The Effect of Silica Dissolution on Reservoir Properties during Alkaline-Surfactant-Polymer (ASP) Flooding
Abstract
Chemical flooding is one of the effective methods to recover large volumes of oil from sandstone formations after primary depletion. However, silica dissolution often occurs during Alkaline-Surfactant-Polymer (ASP) flooding, affecting the petro-physical properties of the formation. To address this issue, samples from Berea sandstone formations were treated with various brine solutions, through static tube tests and core flooding experiments. Analytical tests such as DR/2800 spectrophotometer and scanning electron microscope were used to evaluate the silica solubility and the alteration in mineral content. The results indicated that the silicate contents decreased after the saturation due to silica solubility in the solution. Increasing brine salinity to 40,000 ppm and introducing Magnesium and Calcium ions to the solution, reduces the silicate contents by 5.03% and 7.32%. Moreover, saturating the samples with ASP solution, further reduced the silicate contents by 14.86%. This reduction is associated with a relative increase in silica solubility and pH of the solution. Silica dissolution affects the pore microstructure, which resulted in increasing the porosity and pore volume after the core flooding. The injection of the ASP solution increased the porosity by 5.83%, thus the pore volume increased from 17.72 to 18.76 cc. This is associated with the high silica solubility and the increase of solution pH in the ASP solution. The permeability of the samples generally reduced after the core flooding, due to the silica solubility. However, injecting the ASP solution, resulted in a major reduction of the permeability by more than 75%. These changes in the petro-physical properties can lead to severe formation damage and affect hydrocarbon production. This study assists in understanding the impact of silica dissolution during ASP treatment and addresses the factors involved. Efficient utilization of chemical flooding can help mitigating silicate scaling within the formation and extend field productivity.
Keywords
Silica dissolution, Silica solubility, Chemical EOR, Porosity, Permeability
Introduction
Oil and gas production are the primary energy sources for the world economy today [1,2]. Primary, secondary, and tertiary recovery techniques are widely used to recover more hydrocarbon from depleted reservoirs [3]. Enhanced oil recovery (EOR) is commonly applied method in sandstone reservoirs to improve oil production after secondary recovery [4]. Alkaline-Surfactant-Polymer (ASP) flooding is an EOR method, that combines all the functions of the individual chemicals [5-7]. ASP flooding calls the EOR to the utmost, for its promising results in increasing oil recovery by 20% [8-11]. Surfactants are mainly used to reduce the water/oil interfacial tension (IFT), which leads to alter the wetting-phase and stabilize foam [5,12]. Polymers increase the solution viscosity and enhance its mobility ratio. This is crucial to achieving good sweep efficiency of the formation [13,14]. Alkaline increases the solution pH, and forms the acrylic acid, which reduces the adsorption of the anionic surfactant and improves the performance of in situ surfactants [5].
Although chemical flooding was found effective in sandstone reservoirs, the issue of silicate scaling is causing severe problems to the production. The precipitation, accumulation, and deposition of silicate is a challenge to the wellbore stability and flow assurance of hydrocarbons [3,15]. Other issues are related to the salinity and hardness of the ASP solution, which is found to increase the silica solubility at reservoir conditions [16]. Sandstone reservoirs are mainly composed of quartz, which is the most stable form of amorphous silica in nature. Silicate scaling occurs when the concentration of the dissolved silica exceeds the solubility limit at a given temperature and pH [17]. However, the formation of silicate is highly complicated, involving silica dissolution, polymerization, and precipitation with other multivalent ions (Equation 1 and Equation 2) [18-23]. The water/silica interactions change the petro-physical properties of the formation through mineral dissolution, such as porosity and permeability [24,25] reported that the mineral dissolution can significantly change the porosity and permeability of the wellbore. Moreover, several studies have found that silica dissolution is closely related to the pH of the solution, as the alkaline solutions are the main cause of dissolving high portions of silica ions due to the high pH (above 9) promoted around the wellbore, resulting in the dissolution of quartz [19,26,27]. Also, the presence of Ca++ and Mg++ ions in the solution, accelerates the formation of silicate scale, due to the continuous polymerization of amorphous silica.
