Iron Stress Testing of Oilfield Produced Waters and Hydraulic Fracturing Fluids in the Bakken
Abstract
Friction reducers are a major additive in almost all water-based hydraulic fracturing fluids. Despite the important role they play in friction reduction during hydraulic fracturing, they pose a veritable threat to the permeability of tight reservoirs upon precipitation. In the industry today, the potential for such precipitation is seldom assessed and as a result, an additional contributor to the formation damage problem has been given little attention. In this work, the sensitivity and affinity of friction reducers to very low concentrations of ferric ions are exploited to provide a qualitative assessment of their propensity to precipitate in hydraulically fractured hydrocarbon reservoirs. Representative samples of produced water and a routinely utilized hydraulic fracturing fluid recipe were spiked with 50 ppm Iron (III) trichloride and incubated for 24 hours in a water bath at 200 degrees Fahrenheit. The results showed that precipitation of friction reducers in fracturing fluid is highly likely at low concentrations of ferric ions. This precipitate formed turned from yellow to brown color over time upon heating. The test showed presence of fragmented/unfragmented Partially Hydrolyzed Polyacrylamide (PHPAM) in the produced water, with varying degrees of FRgel precipitation depending on the processing treatment given to the water used for hydraulic fracturing. Evidence for the presence of partially hydrolyzed polyacrylamide was demonstrated in one of the produced waters while the rest did not produce any discernable precipitates. The results indicate that formations with lower iron concentrations may be more vulnerable to Fe 3+ -PHPAM precipitation than those with higher ferric ion concentration. Therefore, to mitigate formation damage through precipitation of FRs during hydraulic fracturing, a routine iron stress test like the one described herein is required on both hydraulic fracturing fluid and produced water to determine the vulnerability of the reservoir to formation damage by fragmented/unfragmented anionic PHPAMs.
Keywords
Coordination bond, Colloidal particles, Polyacrylamide, Formation damage, Friction reducer
Introduction
Friction reducers are indispensable in the formulation of water-based hydraulic fracturing fluids and make up anywhere between 0.05 to 0.2% of the composition of these fluids. They reduce friction (slickening) and enhance the viscosity of the hydraulic fracturing fluid. Guar gum was the friction reducer additive of choice for hydraulic fracturing fluid formulation purposes prior to its replacement with polyacrylamide whose usage in frac fluids is gaining grounds [1]. It is used in enhanced oil recovery and in the formulation of hydraulic fracturing fluids [2]. One of the main advantages of PAMs in slick-water formulation is the significant cost advantage as compared to guar gum [3]. Despite the advantages derived from the use of PAM and its derivatives as additives in formulating hydraulic fracturing fluids, their precipitation in tight reservoirs may lead to partial or complete blockage of pore throats and unpropped fractures in reservoir rocks and also cause significant formation damage to proppant packs. Their usage therefore remains a hydrocarbon production challenge. In North Dakota, most of the hydrocarbons are produced through hydraulic fracturing of low permeability and porosity shales and sandstones using produced water contaminated with HPAM [4] and so the problem of formation damage through friction reducer gel precipitation can be potentially higher. Field studies have shown that unbroken gels in fracturing fluids could lower the overall gas production rate by 30% and reduce initial gas rates by 80% [5]. Once a well has been damaged through a contribution from this kind of precipitation, it may cost a significant amount of money to remediate. Knowledge of the conditions under which these friction reducers precipitate in the reservoir is important to implement proactive control strategies to reduce their impact on formation damage. The chemical characteristics of FRs (mostly PHPAM used in Bakken HFF) vis-a-vis their precipitation can be understood from its molecular structure and the resulting interaction between this polymeric compound and the array of ionic species in the reservoir environment. The presence of iron(III) is of particular concern [6]. The dissolution of pyrite from reservoir rock releases iron(II) which is subsequently oxidized to ferric ion at low pH. The reactions involved are shown in equation (1) and (2) below.
When the pH of the surrounding fluid mixture is low (within the 2-3 range), Iron(II) is slowly converted to iron(III) in the presence of oxygen [7] and in this state, it achieves higher thermodynamic stability [8,9]. At pH values above 5, this conversion is faster. At any of the two indicated pH ranges, the reaction for this redox transformation is shown below.
The synthesis and resulting chemical structure analysis of PHPAM are essential for comprehending the interaction between ferric ions and PHPAM.
