in Arabidopsis thaliana activation, suppressed the LRR receptor-like serine (FLSE2). It showed that genes regulated by RLP23 are only a fraction of those genes, which express differentially on the activation of FLS2 [31].
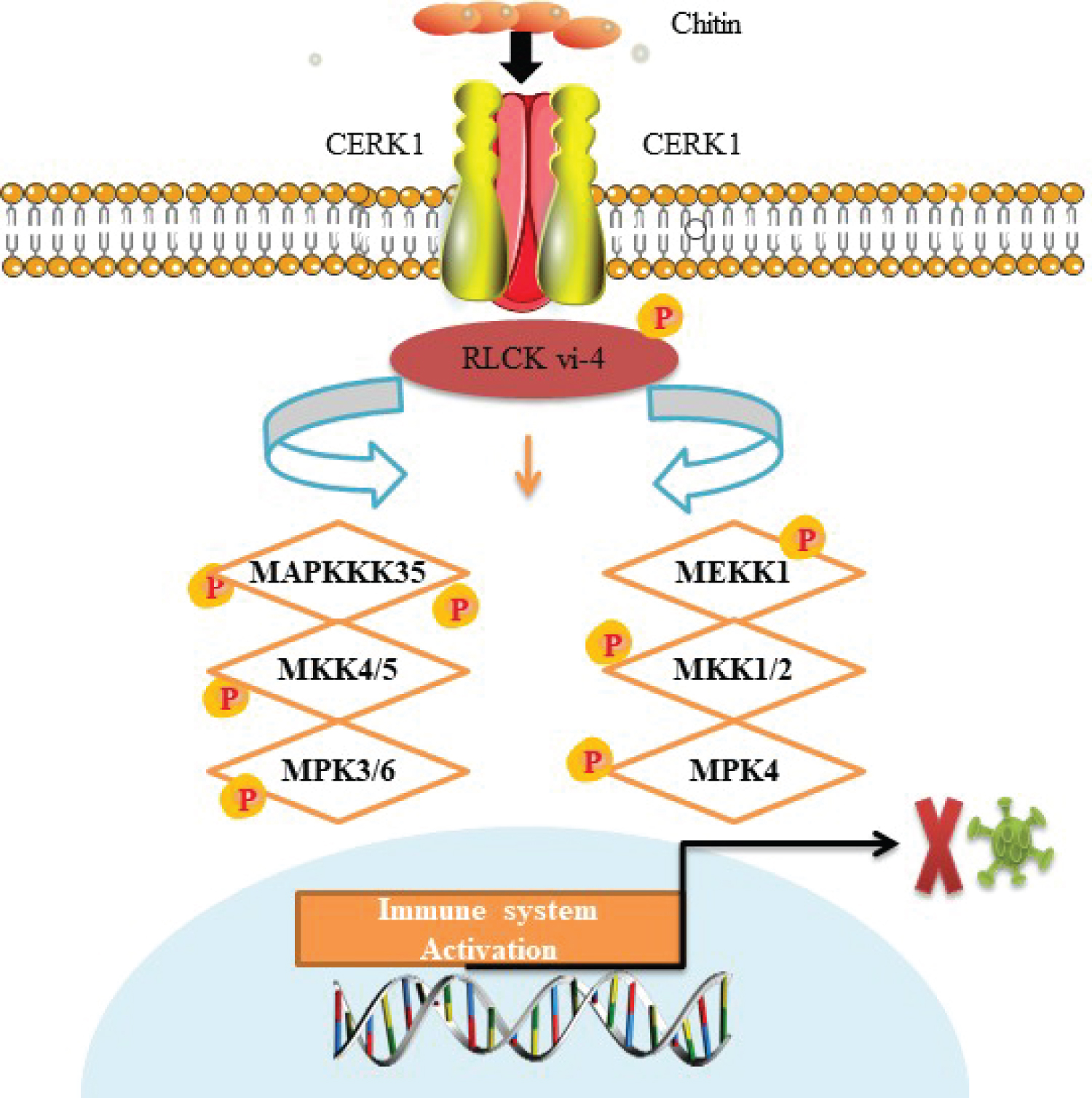
In another study, it was demonstrated that wall associated kinase 1 in Solanum lycopersicum (SlWak1) plays an important role in pattern recognition receptors-triggered immunity (PTI) through transcription factor interference [32]. Recently in transcriptomic analysis, it has been found that in RP-type immune receptors regulation, EDS1, a conserved lipase like protein act as a signal transducer between intracellular NLR receptors activated by pathogen and transcription function and causes host cell death [33,34]. Mia found that CERK1, a lysine motif receptor-kinase plays important role in signaling chitin elicitor in Arabidopsis thaliana. CERK1 is a cell membrane binding protein having three Lysine motifs in extracellular domain and one serine/threonine kinase domain inside cell plasma with auto phosphorylation/myelin activity of protein kinase, which shows that CERK1 has a specific role in pathogen-associated molecular patterns (PAMP) association in plant cells [35,36]. ANXUR receptor-like kinases are cell surface-resident pattern recognized receptors that help plant to respond during microbial attack with nucleotide binding domain having leucine rich repeat proteins. It was identified that ANXUR1, a malectin-like domain having receptor like kinase together with its ANX2 negative homolog in Arabidopsis thaliana [37]. Other aspect of these immunological response explain that, to counter the pathogen mediated pathogenesis, plants have evolved intracellular immune receptors, which are nucleotide-binding leucine- rich repeat domain-containing receptors (NLRs), to detect cytoplasmic effector activity and trigger powerful immune responses [38].
