Seven Day Anhepatic Survival in a 19 Month Old Child: An Interdisciplinary Challenge
Abstract
Objective
Today, numerous patients with acute liver failure die due to shortage of donor organs and long waiting lists. We describe the paediatric intensive care and surgical management of repeated split liver transplantation with the so far longest reported anhepatic survival of 168 hours after total hepatectomy in a 19 month old child.
Case
A 19 month old child with acute liver failure (ALF) received an adult left liver lobe. Upon reperfusion the graft showed complete perfusion failure with massive swelling of the donor-organ necessitating immediate total hepatectomy. Disseminated coagulopathy led to acute haemorrhage requiring mass-transfusion over 3 days. Factor XIII and tranexamic-acid were used to treat hyper-fibrinolysis.
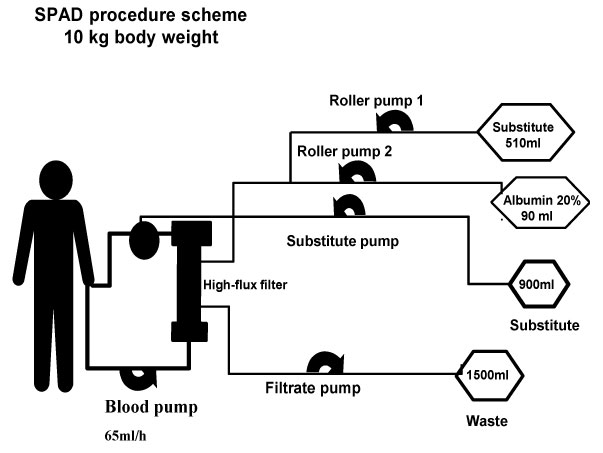
Immediately after hepatectomy the child was re-listed for high urgency transplantation. Single-Pass-Albumin-Veno-Venous-Hemodiafiltration/-filtration (SPAD) with total turnover rates up to 150 ml/kg/hour was initiated to maintain ammonium, bile-acids, bilirubin, amino acids and cerebral perfusion within thresholds until a matching split organ was available on day eight.
Overall 16 surgical interventions, including two re-transplantations, one with cavo-portal anastomosis due to portal vein thrombosis, an abdominal wall closure with dermal-porcine-collagen and skin-mesh-grafts were necessary. Anticoagulation with argatroban, 5 weeks on mechanical ventilation, antibacterial and antimycotic treatment of multiple infections were required.
The boy was finally discharged home after 6 months. At 2 year follow up the child presented in good health with a well functioning graft without any neurologic sequelae.
Conclusion
A skilled interdisciplinary team of cooperating paediatric and surgical specialists and modern techniques, as SPAD, special surgical procedures and new materials, made the survival in this devastating liver transplantation-course possible.
Keywords
Anhepatic, Total hepatectomy, Primary non-functioning graft, Single pass albumin dialysis, SPAD, Portocaval shunt, Cavo-portal anastomosis, Cerebral hyperperfusion, Doppler-Ultrasound, Dermal porcine collagen, Liver transplantation
Introduction
Primary liver graft non-function after liver transplantation is an acute life threatening event for the recipient and is associated with high mortality in children and adults [1-6]. Sieders, et al. reported a 27% loss of liver grafts early after transplantation in children, the majority during the first 3 days post transplantation [2]. Despite the use of hemofiltration the time until re-transplantation or death is very short. 24-67 hours of anhepatic survival were reported until today. However, survival after a second liver transplantation is poor in children (33%) [2,7,8].
Little is known about survival in anhepatic children under Single-Pass-Albumin-veno-venous-haemo-Dialysis (SPAD) nor blood levels or clearance of amino acids or any medication [9].
Case Report
We publish this case after the parents gave their written consent.
A 19 month old boy with 10 kg body weight and acute liver failure of unknown origin received a left liver lobe of a deceased adult donor. On reperfusion, the graft showed a complete perfusion failure with massive swelling and had to be removed immediately. The histology of the explanted transplant organ revealed a profound steatosis and in addition a toxic liver injury of unknown origin, which might have led to an organ swelling and thereby an impairment of venous reflow further increasing swelling.