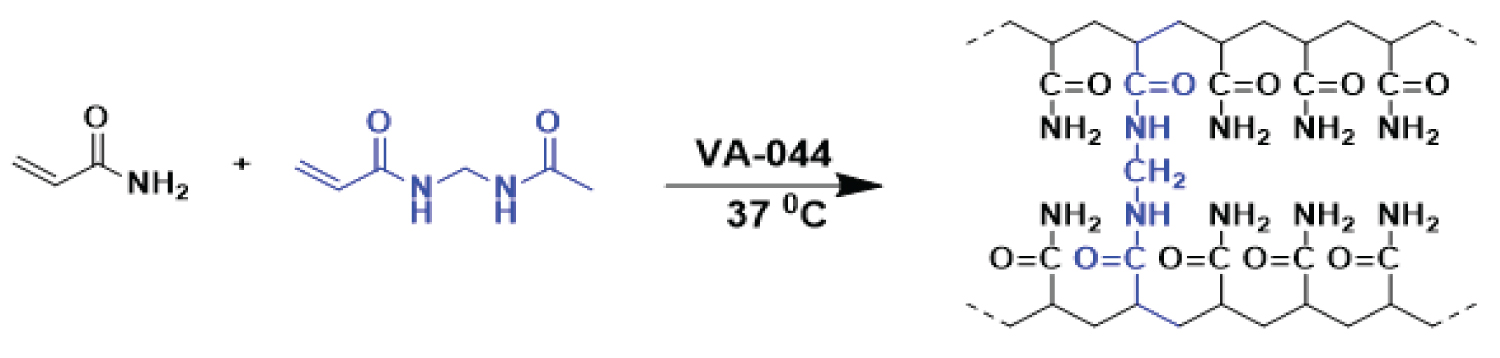
Polyacrylamide is a linear - chain water - soluble polymer with the formula -CH 2 CHCONH 2 - [10]. Different types of PAMs are synthesized via free radical polymerization of acrylamide (monomer) using an initiator under a specific temperature [11]. The polymerization of partially hydrolyzed polyacrylamide (PHPA) used in HF is shown in Figure 1.
The structure of PHPAM consists of a chain of acrylamide monomers with random acrylate monomers depending on the degree of hydrolysis [12]. This 3D structure has a backbone comprising of -COOH, -NH 2 and the double bond (Figure 1). These functionalities on the backbone of the linear PHPAM are electron-dense and serve as attachment sites for electron deficient inorganic chemical entities in the environment of the polymer.
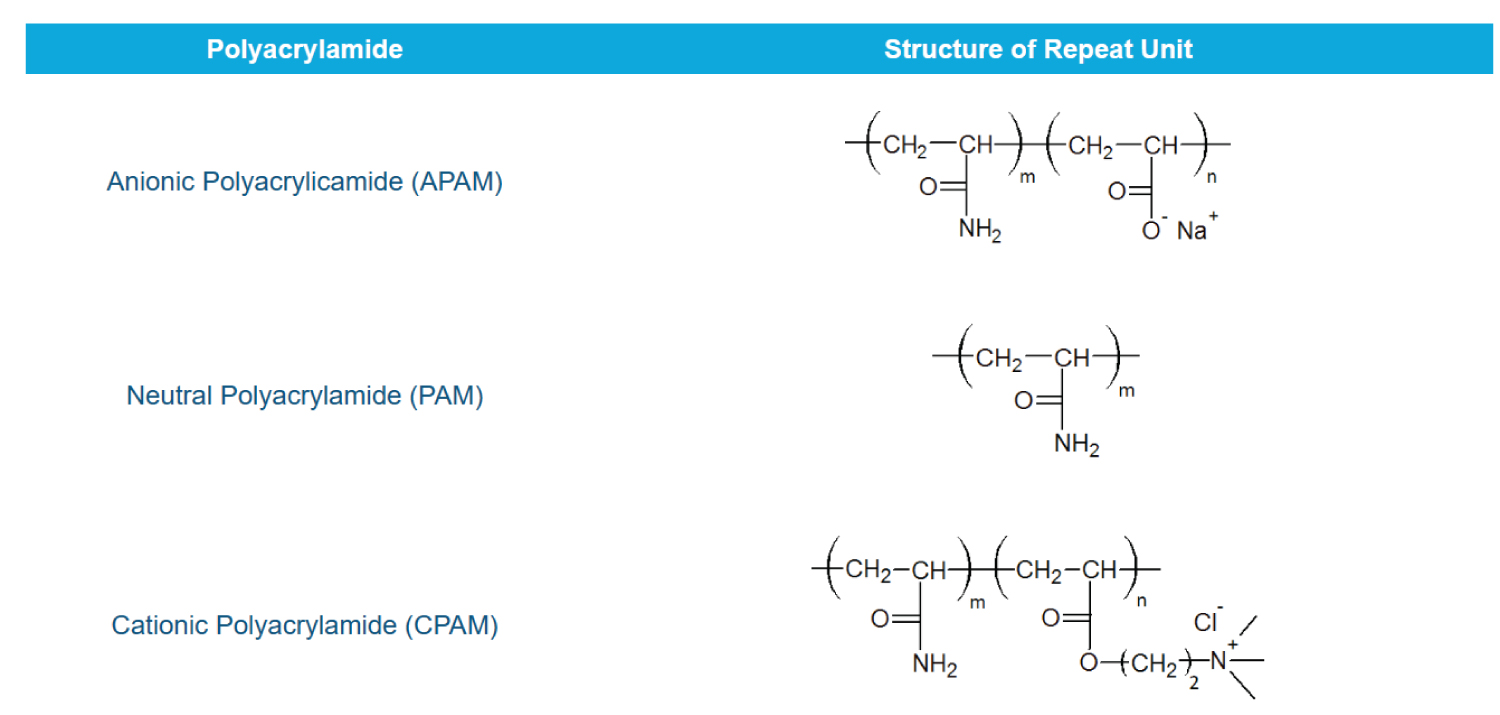
In recent years researchers have focused on the design of several types of PAMs and non-ionic, anionic, and cationic PAMs have been synthesized and characterized. The molecular structure of these PAMs (polyacrylamide) variants is shown in Figure 2.
High viscosity PHPAM is the most used friction reducer in the Bakken and is anionic. In produced waters, concentration of PHPAM residues lie between 10-1000 mg/L [13].
Precipitation of PHPAM in the reservoir is a huge challenge to the industry and it is important to find out the contribution of the reservoir environment to this phenomenon. The degradation of partially hydrolyzed polyacrylamide under harsh reservoir conditions (e.g., temperature and salinity) has been experimented [14]. Recently, the precipitation of PHPAM in the presence of iron in the reservoir is gaining traction. This stems from the realization that, about 60% of the reservoirs (conventional & unconventional) drained through wells in the USA contain concentrations of total iron exceeding 50 mg/L.