In early days of research on RLCK, findings of numerous supportive studies have established the facts about the essential contribution of these cytoplasmic kinases in pathogen associated molecular patterns-triggered immunity (PAMP-TI). These findings helped in developing the basic understand about function and ways of action and revealed the cascaded of signal transduction, activation of cellular kinase proteins traffic to generate immune response against the entry of any pathogen [39-43]. They proved that these cytoplasmic kinases, especially PBL13 kinase oppositely work in regulation of plant innate immunity under pathogen attack of bacteria and show association with RBOHD before the perception of pathogens indicating that PBL13 causes inhibition of inappropriate defense system activation when pathogen is absent. Figure 1, gives figurative description of RLKs role in plants immunity against pathogen attack.
RKs and Plant Growth/ Development
There are different receptor like cytoplasmic kinases that show their capability in embryo germination and help plant in cell division, differentiation and other chemical as well as physical mechanisms through their specialized activation/monitoring systems [43]. This process start with the seed germination process where a little embryo breaks dormancy and stored food is also converted into useable energy source for its growth activities. It has been found that a mutation of CRINKLY4 gene, which codes for putative receptor kinase in signaling during cell differentiation, could cause abnormality in leaf epidermis such as cell shape and size in developing endosperm. It reveals the role cr4 gene involvement in controlling the growth of plants [44]. In another study, it was evaluated that a receptor kinase CLAVATA (CLV1) in Arabidopsis thaliana contains an extracellular domain consisting of 21 leucine rich repeats, one cytoplasmic kinase domain and a transmembrane domain which regulate the identity and behavior of stem cells during plant vegetative shoot growth in shoot meristem, florescence meristem and inflorescence meristem [45]. Meristems have a common domain in central zone and peripheral zone that help them in organization center signaling in cell differentiation and characterization in new organ formation. The homeodomain expression factor WUSCHEL (WUS) in organization center is necessary for regulation of stem cells of central zone whose mutation leads to expansion of central zone of meristem causing a band-like stem, flowers and floral organs such as club-shaped siliques [46]. CLV gene plays central role in signal transduction by CLV1 receptor kinase. CLV1 receptor kinases activated by small ligand binding molecules CLV3, which is secreted by central zone stem cells. Findings reveal that mutation in anyone of these mentioned genes can cause defects in development of stem cells and hence, control the growth of plant [47]. CLV3 is the member of endosperm surrounding region (CLE) gene family, which codes for small amino acid peptides having a conserved C-terminal sequence and CLE motif containing specific receptors for signaling. Molecular analysis reveals that overexpression of CLE peptides leads to retardation of root growth and premature root meristem differentiation that indicates a pathway related to CLV [47,48]. Similarly, it has been reported that a gene family member of cellulose synthase CESA1/RSW1 regulates the growth of root and shoot cell walls and determines cell shape during cell division and expansion in embryo cells of Arabidopsis [49]. During research on RLCKs function in rice callus, Sun, et al. in 2015 found that a temperature sensitive 290-kDa complex OsSec18 in rice embryo cells facilitating a conserved ATPase protein, plays important role in plant height and 1000-grain seed weight. They also found that Os60sP0 is the component of OsSec18 that plays role in vacuolar morphology by production of fusion proteins in rice endosperm [50]. While working on non-cell-autonomous proteins (NCAPs) role in cell-to-cell movement of CmPP16-1 in pumpkin (Cucurbita maxima), researcher reported that pumpkin phloem sap movement is facilitated by NCAPP1 role in posttranscriptional modification of phloem sap proteins [51]. Furthermore, they found that a glutathione S-transferase (GST)-CmPP16-1 fusion protein system consisted of 36 amino acid peptide is required for cell-to-cell movement capability of glycosylation precognitive motif for phosphorylation in consistence with GST proteins in plasmodesmata transport [51,52]. During carotene biosynthesis in Arabidopsis thaliana, there are some rate-limiting enzyme known as phytoene synthase that regulates carotene biosynthesis by its role in posttranscriptional modification of OR (ORANGE) protein that regulates chromoplast differentiation [53]. If At-OR is overexpressed then amount of biological active phytoene synthase is also increased which demonstrates the regulation of carotene biosynthesis [54]. During research on chloroplast biosynthesis regulation in maize, researchers found that ZmRH3 in maize and AtRH3 in Arabidopsis plays important role in binding with two mitochondrial RH proteins (PMH-1 and PMH-2) which function in splicing of introns. Additionally, they also found that AtRH3 null mutation caused death of embryo and weak expression of this protein produced pale green plant by regulation of chloroplast biosynthesis 196 [55,56].
RKs and Stress Conditions
During its life cycle, a plant faces more biotic as well as abiotic stress from its environment than any other organism because it cannot move or act physically in response to any stress [57]. A plant at ground faces high and cold temperature, drought, water logging, ultraviolet radiations, salinity & alkalinity, chemical hypoxia and lowering and some physical damages from animals, birds and insect pathogens [58]. As like response to pathogen attack, plants also response to any stress either in the form of producing different chemical products or proteins that work to save the plant from damage [59-61]. It has been evaluated that these secreted enzymes working for the plant during stress condition are known as universal stress proteins. Their structures shared similarity with UspA, UspC, UspD, UspF, UspG but belong to different subfamilies [62,63] and help the plants to survive in extremely unfavourable conditions. Findings of different studies demonstrate that during unbalanced nutrient availability, high or low pH, heavy metals stress or some other harsh conditions, plants enhance the production of UspA that is serine/threonine coded protein, which controls the phosphorylation process [62,64].