A temporary end to side porto-caval shunt was established to allow portal vein flow. Diffuse intra-abdominal bleeding occurred due to disseminated intravascular coagulopathy requiring PPSB containing clotting factor II -Prothrombin, factor VII - Prokonvertin, factor X - Stuart-Prower-Factor, factor IX - antihaemophile Factor B, fresh frozen plasma and mass transfusion.
Bridging to re-transplantation
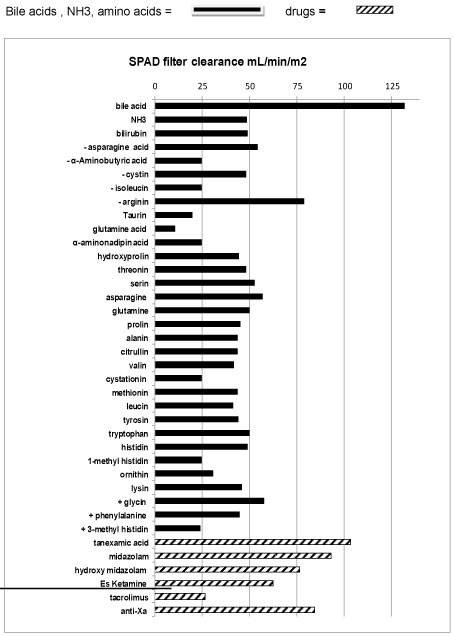
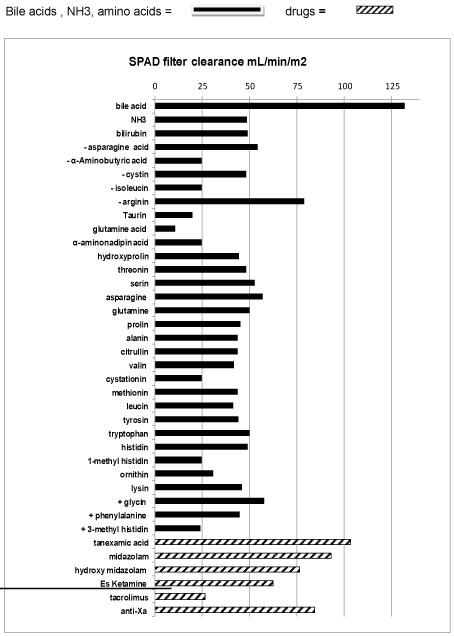
A Shaldon catheter was placed and the child was admitted to our paediatric intensive care unit (PICU) ventilated, with on-going intra-abdominal bleeding. As no liver function could be expected in an anhepatic child, SPAD was instituted immediately, as previously described [9]. SPAD was used with turnover rates up to 60 ml/kg/h albumin solution against the stream, and up to 90 ml/kg/h hemofiltration rate for seven days. These high rates were necessary to maintain ammonium (< 150 µmol/L), bile acids, bilirubin (< 10 mg/dL), amino-acids and cerebral perfusion within thresholds. Pre and post filter levels of all medication, bile acid, bilirubin, ammonium (NH3) and amino-acids were measured and SPAD clearance (mL/m2) was calculated by the formula: (Pre filter concentration-post filter concentration) blood flow (mL/min)/((pre-filter concentration x m2 body surface) (Figure 1). The filter was changed every 24 hours. No extra fluids (Ultrafiltration) were withdrawn by SPAD due to the remaining good renal function in this child. Packed cells (300 ml/kg/d), platelets and 300 ml/kg/d fresh frozen plasma (FFP) were substituted continuously to replace blood losses due to coagulopathy and diffuse haemorrhage during the first two days. Factor XIII replacement and tranexamic-acid was titrated up to 30 mg/kg until levels of D-Dimers decreased and hyper-fibrinolysis was solved. Thereafter, 40-80 ml/kg/d fresh frozen plasma was continuously replaced in combination with low dose heparin to avoid further hyper-fibrinolysis related to hemodiafiltration and to decrease the risk of further bleedings.
On the third anhepatic day, after haemorrhage and blood losses have stopped, SPAD was discontinued for 4 hours to remove the intra-abdominal hematoma causing abdominal compartment syndrome and profound bowel oedema. Thereafter the abdominal wall was extended with a Goretex-patch to avoid further abdominal organ compression.