Many ASP flooded oil reservoirs worldwide had experienced silica scaling problems that affect the productivity of the field. Several factors are directly involved in forming silicate scaling, and damaging the formation, such as brine salinity, ASP concentrations, pH, and temperature [7,16,20,22,28]. Injection of the alkaline solution in oil reservoir leads to mineral dissolution and precipitation, possibly resulting in changes in permeability and porosity, and consequently altering solution pH [20,28]. The alkaline solution causes two main changes in the reservoir; firstly, it affects the presented fluids by decreasing the mobility ratio causing emulsification. Secondly, it promotes a high pH environment, leading to dissolving minerals such as quartz, feldspar, and clays [29]. Several scholars have studied the formation of silicate scaling during water flood, confirming the possible reservoir damage caused by the precipitation of silica ions [30-32]. A recent study by [32] reported a reduction in flow rates during water flooding accompanied by a significant reduction in permeability due to silica precipitation.
However, the impact of ASP solution on silicate scaling is not fully understood, and only limited studies have evaluated the implications of silica dissolution on reservoir properties during ASP flood. In this regard, this paper investigates the changes in porosity and permeability of Berea sandstone formation during ASP flooding. Static and core flooding experiments were employed to determine the silica solubility. Several analytical methods were used including field emission scanning electron microscopy (FESEM), energy dispersed X-ray (EDX), and DR/2800 spectrophotometer. The achieved results provide a good understanding of the behavior of silica dissolution during the ASP flood and its implications on reservoir properties.
Methodology
Samples and materials
Core samples from Berea sandstone formations were used in this study and treated with various synthetic brine solutions, to evaluate the effect of silica dissolution on porosity and permeability during ASP injection. Samples were used as received from Kocurek Industries INC. Hard Rock Division (Caldwell, Texas), with no additional treatment. Core samples were prepared in various sizes to meet the test requirements. The bulk sample was crushed into powders, and then sieved into sizes of (< 75 μm) to fulfil the sampling requirements for silica solubility tests [33]. Fragments of the core sample were polished with 1500 silicon carbide grit and cleaned with ethanol to be used for SEM analysis. This process ensures the removal of any contaminates after grinding [34]. Whole core plugs (1.5 × 3 inches') were used for core flooding experiments. Then several samples were separated from the bulk powder, and saturated into various brine solutions as highlighted next. It should be noted that, all samples were preserved in polyethylene bags, to avoid oxidation.
Sodium Chloride, Magnesium Chloride, and Calcium Chloride were used to prepare the brine solutions. While Sodium Metaborate, Alpha Olefin Sulfonate (AOS), and SH copolymer were used to prepare the ASP solution. All chemicals were used as received from the suppliers. Four synthetic brine solutions were used; accordingly, 1) Distilled water (DW), 2) 40,000 ppm synthetic brine, 3) 59,940 ppm hard brine (Table 1), and 4) ASP solution with 0.8% alkali, 0.8% surfactant and 2000 ppm copolymer concentrations (Figure 1). These solutions were prepared to mimic the injection scheme for ASP flooding in oil fields. DW and 40,000 ppm synthetic brine (prepared using NaCl only) were used to evaluate the impact of salinity. Magnesium Chloride and Calcium Chloride were used to prepare the 59,940 ppm hard brine as suggested by [16]. ASP solution was prepared based on the work of [35], who reported a high oil recovery percentage.