In view of the realization that ferric ions can form complexes with PHPAM and precipitate in the form of a gel in the reservoir and damage the formation permeability, there is an urgent need for a simple, yet effective test to detect the presence of partially hydrolyzed polyacrylamide in produced waters and hydraulic fracturing fluids. This test is conducted on hydraulic fracturing fluids and produced waters, and it qualitatively detects the presence and ease with which friction reducers in these liquids will precipitate out of solution when in contact with low concentrations of ferric ions under reservoir conditions. In other words, the vulnerability of the fluid to get particles of precipitated FRs in solution gives an idea of the “stress” of the fluid. Less stressed fluids are less prone to PHPAM precipitation. To determine this stress, an iron stress test is conducted, and acts as diagnostic test for the presence of PHPAMs in produced waters (in broken or unbroken form) and hydraulic fracturing fluids and how easily they can precipitate out of these liquids.
In this article, a stress test on samples of produced waters and an industrially formulated slick-water sample (hydraulic fracturing fluid) used for hydraulic fracturing is designed to test the hypothesis that, some hydraulic fracturing fluids and produced waters containing broken/unbroken fragments of PHPAM in the Bakken could be iron- stressed samples and as a result, may potentially contribute to formation damage to proppant pack and unpropped fractures due to precipitation of a gel-like Fe- PHPAM complex.
Experimental Analysis
Produced water and hydraulic fracturing fluid samples
Four Produced water samples at different were collected from the Williston Basin Counties. These included.
i. An unfiltered and untreated water produced water sample.
ii. A filtered but untreated water produced water sample.
iii. A treated and filtered produced water sample.
iv. A hydraulic fracturing fluid sample.
v. 30 grams of FeCl 3
Preparation of 50 mg/L FeCl 3 stock solution
A 50 mg quantity of FeCl 3 powder was carefully weighed using a METTLER TOLEDO high precision balance and dissolved in 1 liter of distilled water. The resulting solution was subjected to magnetic stirring at 1500 rpm to achieve a uniform and homogeneous solution of 50 mg/L of FeCl 3 .