To cope with drought conditions in G. hirsutum, a gene NCED (9-cis-epoxycarotenoid- deoxygenase) regulated the production of abscisic acid (ABA) which in turn regulate the physiological function in the prevailed condition manage the drought condition [65]. As like, NCED, DELLA protein kinase is responsible for signaling and controlled production of gibberellic acid under stress conditions and regulate other physiological functions [66,67]. In published literature, studies reported that during stress conditions, there are different receptor kinases which sense signal from environment and sends directions for the production o enzyme proteins which can help plant to survive, however the number and types of genes which code for these kinase proteins and their way of response to environment varies from plant to plant [68-71]. Rice plant shows special mechanism to absorb the stress condition by the expression of different OsRLCKs, which help plant during stress, immunity and reproduction. Most of these receptor-like cytoplasmic kinases work for plant survival especially during growth and developmental stages and in response to abiotic stress QTLs [72-74] It was reported that in transgenic Arabidopsis plants OsSAP11 and OsRLCK253 (obtained from rice) help the plant in drought and salt stress through signaling pathway by effecting many endogenous genes [75]. Ambavaram, et al. in 2014 found that a receptor like cytoplasmic kinase known as GUDK (growth under drought kinase) by signaling through phosphorylation and activation controls the expression of OsAP37 under drought stress [76].
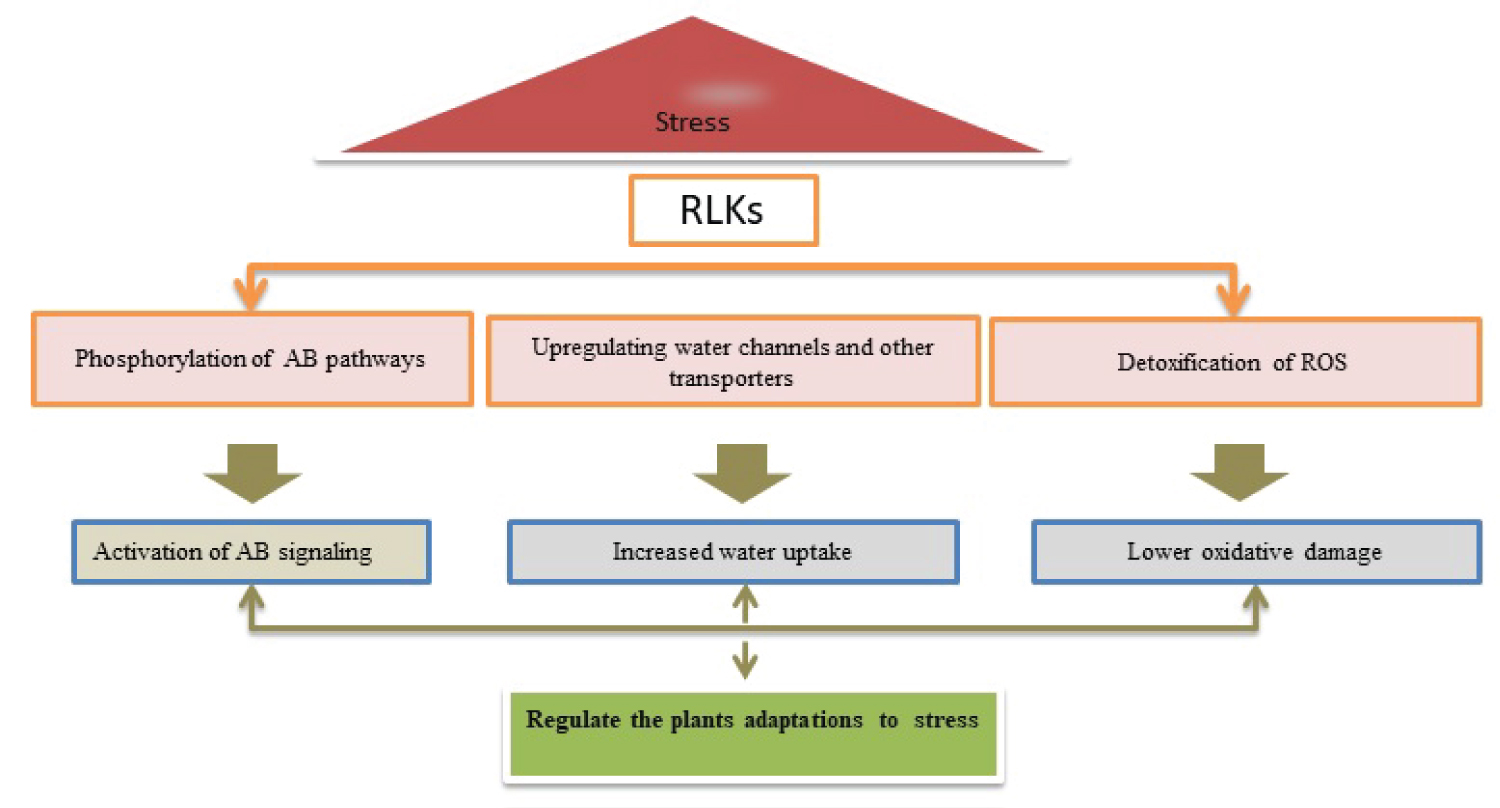
In rice, a receptor-like cytoplasmic kinase XCRK increases the antioxidant capacity and Xanthomonas oryzae pv .oryzicola (Xoc) tolerance by production of ABA [77]. Figure 2 demonstrates the mechanism of RKs proteins to regulate the different stress conditions and support normal life continuity in plants.