Experimental design
Three types of experiments were conducted in this study. 1) Static experiments; test tubes were used to saturate eight grams of the crushed core sample in 30 mL of synthetic brine for 3 days to achieve equilibrium (Figure 1). It should be mentioned that we assume that the silica/brine reaction rate is not affected by the fluid flow, and the surface area of the sample does not drop significantly as dissolution proceeds [36]. After equilibrium, silica solubility was detected in the brine solution. 2) Characterization tests; the alteration of silicate minerals was determined using Supra 55 VP Zeiss Variable Pressure scanning electron microscope with energy dispersive spectrometer (SEM/EDX) before and after the saturation, at a resolution of 2 nm at 30 kV. Silica solubility was measured based on the Silicomolybdate method using DR/2800 Spectrophotometer at room temperature. This method allows us to detect silica concentration in solutions at high ranges in (mg/L). 3) Core flooding were performed using Benchtop Permeability System (BPS-805) at room temperature. The alteration in rock properties was evaluated using the PoroPerm system before and after flooding at 25 ℃. Before the core flooding; samples were dried at 80 ℃, and then saturated under a vacuum pump at ambient temperature with the synthetic brine for one day for sufficient saturation. The saturated core sample was placed in the core holder and the brine solution was injected at an injection rate of 0.2 cc/min until deferential pressure stabilized. The core sample was then removed from the chamber, dried, and evaluated again using the PoroPerm system. Moreover, silica solubility of the outlet solution was detected and compared with the static experiments.
Results and Discussion
SEM/EDX analysis
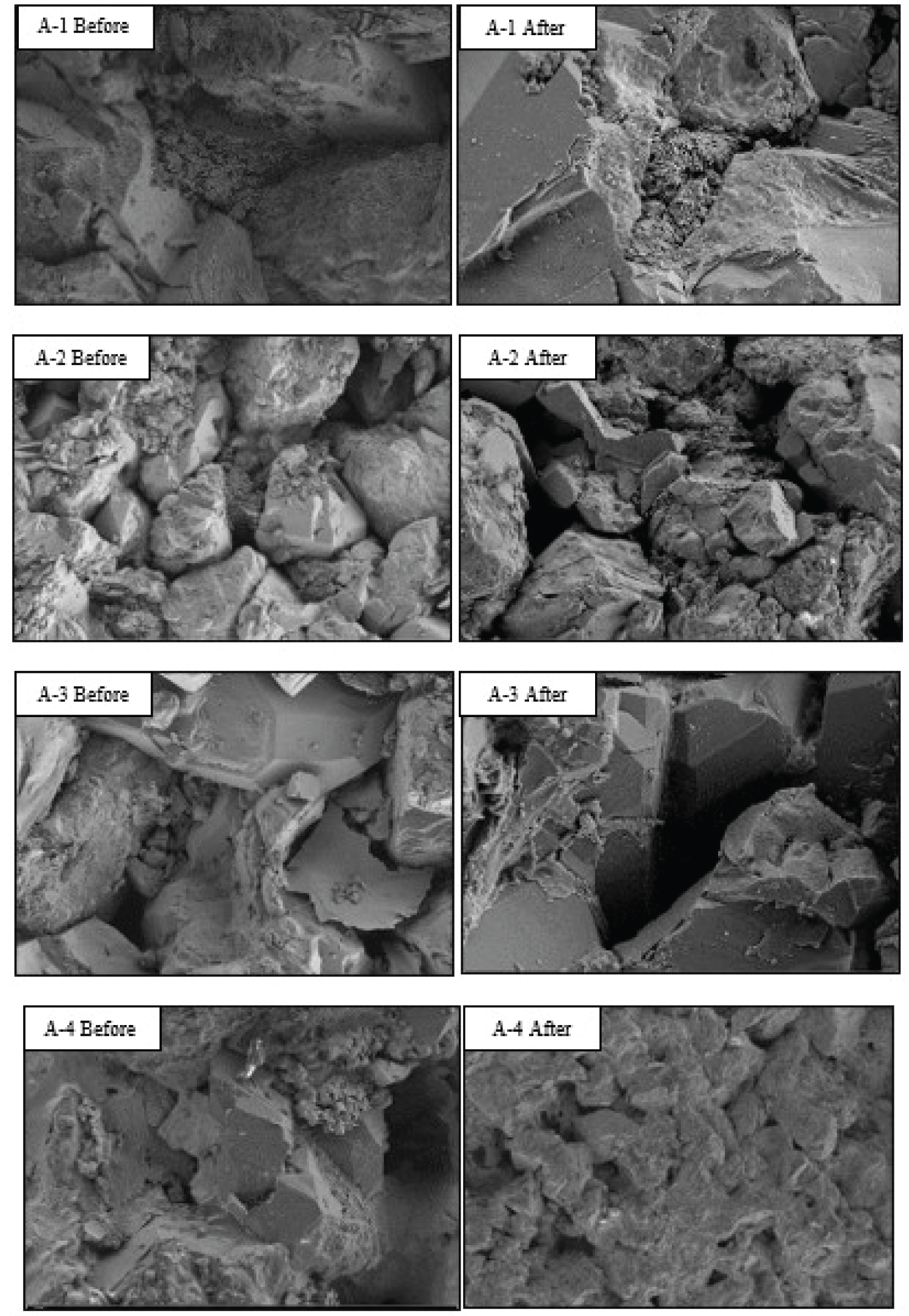
The SEM images of the samples before and after saturation are shown in Figure 2. Quartz grains are the most dominant component in the samples and vary in appearance from sub-rounded to well-rounded grains. These grains are covered by a thin layer of amorphous silica cement, with spread micro-cracks. However, after treatment, various changes in the surface morphology were observed. Mainly, the silica film around the quartz grains was removed after saturation due to the dissolved silica into the solution. Several dark spots appeared after the saturation, and the grains' surface became more smooth.