Methodology
1. A 30-milliliter portion of each of the fluids was placed inside round bottom flasks and labelled WT, McK-treated, MUU, MTF, and FF as shown in Figure 3.
2. An equivalent volume of 30 mls of the FeCl 3 solution was used to spike each of the produced water samples and hydraulic fracturing fluids in the flasks, and the mixture allowed to rest for 3 minutes (see Figure 4).
3. The flasks were then placed in an oven for 6hrs at a temperature of 150⁰F (see Figure 4).
4. After the incubation period of 24 hrs, the flasks were removed from the heater and the results are shown in Figure 4
Results/Discussion
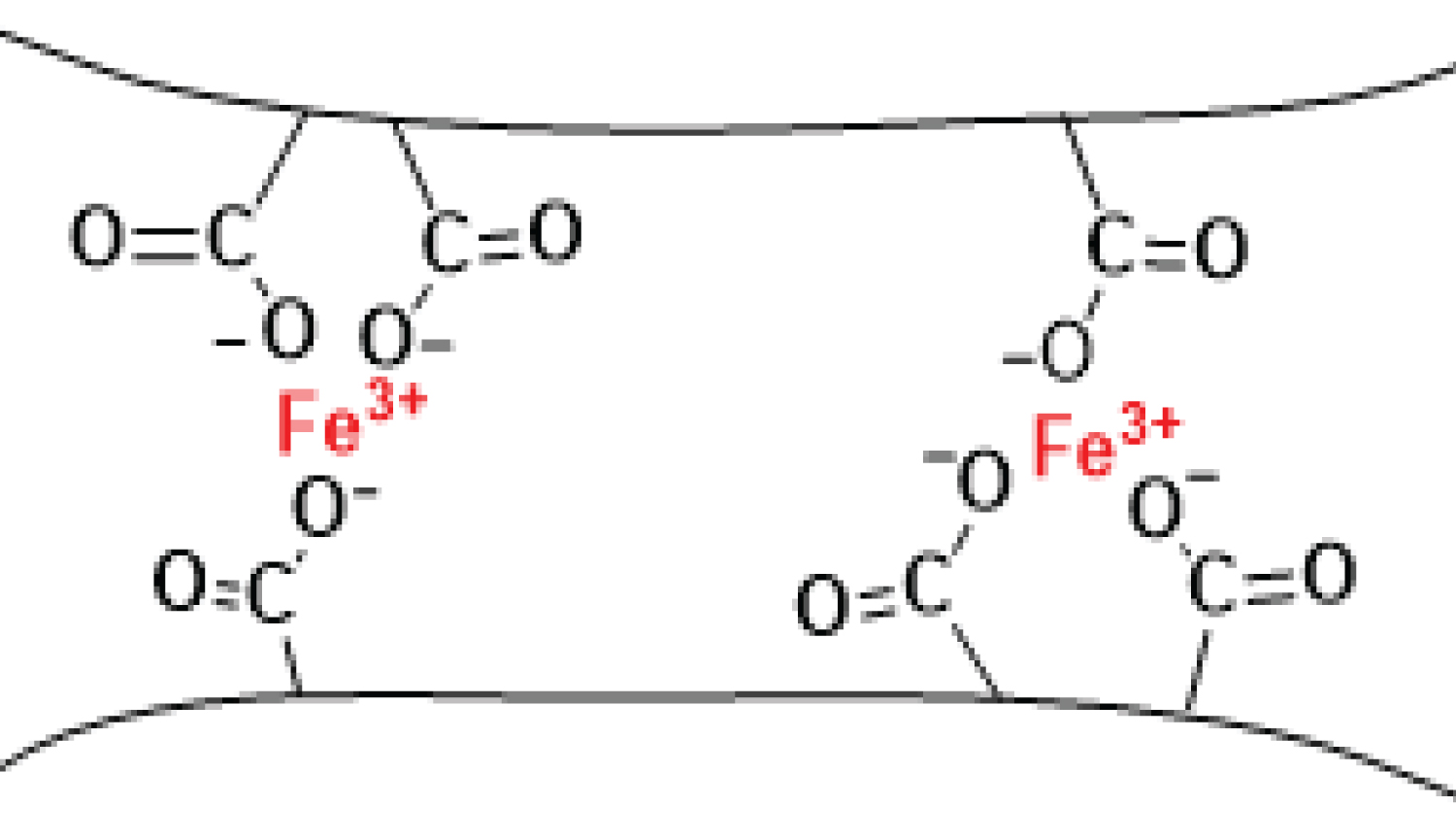
Due to the presence of these reactive sites on the PHPAM molecule, it can effectively bind metal ions through robust coordination bonds. Formation fluids and oilfield produced waters commonly contain elevated concentrations of various anions such as Ca 2+ , Mg 2+ , Zn 2+ , Fe 3+ , among others. These anions, being electron deficient, can form coordinate bonds with each of the electron-rich donor sites on the PHPAM backbone, resulting in the formation of a gel-like precipitate (Figure 5). The reaction involves the displacement of Na + ion by Fe 3+ .