RKs and Plant Reproduction
For the continuity of life, in mechanism of sexual reproduction in plants, gametes fusion of male and female parts of same (monoecious) or separate (dioecious) plants take place by the different membrane receptor-like kinases and mediate the process of fertilization to zygote and embryo formation [78]. There is increased size of evidence about the role of receptor kinases in the reproduction of plants. Any kind of abnormity in these specific kinase proteins may halt the process of gametes formation, fertilization and hence, determine there production of plants. Recent findings revealed that a pollen receptor-like kinase1 PRK1 having conserved amino acids similarity with 69-kD protein found only on pollen microsomal membrane is compulsory for normal development of pollen and growth of pollen tube [79,80]. Researchers further elaborated that development of pollen is a highly coordinated and complicated biological process that involves different receptor like kinases, has special function in signal transduction in an organized way during pollen development and pollen tube growth in Petunia inflate [81-82]. A number of proteins and signaling molecules are involved in regulation of pollen development and any mutation of genes results in the formation of defective proteins and signaling molecules along with some environmental factors leading to male infertility [83,85]. During pollen production there are many genes, which code specific proteins and enzyme kinases responsible for health and viability of pollens during pollination until fertilization [37,85]. Some genes only express during specific times of plant development such as in maize ZmSTK gene family coding for serine/threonine enzyme kinase express only during pollen formation and its mutation causes severe protein disruption in pollen development [86]. Gene ontology (GO) functions and molecular analysis show that ZmSTK1 & ZmSTK2 mutant paralogs are expressed in pollen development and germination and cause damage during pollen transmission. Zmstk1 single mutant causes less damage as compared to double mutant and zmstk2 even has less effect on pollen damage but when both zmstk1 & zmstk2 combine, zmstk2 acts as enhancer of zmstk1 expression and damage is so severe that double mutant pollens cannot survive for fertilization and likely, no seeds are formed in offspring [86-89].
Wang, et al. in 2017 while working on maize pollen development reported that ZmSTK2-USP promoter gene is expressed only in mature pollens but not in any other floral or vegetative tissue. ZmPti1a in maize expresses only in pollen development and present only on plasma membrane of pollen that plays an important role in signaling pathway [90,91]. In Arabidopsis thaliana there are more than 23 different receptor like kinases that express during development of pollens and growth of pollen tube [79]. Receptor like cytoplasmic kinases LIP1 and LIP2 working for the pollen tube guidance into the micropyle during pollination and in case of any mutation the pollen tube cannot be guided into the micropyle and lead to retardation of fertilization [92]. While working on pollen tube guidance into the female gametophyte, there are findings which reveal that there are certain RLCKs which are involved in pollen tube development and guidance [93]. In coordination with paralogs of female factors, FERONIA/SIRENE, inhibits the rupture of pollen tube before reaching its destination of fertilization in synergid cell. Any mutation in these genes lead to pollen’s failure to survive and halted the most important process of fertilization due to lack of signaling proteins required for reception [94,95]. Homologs AtIPK2α & AtIPK2β proteins are required in process of embryogenesis. In Arabidopsis thaliana, there are two closely related homologs AtIPK2α & AtIPK2β, which were observed to take part in auxiliary shoot branching, root growth and during abiotic stress response. But recently in 2015 Zhan, et al. found that they also play role in development of pollens, pollen tube guidance and in process of embryogenesis [96,97].
Glycosylphosphatidylinositol (GPI) has equal importance in both kingdom Animalia and plantae. It is well evaluated that in tomato plant, GPI serves as a transmembrane anchoring domain for protein binding that is produced in endoplasmic reticulum by additional monosaccharide, fatty acids and some phospho-ethanolamine attachment. In case of any deficiency of GPI production, same as in animals and microbes, the embryogenesis and cell division is blocked leading to embryo lethality and blocked cell growth in plants [98]. In Arabidopsis thaliana, SETH1 and SETH2 sister genes encode for two conserved proteins are involved in several vital functions like GPI biosynthesis, cell wall synthesis, shaping, intracellular signaling and pollen tube growth. In gremlins, genetic testing reveals that mutant seth1 and seth2 lead to inhibition of pollen function and pollen transmission resulting in reduction of pollen tube growth, pollen germination and cellulose deposition along with other irregularities in metabolism [99,100].
LBD (Lateral organ boundaries domains) are some proteins responsible for regulation of biological processes of development of lateral organs in plant and morphogenesis, immune response, regeneration and development of pollen [101,102]. While working on rice heading date, it has been reported that in rice, OsLBD37 & OsLBD38 are two homologous proteins, convoluted in regulation of pollen heading date [103]. The overexpression of these two proteins localized in nucleus differently lead to delay in heading date and increase in yield by suppression of expression of Hd3a and RFT1 florigen genes, which regulate the heading date Ehd1 [104]. Li, et al. in 2017 while working on Arabidopsis anther cell reported that a somatic embryogenesis receptor-like kinase1 SERK1 and SERK2 and leucine rich repeats receptor like kinases play role in cell differentiation during anther development in floral regions [105].
As most suitable model in plant sciences, In 2017, while working on growth of pollen tube and root tip growth in Arabidopsis, Schoenaers and his co-workers found that ERULUS (ERU) gene is expressed only in pollen tube and root hairs, codes for a receptor like kinase that played special role in pollen tube growth. Furthermore, it also has a special link with Ca+2 concentration as pollen tube growth is directly related to calcium availability and in case of calcium deficiency the pollen tube growth and fertilization in ovules is also decreased about 37% as compared to wild type [106].
In following years, in Arabidopsis plant, it was explored that two receptor like kinases BUPS1 and BUPS2 along with their peptide ligands RAFL4 and RAFL19 interacting with each other are shown to play a special role in cell-to-cell signaling during fertilization. They are expressed during pollen tube growth and responsible for pollen tube integrity until safe fertilization. BUPS1 & BUPS2 make interaction with ANXUR1 and ANXUR2 through extracellular domain and bind with RALF4 and RALF19 [107].