The mineral element contents before and after the saturation are given in Table 2. The EDX analysis shows considerable changes in silicate contents after the saturation, which confirms the silica dissolution. Sample A1 shows minor changes after saturating with DW, however, increasing the salinity to 40,000 ppm has increased the silica dissolution in Sample A2. As a result, the silicate contents reduced by 5.03%, due to the presence of Cl- ions in the solution and increasing the degree of hydration. Introducing Mg2+ and Ca2+ ions to the brine in Sample A3, have further increased silica dissolution, and the silicate contents reduced by 7.32%. This is related to the depletion of the silicic acid by Mg2+ and Ca2+ minerals, resulting in dissolving more silica [37]. Sample A4 was treated with the ASP solution and indicated the highest reduction in silicate contents by 14.86%. This is attributed mainly to the high pH environment created by the alkaline, which allows the brine to dissolve more silica. Besides the additional impact of surfactant in displacing water molecules from silica surface, thus increasing the silica dissolution.
Silica solubility measurements
Table 3 presents the silica solubility results for the treated samples with different synthetic brine solutions. In the static tests, crushed core samples indicated relatively higher silica solubility than the core chips. This is related to the size of the grain particles and the surface area, as the small grain particles are more soluble in brine. Saturating Sample A1 in DW indicated a solubility of 4.5 mg/L. The silica solubility increased to 8.3 mg/L when the brine salinity increased to 40,000 ppm for Sample A2. This is attributed to the effect of the degree of hydration. When the crystalline silica grains are contacted with the brine, they contain the water inside the crystals, which is known as the water of hydration. The amount of this water decreases with the increasing salt contents, thus the high hydrated particles have low silica solubility which has low salt contents. At the presence of Mg2+ and Ca2+ ions, the silica dissolution increased to 15.5 mg/L, meanwhile, the increase of pH to 8.3, contributed to dissolving more silica from Sample A3.
Saturating Sample A4 in the ASP solution indicated a high silica solubility value of 48.2 mg/L. The significant increase in the solution pH above 9, makes the silica more soluble [38]. This increase in the pH leads to depleting the silicic acid, and enforces the system to re-equilibrate, resulting in dissolving additional silica. The measured silica dissolution from the core flooding experiments is aligned with the static tests. However, the measured value of soluble silica at the outlet solution is relatively higher than the corresponding amount during the static test. This is related to the equilibrium time, as the flooded core samples require more time to reach the equilibrium conditions, thus more silica is dissolved. Besides the continuous fluid flow in the flooded core sample ensures covering more surface area, compared to the static tests. In summary, silica solubility is highly affected by the brine salinity and the presence of hard minerals. During the ASP flood, the high pH environment created by the alkaline contributes to dissolve more silicate minerals, which can affect the reservoir properties and hydrocarbon production.