Illustration of coordinate bonding of Fe 3+ with PHPAM (Note that the Na + in the anionic PHPAM shown in Figure 2 above has been displaced by Fe 3+ . Except for the carboxylic acid group involved in the reaction, the other functional groups are not shown on the polymer back bone for simplicity and clarity) (Figure 6).
Iron(III) has been shown to have the highest strength of coordination with PHPAM when compared to other ions like Mg 2+ ,Zn 2+ and Mg 2+ and Al 3+ (Si et al., 2021). While these ions can also form precipitates with PHPAM, it has been shown by FTIR and UV-vis spectroscopic measurements that it is the metal-coordination bonds between ferric ions and carboxylic groups of the polyacrylamide ligand, which forms the most stable gel (Wei et al., 2013). The unique stability of this gel-like precipitate compared to those formed by the other competing cations serves as the foundation for the iron stress test, as mentioned earlier. The less stable precipitates with the other metallic ions lack a discernible color, which hinders their detection in produced water or hydraulic fracturing fluid (Figure 7).
While the other produced water samples tested did not produce a positive result for the presence of PHPAM, the hydraulic fracturing fluid and McKenzie Produced water sample show evidence of the presence of full or fragmented PHPAM and are there in an iron stressed state. (Figure 8a, Figure 8b and Figure 8c) below.
The strength of the coordination bond between PHPAM and the interacting ion depends upon the ion's valency. Therefore, cations like Ca 2+ form stronger bonds with the FR polymer than singly charge ions. For any pair of co-existing ions in the environment of the polymer having the same valency, the cation with the higher charge density forms a stronger coordination bond with the polymer. As such, Fe 3+ forms a stronger coordination bond and a more stable complex (precipitate) than any of the divalent cations mentioned above (Figure 9).
However, the strength of the coordination bond between PHPAM and Fe 3+ relies on the concentration of Fe 3+ present in the solution and on the temperature of the reservoir [15]. Molecular dynamic simulation studies have revealed that, when the concentration of ferric ions in the solution is high, the bond strength between PHPAM and the ferric ion is lower compared to situations with lower ferric ion concentration (Andres A. Geraldo; Ivan Moncayo-Riasco; Rafael Ribadeneira; 2022). This relationship between concentration and coordination bond strength can be explained by positing that high concentrations of ferric ions counteract one another and neutralize the cation charge. Coupled with inadequate hydration of water molecules, direct access to the electron-rich oxygen atoms located on the carboxylic acid functionality on the PHPAM backbone is limited. As a result, the bond formed is weak. Conversely, under conditions of low ferric ion concentration, solvation of the Fe 3+ is better and there are no hindrances to accessing these electron-rich sites on the PHPAM molecule. Consequently, the bond becomes stronger, resulting in a more stable precipitate. The addition of the Fe 3+ ions to polymer solutions caused immediate crosslinking.
Conclusions
In this work, the unique ability of ferric ions to form a stable gel-like complex upon interaction with PHPAM was investigated. The following conclusions were drawn:
1. The analysis of the produced water samples from McKenzie County and hydraulic fracturing fluid revealed the existence of both fragmented and unfragmented partially hydrolyzed polyacrylamide. This presence was confirmed by the observation of a gel-like precipitate, which underwent a color transition from yellow to brown when subjected to heat. Only these tested samples of produced water and hydraulic fracturing fluid were found to be in iron-stressed condition, indicating their likelihood of generating the gel-like precipitate, and as a result, can block reservoir rock pore throats and hydraulic fractures, leading to detrimental formation damage.
2. PHHPAM was detected in a treated and produced water sample (McKenzie) indicating that conventional methods of treating produced water re-purposed for hydraulic fracturing do not completely remove PHPAM from these liquids. This agrees with the observations made by Al-Sabahi, et al. [4]. Usage of such produced waters may contribute to the formation damage problem through the precipitation of partially hydrolyzed polyacrylamide complexes.