Conclusion and Future Prospective
RKs are key regulators to plants adaptation to environment, reproduction, growth and defense. Going through literature of years of research on RKs, it has been well understood that, these proteins are especially designed to receive their ligand and plays cameral role in regulating the signaling mechanisms of defense strategy, sexual reproduction, and growth and stress conditions. A growing literature also demonstrates that RKs share similar structural organization with each other to make adopting complexes like SERKs and SOBIR and expand the RKs pathways involvement by using the RLCKs as signal transducers to adopt common signaling nodes that link them to downstream signaling cascade of cellular response level. Current understandings on the RKs role in plant innate immunity studies indicate that RLCKs regulate the defense mechanism through the variety of signaling nodes including G protein and orchestrate a variety of immune responses according to type of pathogen attack. In conclusion, we stated that, nature granted the land plants an evolutionarily evolved special cellular protein network in the form of RKs to regulate signal peptides to coordinate the growth, sexual reproduction and develop downstream signaling nodes to orchestrate a complex array of defense against microbial pathogens or to face any drought condition. However, still great deal is remaining to connect the inadequate knowledge concerning how this sophisticated machinery receives stimuli from external world and performs operation of RLCK-dependent signaling. Advanced level studies are needed to explore the risk factor of RKs redundancy in crucial cellular signaling pathways and to develop better understanding about the substrates of these proteins especially RLCKs. To enable the plant to cope the rising pathogen burdens, we are in urgent need to identify new PRRs by ever-expanding genome information from both plant and microbes; we need to unravel the immune receptors repertoire, their corresponding PAMP and clear understanding about the host-pathogen evolution. Furthermore, combining the biochemistry and protein- genomic analysis of mutants in RKs, pathways will be needed to evaluate the impact and difference of expression in those phenotypes.
Future investigations must focus on the expression of RKs in specially treated conditions to elucidate the RKs ligands and substrates as well to explore the activation of different signaling nodes and the way of regulating downstream cellular signaling will significantl advance our understanding about the role of RKs in a plant life.
Acknowledgments
The authors gratefully acknowledge the assistance and motivation energy of Professor Xuebin Zhang to accomplish this manuscript.
Funding
The authors also appreciate the financial support from the National Natural Science Foundation of China, Grant/Award Numbers: 31970323, 31871517, 31601212.
Authors’ contributions
Ansar Javeed conceived the concept of the review and coordinated the project; Xuebin Zhang approved and supervised the writing process; Mehak Sarfraz reviewed literature, extracted data and drafted the manuscript with Ansar Javeed; Muhammad Waqar Khan and Yang Wenqi reviewed the methodology and data analysis; Maqsood Ahmed reviewed and provided intellectual input on the review. All authors have read and approved the final version of the manuscript.
References
- Chen X, Yanglin Ding,Yongqing Yang, et al. (2021) Protein kinases in plant responses to drought, salt, and cold stress. Journal of integrative plant biology 63: 53-78.
- Restrepo-Montoya D, Robert Brueggeman, Phillip E. McClean, et al. (2020) Computational identification of receptor-like kinases “RLK” 375 and receptor-like proteins “RLP” in legumes. BMC genomics 21: 1-17.
- Fan M, Wenjuan Ma, Chen Liu, et al. (2018) Evolution and expression characteristics of receptor-like cytoplasmic protein kinases in maize, rice and Arabidopsis. Int J Mol Sci 19: 3680.
- Shiu SH, Wojciech M Karlowski, Runsun Pan, et al. (2004) Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant cell 16: 1220-1234.
- Nguyen QN, Yang-Seok Lee, Lae-Hyeon Cho, et al. (2015) Genome-wide identification and analysis of Catharanthus roseus RLK1-like kinases in rice. Planta 241: 603-613.
- Vij S,JitenderGiri, Prasant Kumar Dansana, et al. (2008) The receptor-like cytoplasmic kinase (OsRLCK) gene family in rice organization, phylogenetic relationship, and expression during development and stress. Mol plant 1: 732-750.
- Wolf S (2017) Plant cell wall signalling and receptor-like kinases. Biochem J 474: 471-492.
- Hanks SK, THunter, et al.(1995)The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. The FASEB journal 576-596.
- Bi G,Guozhi Bi, Zhaoyang Zhou, et al.(2018)Receptor-like cytoplasmic kinases directly link diverse pattern recognition receptors to the activation of mitogen-activated protein kinase cascades in Arabidopsis. The Plant Cell 30: 1543-1561.
- Liang X and JM Zhou (2018) Receptor-like cytoplasmic kinases: central players in plant receptor kinase–mediated signaling. Annu Rev Plant Biol69: 267-299.
- Elangovan A, ElangovanAllimuthu, Monika Dalal, et al.(2020)Characterization of atypical protein tyrosine kinase (PTK) genes and their role in nabioticstress response in rice. Plants 9: 664.
- Maurer-Stroh S, B Eisenhaber and F Eisenhaber(2002) N-terminal N-myristoylation of proteinsrefinement of the sequence motif and its taxon-specific differences. J MolBiol317: 523-540.
- Podell S and M Gribskov(2004) Predicting N-terminal myristoylation sites in plant proteins.BMC Genomics5: 1-15.
- Majeran W, Jean-Pierre Le Caer ,LalitPonnala, et, al.(2018) Targeted profiling of Arabidopsis thaliana subproteomes illuminates coand posttranslationally N-terminal myristoylatedproteins. Plant Cell 30: 543-562.
- Lin W,Xiyu Ma, Libo Shan, et al.(2013)Bigroles of small kinases: The complex functions of receptor-like cytoplasmic kinases in plant immunity and development. J Integr Plant Biol 55: 1188-1197.
- Yamaguchi K,Kenta Yamada, Kazuya Ishika, et al.A receptor-like cytoplasmic kinase targeted by a plant pathogen effector is directly phosphorylated by the chitin receptor and mediates rice immunity. Cell Host Microbe 13: 347-357.
- Dodds PN and JPRathjen(2010) Plantimmunity towards an integrated view of plant–pathogen interactions. Nat Rev Genet 11: 539-548.
- Doughari J (2015) An overview of plant immunity. J Plant Pathol Microbiol 6: 414 10.4172.
- Pandey D, Rajendran SRCK, Manu Gaur, et al. (2016) Plant defensesignaling and responses against necrotrophic fungal pathogens. Journal of Plant Growth Regulation 35: 1159-1174.
- Gouveia BC, Iara P Calil, João Paulo BM, et al.(2017) Immune receptors and co-receptors in antiviral innate immunity in plants.Front Microbiol 7: 2139.