The effect of silica dissolution on porosity and pore volume
Table 4 shows the alteration of porosity and pore volume after core flooding experiments by Poroperm. The presented results in Table 4 indicated that the porosity and pore volume increased after the core flooding, due to silica solubility. These results support the earlier findings from the static tests and confirm the impact of silica dissolution on the core's properties. The dissolved amount of the silica had minor increases in the porosity of Sample A1 from 21.89% to 22.13%, an increase of 1.1%. The corresponding pore volume increased as well from 18.61 to 18.82 cc. Injecting saline brine with the presence of Mg2+ and Ca2+ minerals further increase the silica solubility. Thus, the porosity of Sample A2 and Sample A3 increased by 1.13% and 3.22% respectively, accompanied by an increase in the pore volumes. Moreover, the injection of the ASP solution increased the porosity of Sample A4 by 5.83%, and subsequently, the pore volume increased from 17.72 to 18.76 cc. This increase is caused by the high amount of dissolved silica and pH value in the ASP solution. These findings are in agreement with the obtained results from static experiments and confirms that silica solubility can alter the porosity and the pore volume of the reservoir. These findings can assist in designing ASP flooding treatments, by considering the influence of silica dissolution.
The effect of silica dissolution on permeability
The effect of silica dissolution on cores' permeability was determined by two methods. 1) Analyzing the changes in the injected pore volume (IPV) with the pressure difference during the flooding experiment (BPS-805), and 2) Using PoroPerm on the dried sample after the flooding test. For the first method, the pressure readings were recorded every 10 minutes, and the corresponding IPV values were calculated based on the equivalent flow rate and porosity. The permeability values were calculated based on Darcy's Law and compared with the obtained results from the PoroPerm test. Table 5 shows the obtained results for permeability alteration after the core flooding experiments for both methods. Before the core flooding experiment, each sample was flooded with a deionized brine solution to acquire the initial value of the core's permeability.
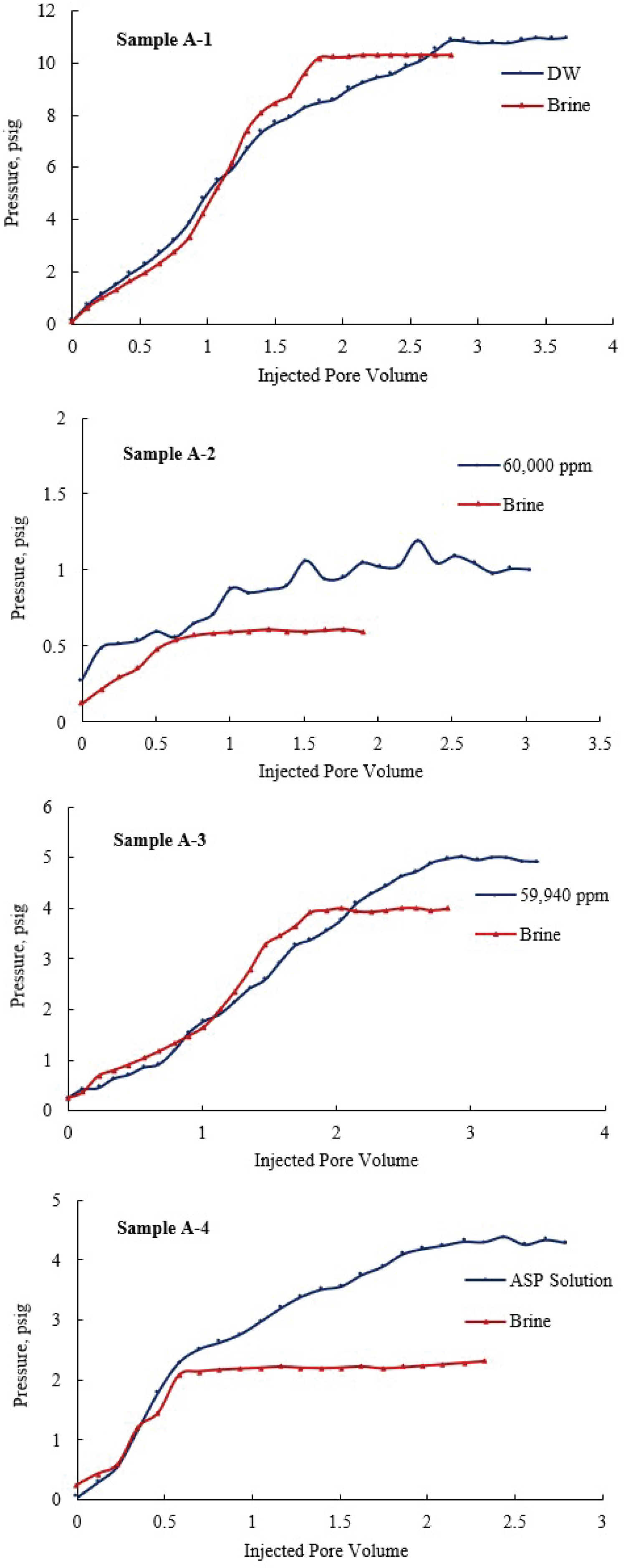
Figure 3 illustrates the relationship between the IPV and the pressure for all samples during the flooding experiment. The pressure difference in Sample A1 started to stabilize at 10 psig after injecting around 2 PV of brine solution. However, around 3 PV of DW was injected to stabilize the pressure at 11psig. The low difference in pressure stabilization value indicates a minor reduction in permeability by 2%. The pressure difference of Sample A2 relatively increased when flooded with the 40,000 ppm brine. Around 1 PV was enough for the pressure to stabilize when the sample was flooded with the brine, while more than 2.5 PV of 40,000 ppm brine was injected for the pressure to stabilize. This results in a significant reduction of the permeability from 55.74 mD to 32.23 mD, a reduction of 42.18%. Similarly, Sample A3 indicated a permeability reduction of 47.79%, resulted from injecting hard brine solution to the sample. Based on the results in Figure 3, the early stages of the flooding showed no significant reduction in permeability value. However, after the injection of 2 PV, the pressure continued to increase and stabilized at 5 psig, after 3 PV of the hard brine was injected. The injection of ASP solution indicated a major reduction in the permeability of Sample A4. The permeability reduced from 131.68 to 31.57 mD, a reduction of more than 75%. Injecting brine solution to sample A4, stabilized the pressure at 2 psig after injecting 0.5 PV. However, the pressure stabilized at 4.5 psig after injecting 2.5 PV of the ASP solution, which led to a significant reduction in the permeability.