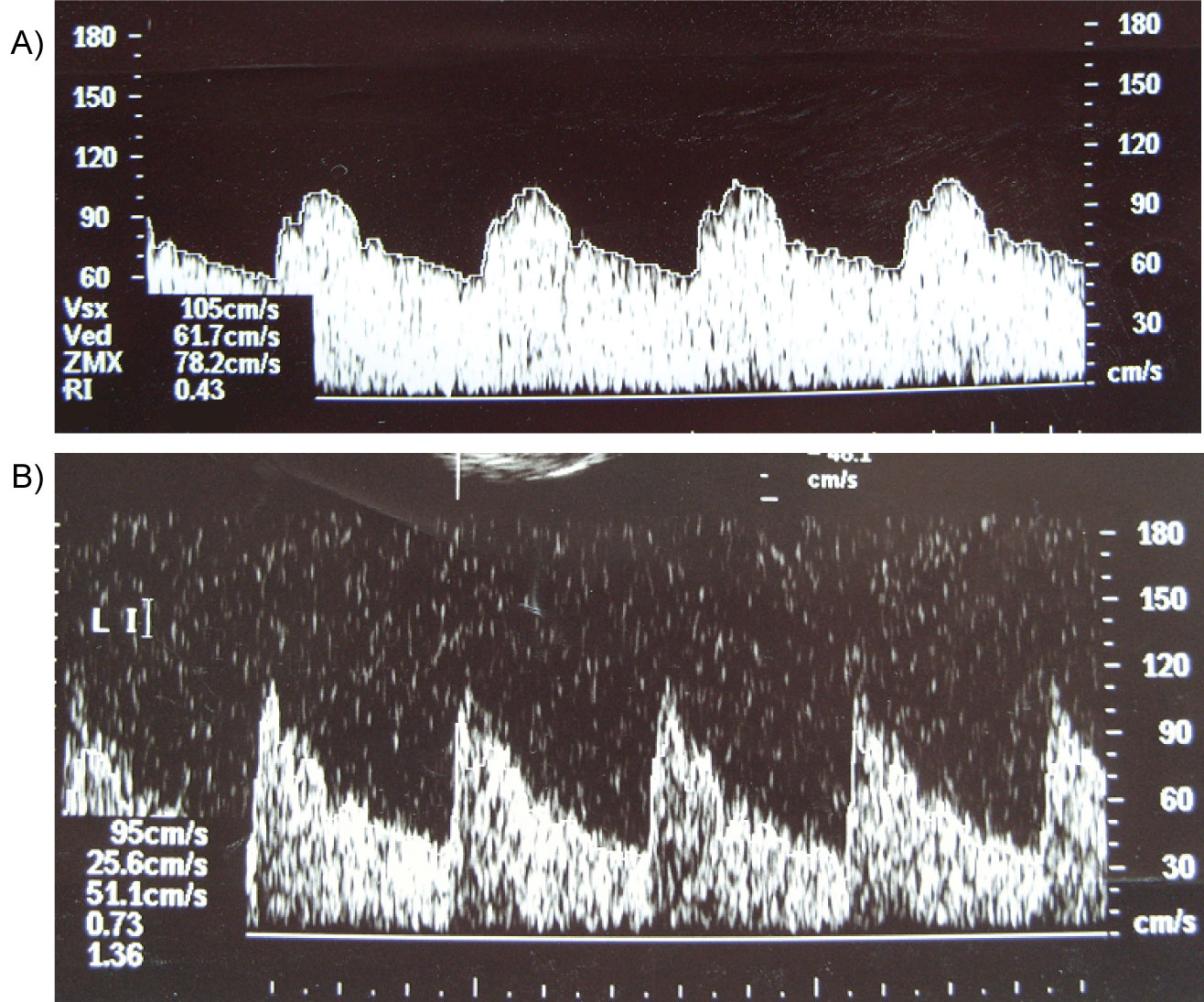
On re-admission to PICU Doppler ultrasound of the middle cerebral artery of the child showed severe cerebral hyperaemia indicated by low resistive index of 0.45. Five hours after restart of SPAD the cerebral blood flow normalized (Figure 2).