- Sun L and J Zhang (2020) Regulatory role of receptor-like cytoplasmic kinases in early immune signaling events in plants. FEMS Microbiol Rev 44: 845-856.
- Couto D, Roda Niebergall, Xiangxiu Liang, et al. (2016) The Arabidopsis protein phosphatase PP2C38 negatively regulates the central immune kinase BIK1. PLoS Pathog 12: 1005811.
- Gust AA, R Pruitt, T Nürnberger, et al. (2017) Sensing danger key to activating plant immunity. Trends Plant Sci 22: 779-791.
- Tang D, G Wang, J-M Zhou, et al.(2017) Receptor kinases in plant-pathogen interactions more than pattern recognition. Plant Cell. 29: 618-637.
- Yu X, Baomin Feng, Ping He, et al. (2017) From chaos to harmony responses and signaling upon microbial pattern recognition. Annu Rev Phytopathol. 55: 109-137.
- Li L, Yufei Yu, Zhaoyang Zhou, et al. (2016) Plant pattern-recognition receptors controlling innate immunity. Science China Life Sciences. 59: 878-888.
- Qi J, Jinlong Wang, Zhizhong Gong, et al. (2017) Apoplastic ROS signaling in plant immunity. Curr Opin Plant Biol 38: 92-100.
- Zhang H, Changhai Chen, Lulu Li, et al. (2021) A rice LRR receptor-like protein associates with its adaptor kinase OsSOBIR1 to mediate plant immunity against viral infection. Plant Biotechnol J 19: 2319-2332.
- Hegenauer V, Peter Slaby, Max Korner, et al. (2020) The tomato receptor CuRe1 senses a cell wall protein to identify Cuscuta as a pathogen. Nat Commun 11: 1-7.
- Duriez P, Sonia Vautrin, Marie-Christine Auriac, et al. (2019) A receptor-like kinase enhances sunflower resistance to Orobanche cumana. Nat Plants 5: 1211-1215.
- Wan WL, Lisha Zhang, Rory Pruitt, et al. (2019) Comparing Arabidopsis receptor kinase and receptor protein-mediated immune signaling reveals BIK1-dependent differences. New Phytol 221: 2080-2095.
- Zhang N, Marina A Pombo, Hernan G Rosli, et al. (2020) Tomato wall-associated kinase SlWak1 acts in an Fls2-and Fls3-dependent manner to promote apoplastic immune responses to Pseudomonas syringae. Plant Physiol 183: 1869-1882
- Dongus J A and J E Parker (2021) EDS1 signalling At the nexus of intracellular and surface receptor immunity Curr Opin Plant Biol 62: 102039.
- Sun X, Dmitry Lapin, Joanna M Feehan, et al. (2021) Pathogen effector recognition-dependent association of NRG1 with EDS1 and 448 SAG101 in TNL receptor immunity. Nat Commun 12: 1-15.
- Cao Y, Yan Liang, Kiwamu Tanaka, et al. (2014) The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin induced complex with related kinase CERK1. Elife 3: 3766.
- Hammoudi V (2021) The art of passing unnoticed: pathogenic fungi remain incognito thanks to EWCA effectors. Plant Cell 33: 1097-1098.
- Mang H, Baomin Feng, Zhangjian Hu, et al. (2017) Differential regulation of two-tiered plant immunity and sexual reproduction by ANXUR receptor-like kinases. Plant Cell 29: 3140-3156.
- Jones JD, RE Vance, JL Dangl, et al. (2016) Intracellular innate immune surveillance devices in plants and animals. Science 354(6316).
- Sun L, Jun Qin, Kailun Wang, et al. (2017) Expansion of pathogen recognition specificity in plants using pattern recognition receptors and artificially designed decoys. Sci China Life Sci 60: 797-805.
- Bozkurt TO, Sebastian Schornack, Joe Win, et al. (2011) Phytophthora infestans effector AVRblb2 prevents secretion of a plant immune protease at the haustorial interface. Proc Natl Acad Sci U S A 108: 20832-20837.
- Feng F, Fan Yang, Wei Rong, et al. (2012) A Xanthomonas uridine 5'-monophosphate transferase inhibits plant immune kinases. Nature 485: 114-118.
- Lin ZJD, Thomas W H Liebrand, Koste A Yadeta, et al. (2015) PBL13 is a serine/threonine protein kinase that negatively regulates Arabidopsis immune responses. Plant Physiol 169: 2950-2962.
- Komis G, Olga Šamajová, Miroslav Ovecka, et al. (2018) Cell and developmental biology of plant mitogen-activated protein kinases. Annu Rev Plant Biol 69: 237-265.
- Demko V, Eugene Ako, Pierre-François Perroud, et al. (2016) The phenotype of the CRINKLY4 deletion mutant of Physcomitrella patens suggests a broad role in developmental regulation in early land plants. Planta 244: 275-284.
- Jones DS, Amala John, Kylie R VanDerMolen, et al. (2021) CLAVATA signaling ensures reproductive development in plants across thermal environments. Curr Biol 31: 220-227.
- Jha P, SJ Ochatt and V Kumar (2020) WUSCHEL: a master regulator in plant growth signaling. Plant cell Rep 39: 431-444.
- Khan SU, Muhammad Hafeez U Khan, Sunny Ahmar, et al. (2021) Comprehensive study and multipurpose role of the CLV3/ESR-related (CLE) genes family in plant growth and development. Journal of Cellular Physiol 236: 2298-2317.
- Lin H, Wei Wang, Xiugui Chen, et al. (2021) Molecular Traits and Functional Analysis of the CLAVATA3/Endosperm 482 Surrounding Region-Related Small Signaling Peptides in Three Species of Gossypium Genus. Front Plant Sci 12: 1016.