Based on the obtained results, the injection of ASP solution resulted in a major reduction of permeability, due to the massive precipitation of the soluble silica within the porous media. However, there is a noticeable difference in the permeability values acquired by the PoroPerm system and the brine injection method (Table 5). Normally, the brine permeability is consistently lower than the gas permeability, as the gas measurements are carried out on a dry core sample, while the brine measurements are performed on saturated samples. Some empirical relationships were developed to relate the gas permeability values to the brine permeability values [39]. Nevertheless, both methods confirm the high reduction of permeability associated with injecting ASP solution.
The changes in the core's properties increase the risk of damaging the wellbore of an ASP flooded well and affect the production of hydrocarbons. Therefore, successful designing of ASP treatment can mitigate the impact of silicate scaling within the formation and extend the production lifecycle. This study investigated some factors that have a significant effect on silica dissolution in sandstone formations. By controlling these factors, silicate scaling can be delayed, mitigated, or inhibited at early stages, to ensure a longer lifetime for the field productivity.
Conclusion
In this paper, samples from Berea sandstone formation were used to investigate the effect of silica dissolution on porosity and permeability during ASP flood. Core flooding and static tests showed that the silicate contents decreased after the saturation, associated with a relative increase in silica solubility and pH of the solution. Increasing brine salinity and hardness increases the soluble silica in the solution, due to the depletion of the silicic acid by Mg2+ and Ca2+ minerals. Moreover, the injection of the ASP solution indicated a relatively high silica solubility of 48.2 mg/L, with a reduction in silicate contents by 14.86%. This reduction is related to the high pH environment created by the alkaline solution. The pore microstructure properties were altered after the injection of the ASP solution. The pore volume increased from 17.72 to 18.76 cc after the core flooding experiment, thus the porosity increased by 5.83%. This is directly related to the high silica solubility and the increase of solution pH in the ASP solution. Furthermore, the permeability of the samples generally reduced after the core flooding experiment, due to the impact of silica dissolution. A significant reduction by 75% occurred with the injection of the ASP solution. Silica dissolution is one of the critical factors that affect production wells and leads to severe formation damage. Therefore, addressing the effect of silica dissolution on the petro-physical properties of sandstone formations is crucial for effective chemical flood treatment. The achieved results in this study assist in understanding the silica solubility behavior, and the factors involved, which can help to mitigate silicate scaling and extend field productivity.