3. Low ferric ion concentrations have a higher tendency to induce the precipitation of the gel-like complex rather than higher concentrations of ferric ions. This is important because it can determine the dosage of iron control agents to be included in the design of hydraulic fracturing fluids. That is to say, the optimal ferric ion concentration for maximum gel-like material precipitation must be determined to design a low iron stress fracturing fluid that minimizes formation damage in the hydrocarbon reservoir.
4. Iron stress tests are simple to carry out and should be performed routinely to help control ferric ion-induced hydrocarbon reservoir precipitation of broken/unbroken PHPAM hydraulic fracturing re-purposed produced water, and hydraulic fracturing fluid to understand the risk involved in utilizing such waters for hydraulic fracturing.
5. The present study contributes to the growing body of knowledge regarding the coordination properties of Iron(III) with PHPAM. The demonstrated highest coordination strength and formation of stable metal-coordination bonds between Iron(III) ions and the polyacrylamide ligand emphasize its potential superiority over other ions. These results provide a solid foundation for future investigations aiming to optimize and leverage the unique properties of Iron(III) for various practical applications, highlighting the importance of further research in this area.
Acknowledgements
We thank the Department of Petroleum Engineering of the University of North Dakota for providing the produced water samples used in this work. We also acknowledge the contributions of an industrial partner for providing the hydraulic fracturing fluid sample.
We gratefully acknowledge the help of the North Dakota Industrial Commission for providing financial support to accomplish this work.
Conflict of Interest
The authors declare that there are no conflicts of interests.
Data Availability
The results which were generated during this work and supporting its main findings are obtainable from the corresponding authors upon reasonable request.
References
- Hu J, Sun R, Zhang Y (2020) Investigating the horizontal well performance under the combination of micro-fractures and dynamic capillary pressure in tight oil reservoirs. Fuel 269: 117375.
- X Li, Z Xu, H Yin, et al. (2017) Comparative studies on enhanced oil recovery: thermoviscosifying polymer versus polyacrylamide. Energy Fuels 31: 2479-2487.
- Chen Z (2016) Polyacrylamide and its derivatives for oil recovery.
- Al Sabahi J, Bora T, Claereboudt M, et al. (2018) Visible light photocatalytic degradation of HPAM polymer in oil produced water using supported zinc oxide nanorods. Chemical Engineering Journal 351: 56-64.
- Voneiff GW, Robinson BM, Holditch SA, et al. (1996) The effects of unbroken fracture fluid on gas well performance. SPE Prod Facil 11: 223-229.
- Hazra S, Madrid V, Luzan T, et al. (2019) Correlating the performance of friction reducers with source water chemistry.
- Croot PL, Heller MI (2012) The importance of kinetics and redox in the biogeochemical cycling of iron in the surface ocean. Front Microbiol 3: 1-15.
- Rose AL, Waite TD (2002) Kinetic Model for Fe(II) oxidation in seawater in the absence and presence of natural organic matter.Environ Sci Technol 36: 433-444.
- Santana-Casiano JM, González-Dávila M, Millero FJ, et al. (2005) Oxidation of nanomolar levels of Fe (II) with oxygen in natural waters. Environ Sci Technol 39: 2073-2079.
- Wever D A Z, Picchioni F, Boethius A.A. (2011) Polymers for enhanced oil recovery: A paradigm for structure - property relationship in aqueous solution. Prog Polym Sci 36: 1558-1628.
- Watkins JJ, Mc Carthy TJ (1995) Polymerization of styrene in supercritical CO2- swollen poly(chlorotrifluoroethylene). Macromolecules 28: 4067-4074.
- Thomas A, Gaillard N, Favero C, et al. (2012) Some key features to consider when studying acrylamide-Based polymers for chemical enhanced oil recovery. Oil Gas Sci Technol 67: 887-902.
- Xiong B, Loss RD, Shields D, et al . (2018) Polyacrylamide degradation and its implications in environmental systems. npj Clean Water 1: 17.
- Moradi-Araghi A (2000) A review of thermally stable gels for fluid diversion in petroleum production. J Pet Sci Eng 26: 1-10.
- Ryles RG (1988) Chemical stability limits of water-soluble polymers used in oil recovery processes. SPE Reserv Eng 3: 23-34.
Corresponding Author
Claudius Epie Njie, Department of Petroleum Engineering, University of North Dakota, Collaborative Energy Complex Room 113, 2844 Campus Rd Stop 8154, Grand Forks ND 58202-8153, USA.
Copyright
© 2023 Njie CE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.