- Daras G, Dimitris Templalexis, Fengoula Avgeri, et al. (2021) Updating Insights into the Catalytic Domain Properties of Plant Cellulose synthase (CesA) and Cellulose synthase-like (Csl) Proteins. Molecules 26: 4335.
- Sun Y, Tingting Ning, Zhenwei Liu, et al. (2015) The OsSec18 complex interacts with P0 (P1-P2) 2 to regulate vacuolar morphology in rice endosperm cell. BMC plant boil 15: 1-9.
- Ham BK and WJ Lucas (2017) Phloem-mobile RNAs as systemic signaling agents. Annual rev plant boil 68: 173-195.
- Nawaz MA, Muhammad Azher, Chen, et al. (2018) Improving vanadium stress tolerance of watermelon by grafting onto bottle gourd and pumpkin rootstock. Plant Growth Regulation 85: 41-56.
- Zhou X, Ralf Welsch, Yong Yang, et al (2015) Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc Natl Acad Sci U S A 112: 3558-3563.
- Park S, Ho Soo Kim, Young Jun Jung, et al. (2016) Orange protein has a role in phytoene synthase stabilization in sweetpotato. Sci Rep 6: 33563.
- Ahsan N, Mingjie Chen, Fernanda Salvato, et al. (2017) Comparative proteomic analysis provides insight into the biological role of protein phosphatase inhibitor-2 from Arabidopsis. J Proteomics 165: 51- 60.
- Nidumukkala S, Lavanya Tayi, Rajani Kant Chittela, et al. (2019) DEAD box helicases as promising molecular tools for engineering abiotic stress tolerance in plants. Crit Rev Biotechnol 39: 395-407.
- Nguyen HC, Kuan-Hung Lin, Shin-Lon Ho, et al. (2018) Enhancing the abiotic stress tolerance of plants: from chemical treatment to biotechnological approaches. Physiol Plant 164: 452-466.
- Pereira A (2016) Plant abiotic stress challenges from the changing environment. Front Plant Sci 7: 1123.
- Kaleem F, Ghulam Shabir, Kashif Aslam, et al. (2018) An overview of the genetics of plant response to salt stress: present status and the way forward. Appl Biochem Biotechnol 186: 306-334.
- Ahanger MA, Nisha Singh Tomar 1, Megha Tittal, et al. (2017) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants 23: 731-744.
- Hirayama T and K Shinozaki (2010) Research on plant abiotic stress responses in the post-genome era: Past present and future. Plant J 61: 1041-1052.
- Chi YH, Sung Sun Koo, Hun Taek Oh, et al. (2019) The physiological functions of universal stress proteins and their molecular mechanism to protect plants from environmental stresses. Front Plant Sci 10: 750.
- Feder ME, DA Parsell and SL Lindquist (2020) The stress response and stress proteins. In: Cell biology of trauma. CRC Press 177-191.
- Cui X, Pingying Zhang, Yafan Hu, et al. (2021) Genome-wide analysis of the Universal stress protein A gene family in Vitis and expression in response to abiotic stress. Plant Physiol Biochem 165: 57-70.
- Pei X, Xiaoyang Wang, Guoyong Fu, et al. (2021) Identification and functional analysis of 9-cis-epoxy carotenoid dioxygenase (NCED) homologs in G hirsutum. International Journal of Biological Macromolecules 182: 298-310.
- Zhu Z, Yang Ding, Jinhong Zhao, et al (2016) Effects of postharvest gibberellic acid treatment on chilling tolerance in cold- stored tomato (Solanum lycopersicum L) fruit. Food and Bioprocess Technology 9: 1202-1209.
- Wang H, Jinjing Pan, Yang Li, et al. (2016) The DELLA-CONSTANS transcription factor cascade integrates gibberellic acid and photoperiod signaling to regulate flowering. Plant Physiol 172: 479-488.
- Lee HY and K Back (2016) Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J Pineal Res 60: 327-335.
- Zhang M, Jianbin Su, Yan Zhang, et al. (2018) Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Current opinion in plant biology 45: 1-10.
- Aranda-Sicilia MN, Yuri Trusov, Natsumi Maruta, et al. (2015) Heterotrimeric G proteins interact with defense-related receptor- like kinases in Arabidopsis. J Plant Physiol 188: 44-48.
- Ramirez-Prado JS, Aala A Abulfaraj, Naganand Rayapuram, et al. (2018) Plant immunity: from signaling to epigenetic control of defense. Trends Plant Sci 23: 833-844.
- Zhang H, Zhai N, Ma X, et al. (2021) Overexpression of OsRLCK241 confers enhanced salt and drought tolerance in transgenic rice (Oryza sativa L). Gene 768: 145278.
- Wang J, Wu G, Peng C, et al. (2016) The receptor-like cytoplasmic kinase OsRLCK102 regulates XA21-mediated immunity and plant development in rice. Plant Molecular Biology Reporter 34: 628-637.
- Sevanthi AM, C Prakash and P Shanmugavadivel (2019) Recent progress in rice varietal development for abiotic stress tolerance. Advances in rice research for abiotic stress tolerance 2019: 47-68.
- Kothari KS, Prasant K Dansana, Jitender Giri, et al. (2016) Rice stress associated protein 1 (OsSAP1) interacts with aminotransferase (OsAMTR1) and pathogenesis-related 1a protein (OsSCP) and regulates abiotic stress responses. Front Plant Sci 7: 1057.
- Ambavaram MM, Supratim Basu, Arjun Krishnan, et al. (2014) Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat commun 5: 1-14.
- Zhang Y, Guo X, Cui Y, et al. (2017) Overexpression of the receptor-like cytoplasmic kinase gene XCRK enhances Xoc and oxidative stress tolerance in rice. Journal of Plant Biology 60: 523-532.
- Dresselhaus T and MA Johnson (2018) Reproduction: Plant parentage a trios. Curr Biol 28: R28-R30.