Supportive treatment
Midazolame and Es-ketamine were used for sedation. Glucose, electrolytes and water were given enterally. Parenteral nutrition containing 10 g/kg/d glucose, 0.5 g/kg/dlipids containing MCT fat, 0.5-1.0 g/kg/damino-acids , micronutrient substances, and double of the normal substitute dose of water soluble vitamins were continuously administered. The later were used to replace expected high losses of water soluble vitamins due to continuous high flow SPAD (Figure 1). Intravenous antibiotic and antimykotic treatment as well as oral bowel decontamination and lactulose were administered to prevent infections (Figure 3).
After 7 days (168 hours) of SPAD the child received a reduced size left liver lobe, segment two of left liver lobe, from a deceased adult donor [10].
The small portal vein of the recipient did not match the donors small portal segmental vein. The child underwent re-laparotomy three times for low flow (observed by Doppler Ultrasound) associated thrombus to position the liver to allow normal flow and for thrombectomy. After 5 days the portal vein remained occluded, although anticoagulation had been changed from heparin to dalteparin. Graft function slowly improved without portal flow. Ammonium returned to normal values after 10 days, the bilirubin decreased from 12 to 2.2 mg/dl and liver enzymes decreased and were only slightly above the upper range. The boy was extubated 16 days after re-transplantation. Nineteen days after re-transplantation intra-hepatic haemorrhage with compression and occlusion of the liver artery occurred followed by acute deterioration of liver function and hemodynamic instability of the patient. Noradrenaline, blood and volume replacement were required. Therefore, the second graft had to be explanted to stabilize the patient prior to this we had an ongoing discussion whether this child should be re-transplanted a third time. Because the boy was not sedated and ventilated and had no signs of neurologic sequelae prior to the third deterioration we decided together with the parents, surgeons, gastroenterology, intensive care physicians and our nurses to release an enquiry for high urgency liver transplantation at Eurotransplant. According to our Eurotransplant rules in case of an early transplant organ failure within 4 weeks after transplantation a re-nomination for high urgency re-transplantation is possible.
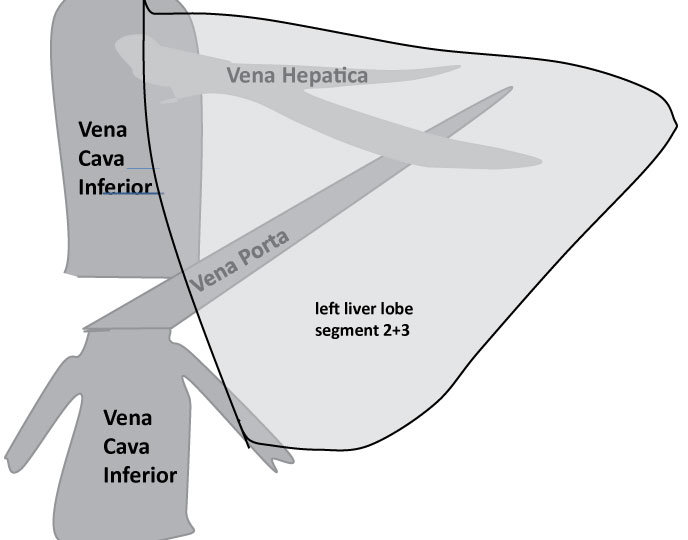
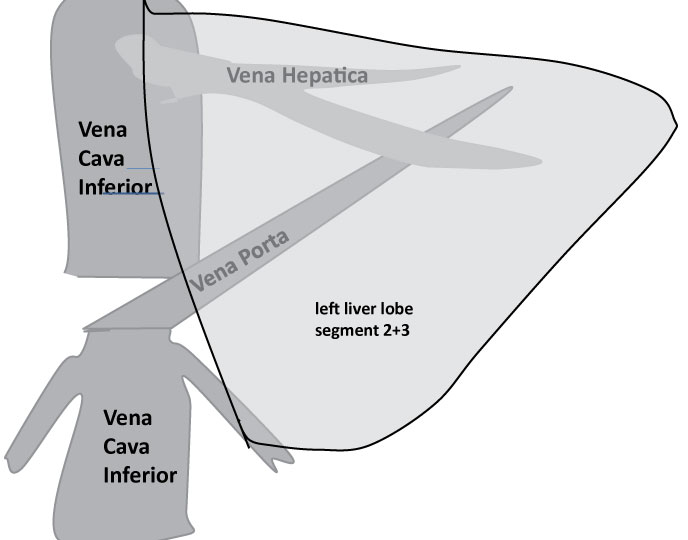
SPAD was restarted in this child. The same intensive care procedure including ventilation as described above resulted in stabilisation of circulation and stop of need of catecholamine application. 24 hours later the child was re-transplanted with a split graft (a left liver lobe of a teenage deceased donor). Due to persistent portal vein thrombosis including the vena mesenterica cavo-portal hemi-transposition (CPHT) was used. The inferior caval vein was attached end-to-end to the left portal vein of the graft to provide sufficient portal vein flow. The cranial end of the inferior vena cava was occluded; the abdominal wall was extended and closed with a Goretex-patch (Figure 4). Argatroban was administered for anticoagulation to anticipate recurrent thrombotic events. Routine swabs were taken. Thereby, bacteria and fungi (candida) were detected in the child´s abdominal drains. Thus, whenever fever occurred, the boy was treated with anti-microbiological agents according to the most recent resistogram. Despite proton-pump inhibitors the boy suffered from duodenal ulcer requiring clipping during an investigative endoscopy. 16 days after the third transplantation the child came off ventilation. He developed lower limb oedema, multiple intra-abdominal lymphoid cysts in the omentum majus and an adhesive ileus with bowel oedema. An extended abdominal surgical revision was necessary to remove these cysts and to re-insert new easy flow drains. The flow in the CPHT of the third liver was extremely sensitive to intra-abdominal pressure and several attempts to tighten and close the abdominal wall failed. The abdomen was revised 13 times in total. The final solution was an extension of the abdominal wall by dermal porcine collagen which served as firm basis for skull skin mesh-graft transplantation.