Authors Declaration
The author declares no competing financial interest.
References
- Cheng Y, Zeng M, Lu Z, et al. (2020) Effects of supercritical CO2 treatment temperatures on mineral composition, pore structure and functional groups of shale: Implications for CO2 sequestration. Sustainability 12: 3927.
- Fatah A, Ben Mahmud H, Bennour Z, (2021) Effect of supercritical CO2 treatment on physical properties and functional groups of shales. Fuel 303: 121310.
- Jing G, Tang S, Li X (2013) Analysis and inhibition of scale accumulation for a producing well in the daqing oilfield. Pet Sci Technol 31: 1772-1777.
- Jeirani Z, Mohamed Jan B, Si Ali B, et al. (2014) Pre-prepared microemulsion flooding in enhanced oil recovery: A review. Pet Sci Technol 32: 180-193.
- Liu Z, Bode V, Hadayati P, et al. (2020) Understanding the stability mechanism of silica nanoparticles: The effect of cations and EOR chemicals. Fuel 280: 118650.
- Nguyen TBN, Bae W, Dang TQC (2014) Improvements of Mixed-surfactants in Alkaline/Surfactant/Polymer Solutions. Pet Sci Technol 32: 1458-1464.
- Sonne J, Kerr S, Miner K. (2012) Application of silicate scale inhibitors for asp flooded oilfields : A novel approach to testing and delivery. SPE 154332.
- Elraies KA, Tan IM, Fathaddin MT, et al. (2011) Development of a new polymeric surfactant for chemical enhanced oil recovery. Pet Sci Technol 29: 1521-1528.
- Li JJ, Jiang HQ, Hou JR, et al. (2014) The effects of oil displacement efficiency and conformance efficiency on viscosity of asp flooding in a heterogeneous reservoir. Pet Sci Technol 32: 830-839.
- Pratap M, Gauma MS (2004) Field implementation of Alkaline-Surfactant-Polymer (ASP) flooding : A maiden effort in India. SPE Asia Pacific Oil and Gas Conference and Exhibition 88455.
- Yan HY, Xiao M, Zhang ZZ, et al. (2014) Remediation of oilfield wastewater produced from alkaline/surfactant/polymer flooding by using a combination of coagulation and bioaugmentation. Pet Sci Technol 32: 1521-1528.
- Tagavifar M, Jang SH, Sharma H, et al. (2018) Effect of pH on adsorption of anionic surfactants on limestone: Experimental study and surface complexation modeling. Colloids Surf A: Physicochem Eng Asp 538: 549-558.
- Ahmed FA, Elraies AK (2015) The Effect of Water Salinity and Reservoir Temperature on Silica Dissolution During ASP Flood: Static Model. ICIPEG 2014: 99-107.
- Amjad Z, Zuhl RW (2008) An evaluation of silica scale controle additives for industrial water systems. NACE 08368.
- Arensdorf J, Hoster D, Mcdougall D, et al. (2010) Static and dynamic testing of silicate scale inhibitors. SPE 132212.
- Junin AA, Junin R, Gbadamosi A (2018) Mechanism governing nanoparticle flow behaviour in porous media: insight for enhanced oil recovery applications. International Nano Letters 8: 49-77.
- Almahfood M, Bai B (2018) The synergistic effects of nanoparticle-surfactant nanofluids in EOR applications. J Pet Sci 171: 196-210.