- Muschietti JP and DL Wengier (2018) How many receptor-like kinases are required to operate a pollen tube. Curr Opin Plant Biol 41: 73-82.
- Takeuchi H and T Higashiyama (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis Nature 531: 245-248.
- Kim MJ, Byeong WookJeon, EunkyooOh, et al. (2021) Peptide signaling during plant reproduction Trends in Plant Science 26: 822-835.
- Chai S, Furong Ge, Sha Li, et al (2016) The journey to glory: receptor-like kinases in pollen tube growth Science Bulletin 61: 827-831.
- Stanley RG and HF Linskens (2012) Pollen: biology biochemistry management. Springer Science & Business Media.
- Chen L and Y-G Liu (2014) Male sterility and fertility restoration in crops. Annu Rev Plant Biol 65: 579-606.
- Pu CX, Yong Feng Han, Shu Zhu, et al (2017) The rice receptor-like kinases DWARF AND RUNTISH SPIKELET1 and 2 repress cell death and affect sugar utilization during reproductive development. Plant Cell 29: 70-89.
- Fan M, Chunyu Zhang, Lei Shi, et al. (2018) Zm STK 1 and Zm STK 2 encoding receptor like cytoplasmic kinase are involved in maize pollen development with additive effect. Plant Biotechnol J 16: 1402-1414.
- Qin X, Wenliang Zhang, Xue Dong, et al. (2020) Identification of fertility-related genes for maize CMS-S via Bulked Segregant RNA-Seq. Peer J 8: e10015.
- Javeed A, Wu S, Huang S, et al. (2019) Agrobacterium-Mediated Gene Transformation Of Pollen Specific Gene Stk1 In Maize (Zea Mays). Appl Ecol Environ Res 17: 11789-11802.
- Javeed A, Fan MX, Shi L, et al. (2017) Isolation and characterization of a pollen specific gene ZmSTK2_USP from Zay mays. Appl Ecol Environ Res 16: 487-494.
- Wang D, HeWang, Muhammad Irfan, et al. (2014) Structure and evolution analysis of pollen receptor-like kinase in Zea mays and Arabidopsis thaliana. Computational biology and chemistry 51: 63-70.
- Wang H, Mingxia Fan, Guohong Wang, et al. (2017) Isolation and characterization of a novel pollen-specific promoter in maize (Zea mays L). Genome 60: 485-495.
- Liu J, Sheng Zhong, Xinyang Guo, et al. (2013) Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol 23: 993-998.
- Higashiyama T and Wc Yang (2017) Gametophytic pollen tube guidance: attractant peptides gametic controls and receptors. Plant Physiol 173: 112-121.
- Ge Z, Tabata Bergonci, Yuling Zhao, et al. (2017) Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signalling. Science 358: 1596-1600.
- Liao H, Renjie Tang, Xin Zhang, et al. (2017) FERONIA receptor kinase at the crossroads of hormone signaling and stress responses. Plant Cell Physiol 58: 1143-1150.
- Freed C O Adepoju and G Gillaspy (2020) Can inositol pyrophosphates inform strategies for developing low phytate crops? Plants 9: 115.
- Sang S, Yao Chen, Qiaofeng Yang, et al. (2017) Arabidopsis inositol polyphosphate multikinase delays flowering time through mediating transcriptional activation of FLOWERING LOCUS C. J Exp Bot 68: 5787-5800.
- Bundy MG, Pawel Z Kosentka, Alaina H Willet, et al. (2016) A mutation in the catalytic subunit of the glycosylphosphatidylinositol transamidase disrupts growth fertility and stomata formation. Plant Physiol 171: 974-985.
- Desnoyer N and R Palanivelu (2020) Bridging the GAPs in plant reproduction: a comparison of plant and animal GPI-anchored proteins. Plant Reproduction 33: 129-142.
- Beihammer G, Daniel Maresch, Friedrich Altmann, et al. (2020) Glycosylphosphatidylinositol-anchor synthesis in plants: a glycobiology perspective. Front Plant Sci 11:611188.
- Xu C F Luo and F Hochholdinger (2016) LOB domain proteins: beyond lateral organ boundaries. Trends Plant Sci 21: 159-167.
- Chen WF, Xiao-Bin Wei, Stephane Rety, et al (2019) Structural analysis reveals a “molecular calipers” mechanism for a LATERAL ORGAN BOUNDARIES DOMAIN transcription factor protein from wheat. J Biol Chem 294: 142-156.
- Li C, Shanshan Zhu, Huan Zhang, et al. (2017) OsLBD37 and OsLBD38 two class II type LBD proteins are involved in the regulation of heading date by controlling the expression of Ehd1 in rice. Biochem Biophys Res Commun 486: 720-725.
- Zhao J, Hongyi Chen, Ding Ren, et al. (2015) Genetic interactions between diverged alleles of Early heading date 1 (Ehd1) 615 and Heading date 3a (Hd3a)/RICE FLOWERING LOCUS T1 (RFT 1) control differential 616 heading and contribute to regional adaptation in rice (Oryza sativa). New Phytol 208: 936-948.
- Li Z, Yao Wang, Jian Huang, et al. (2017) Two SERK receptor-like kinases interact with EMS1 to control anther cell fate 619 determination. Plant physiol 173: 326-337.
- Schoenaers S, Daria B, Alex Costa, et al. (2017) The kinase ERULUS controls pollen tube targeting and growth in 621 Arabidopsis thaliana. Front Plant Sci 8: 1942.
- Zhu L, Liang-Cui Chu, Yan Liang, et al. (2018) The Arabidopsis CrRLK1L protein kinases BUPS1 and BUPS2 are required 623 for normal growth of pollen tubes in the pistil. Plant J 95: 474-486.
Corresponding Author
Ansar Javeed, State Key Lab of Crop Stress Adaptation and Improvement, School of Life Sciences, Henan University, Kaifeng, Henan China.
Copyright
© 2022 Javeed A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.