The child experienced several infections with bacteria, candida and CMV virus requiring timely treatment with anti-microbiological agents. Only once volume replacement but no further catecholamine application was necessary during one infection episode.
The boy was discharged home after 6 month, including 4.5 weeks of ventilation and a total of 192 hours of SPAD. At the time of discharge, graft function was normal, and the patient showed no ascites nor peripheral oedema.
2 years after his initial liver transplantation the boy presented with normal liver function and full neurologic recovery in our outpatient clinic.
At the most recent visit (Age: 9 yrs) he is suspected to have varicose veins and therefore micro bleedings and blood loss into his bowel related to his portal vein thrombosis. Nevertheless, so far no signs of a major bleeding are observed. He shows no ascites and no oedema of the lower limbs. His liver function and neurologic development are normal. His parents delayed his school. Attendance by one year because they feared infections related to immunosuppression.
Discussion
SPAD and Molecular-Adsorbent-Recirculation-System (MARS) can decrease water soluble and albumin bound toxins in ALF. Thereby the grade of encephalopathy is reduced, cerebral hyperperfusion decreases and clinical condition stabilizes [11,12]. In this anhepatic child higher turnover rates for SPAD than previously described were necessary to maintain blood levels for bilirubin, NH4, bile-acids, and the majority of amino acids including glutamine and glutamic acid within treatment goals [9]. There by a normal cerebral flow was achieved (in this boy) and catecholamines could be ceased. On SPAD the clearance of drugs, usually metabolized in the liver, was enhanced, which allowed us to use S-ketamine and midazolam despite liver failure. However, the loss of water soluble micronutrients, vitamins and medication like tranexamic acid during SPAD (on top of normal renal function) must to be encountered on treatment course. In addition to the beneficial effect of SPAD in our case, the (unintended) daily "blood/plasma exchange" during the first 3 days with up to 2,4 L per day until the bleeding was under control, might have added to the prolonged survival time without liver.
In the past, LTX was not considered in any case of portal vein thrombosis. The CPA end-to-end technique used in this case to connect the donor portal vein allowed sufficient flow in the presence of portal vein thrombosis. This allowed us firstly to avoid potential portal hypertension which might have been occurred if an arterialisation of the portal vein would have been established and secondary, to avoid hyperammonaemia followed be chronic encephalopathy due blood of the vena mesenterica by passing the liver. Portal vein thrombosis has been described in a total of 53 patients with only three children among them, with a favourable outcome in two of the latter three [13-16]. One of these children had a transient extension of the abdominal wall but no permanent artificial extension of the abdominal wall was used [13,14]. Similar to 22 (ascites)/17 (lower limb oedema) out of 53 patients (including 1 child) reported by Paskonis, et al. with cavo-portal anastomosis our patient suffered profound transient ascites and transient lower limb oedema during the early postoperative course [15]. Children with ALF after LTX carry a high risk for infections per se, therefore the close monitoring and early treatment of all infections was essential for our patient´s survival.