- Beckingham LE (2017) Evaluation of macroscopic porosity-permeability relationships in heterogeneous mineral dissolution and precipitation scenarios. Water Resour Res 53: 10217-10230.
- Bremere I, Kennedy M, Mhyio S, et al. (2000) Prevention of silica scale in membrane systems : Removal of monomer and polymer silica. Desalination 132: 89-100.
- Basbar AEA, Elraies KA, Osgouei RE (2013) Formation silicate scale inhibition during Alkaline Flooding : Static model. SPE 164669.
- Gill JS (1988) Silica scale control. NACE: 226.
- Iler RK (1979) The Occurrence, Dissolution, and Deposition of Silica.
- Sonne J, Miner K, Kerr S (2012) Potential for inhibitor squeeze applicatio n for silicate scale control in asp flood. SPE: 155039.
- Tan Q, You L, Kang Y, et al. (2020) Changes in pore structures and porosity-permeability evolution of saline-lacustrine carbonate reservoir triggered by fresh water-rock reaction. J Hydrol 580: 124375.
- Steinwinder J, Beckingham LE (2019) Role of pore and pore-throat distributions in controlling permeability in heterogeneous mineral dissolution and precipitation scenarios. Water Resour Res 55: 5502-5517.
- Ahmed FA, Elraies AK, Mohammed AA, et al. (2015) An investigation study on the effect of brine composition on silica dissolution. Adv Environ Eng Geosci Eng 188-192.
- Dobbs HA, Degen GD, Berkson ZJ, et al. (2019) Electrochemically enhanced dissolution of silica and alumina in alkaline environments. Langmuir 35: 15651-15660.
- Elraies AK, Ahmed FA, Ayoub AM, et al. (2016) An experimental investigation on the impact of brine composition on silica solubility at high temperature. Int J Mech 12: 90-94.
- Kazempour M, Sundstrom E, Alvarado V (2012) Geochemical modeling and experimental evaluation of high-pH floods: Impact of Water-Rock interactions in sandstone. Fuel 92: 216-230.
- Yupu W, Junde L, Bing L, et al. (2004) Why does scale form in asp flood? how to prevent from it?--a case study of the technology and application of scaling mechanism and inhibition in asp flood pilot area of n-1dx block in daqing. SPE: 87469.
- Ikeda R, Ueda A (2017) Experimental field investigations of inhibitors for controlling silica scale in geothermal brine at the Sumikawa geothermal plant, Akita Prefecture, Japan. Geothermics 70: 305-313.
- Niu Q, Zhang C (2019) Permeability prediction in rocks experiencing mineral precipitation and dissolution: A numerical study. Water Resour Res 55: 3107-3121.
- Yanaze T, Yoo SY, Marumo K, et al. (2019) Prediction of permeability reduction due to silica scale deposition with a geochemical clogging model at Sumikawa Geothermal Power Plant. Geothermics 79: 114-128.
- Fatah A, Bennour Z, Ben H, et al. (2021) Surface wettability alteration of shales exposed to CO 2 : Implication for long-term integrity of geological storage sites. Int J Greenh Gas Control 110: 103426.
- Fatah A, Bennour Z, Ben Mahmud H, et al. (2020) A Review on the influence of CO 2 /shale interaction on shale properties: Implications of CCS in shales. Energies 13: 3200.
- Elraies KA, Kalwar SA (2013) The application of acrylic acid as precipitation inhibitor for asp flooding. J Pet Environ Biotechnol 04: 4-7.
- Bowman RW, Gramms LC, Craycraft RR (2000) High-Silica Waters in Steamflood Operations. SPE 63015.
- Southwick JG (1985) Solubility of silica in alkaline solutions: Implications for alkaline flooding. SPE.
- Swanson BF (1981) A simple correlation between permeabilities and mercury capillary pressure. J Petrol Technol 33: 2498-2504.
Corresponding Author
Ahmed Fatah, Department of Petroleum Engineering, Curtin University; Faculty of Geoscience and Petroleum Engineering, Universiti Teknologi Petronas, Miri 98009, 31750, Tronoh, Malaysia.
Copyright
© 2022 Fatah A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.