Conclusion
This is the longest anhepatic survival reported after total hepatectomy [1,4-6]. Modern techniques, SPAD and special surgical procedures as CPA and innovative materials as dermal porcine collagen, and a skilled interdisciplinary team of cooperating paediatric and surgical specialists can improve outcome in such patients.
References
- B Ringe, N Lübbe, E Kuse, et al. (1993) Total hepatectomy and liver transplantation as two-stage procedure. Ann Surg 218: 3-9.
- Sieders E, Peeters PM, TenVergert EM, et al. (2002) Graft loss after pediatric liver transplantation. Ann Surg 235: 125-132.
- Arora H, Thekkekandam J, Tesche L, et al. (2010) Long-term survival after 67 hours of anhepatic state due to primary liver allograft nonfunction. Liver Transpl 16: 1428-1433.
- Oldhafer KJ, Bornscheuer A, Frühauf NR, et al. (1999) Rescue hepatectomy for initial graft non-function after liver transplantation. Transplantation 67: 1024-1028.
- Bustamante M, Castroagudín JF, Gonzalez-Quintela A, et al. (2000) Intensive care during prolonged anhepatic state after total hepatectomy and porto-caval shunt (two-stage procedure) in surgical complications of liver transplantation. Hepatogastroenterology 47: 1343-1346.
- Detry O, De Roover A, Delwaide J, et al. (2007) Prolonged anhepatic state after early liver graft removal. Hepatogastroenterology 54: 2109-2112.
- Hammer GB, So SK, Al-Uzri A, et al. (1996) Continuous venovenous hemofiltration with dialysis in combination with total hepatectomy and portocaval shunting. Bridge to liver transplantation. Transplantation 62: 130-132.
- Montalti R, Busani S, Masetti M, et al. (2010) Two-stage liver transplantation: An effective procedure in urgent conditions. Clin Transplant 24: 122-126.
- Ringe H, Varnholt V, Zimmering M, et al. (2011) Continuous veno-venous single-pass albumin hemodiafiltration in children with acute liver failure. Pediatr Crit Care Med 12: 257-264.
- Srinivasan P, Vilca-Melendez H, Muiesan P, et al. (1999) Liver transplantation with monosegments. Surgery 126: 10-12.
- Pugliese F, Ruberto F, Perrella SM, et al. (2007) Modifications of intracranial pressure after molecular adsorbent recirculating system treatment in patients with acute liver failure: Case reports. Transplant Proc 39: 2042-2044.
- Novelli G, Rossi M, Pretagostini R, et al. (2003) A 3-year experience with Molecular Adsorbent Recirculating System (MARS): Our results on 63 patients with hepatic failure and color Doppler US evaluation of cerebral perfusion. Liver Int 23: 10-15.
- Kumar N, Atkison P, Fortier MV, et al. (2003) Cavoportal transposition for portal vein thrombosis in a pediatric living-related liver transplantation. Liver Transpl 9: 874-876.
- Verran D, Crawford M, Stormon M, et al. (2004) Liver retransplantation in an infant requiring cavoportal hemi transposition. Pediatr Transplant 8: 416-419.
- Paskonis M, Jurgaitis J, Mehrabi A, et al. (2006) Surgical strategies for liver transplantation in the case of portal vein thrombosis-current role of cavoportal hemitransposition and renoportal anastomosis. Clin Transplant 20: 551-562.
- Tzakis AG, Kirkegaard P, Pinna AD, et al. (1998) Liver transplantation with cavoportal hemitransposition in the presence of diffuse portal vein thrombosis. Transplantation 65: 619-624.
Corresponding Author
Hannelore IG Ringe, Department of Pediatric Intensive and Intermediate Care Medicine, Charité Campus Virchow, Universitätsmedizin Berlin, Germany.
Copyright
© 2019 Ringe H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.