It's a Bit Crowded in Here: Examining Pressure Reactivity in Craniocerebral Disproportion
Abstract
Introduction: Pressure reactivity index (PRx) is a measure used to gain insights into cerebrovascular reactivity. It is generally used in acute settings such as traumatic brain injury and subarachnoid hemorrhage, however there is limited data on its utility in cases of slow compensatory changes in intracranial pressure such as craniocerebral disproportion. Here we illustrate a case of how PRx can provide valuable information in such settings.
Case presentation: PRx was measured in a 10-year-old boy who presented with multiple cranioneuropathies, found to have craniocerebral disproportion and secondary compression of posterior fossa structures. He was taken to surgery for decompressive craniotomy and cranial expansion procedure. He had an acute worsening in PRx, with a slow stabilization over the following days.
Conclusion: This case highlights the importance of monitoring individualized PRx curves, and that autoregulatory curves are not constant between individuals or within same individuals over time. Acute iatrogenic interventions could lead to poor neurological consequences, due to interruption of a chronically compensated state.
Keywords
PRx monitoring, Pediatric neurosurgery, Craniocerebral disproportion
Introduction
Pressure reactivity index (PRx) examines the relationship between mean arterial blood pressure (ABP) and intracranial pressure (ICP) to gain insight into the relationship between cerebral perfusion pressure (CPP) and cerebral blood flow (CBF), or cerebrovascular reactivity. It has been studied most extensively in acute traumatic brain injury in attempts to guide management and assist with prognostication. However, few studies have examined PRx in slow, compensatory changes in cerebral pressure dynamics, such as what may occur in conditions like craniocerebral disproportion [1]. Here we present a case of craniocerebral disproportion, and the re-normalization of cerebrovascular reactivity after iatrogenic disruption of a compensated state.
Case Presentation
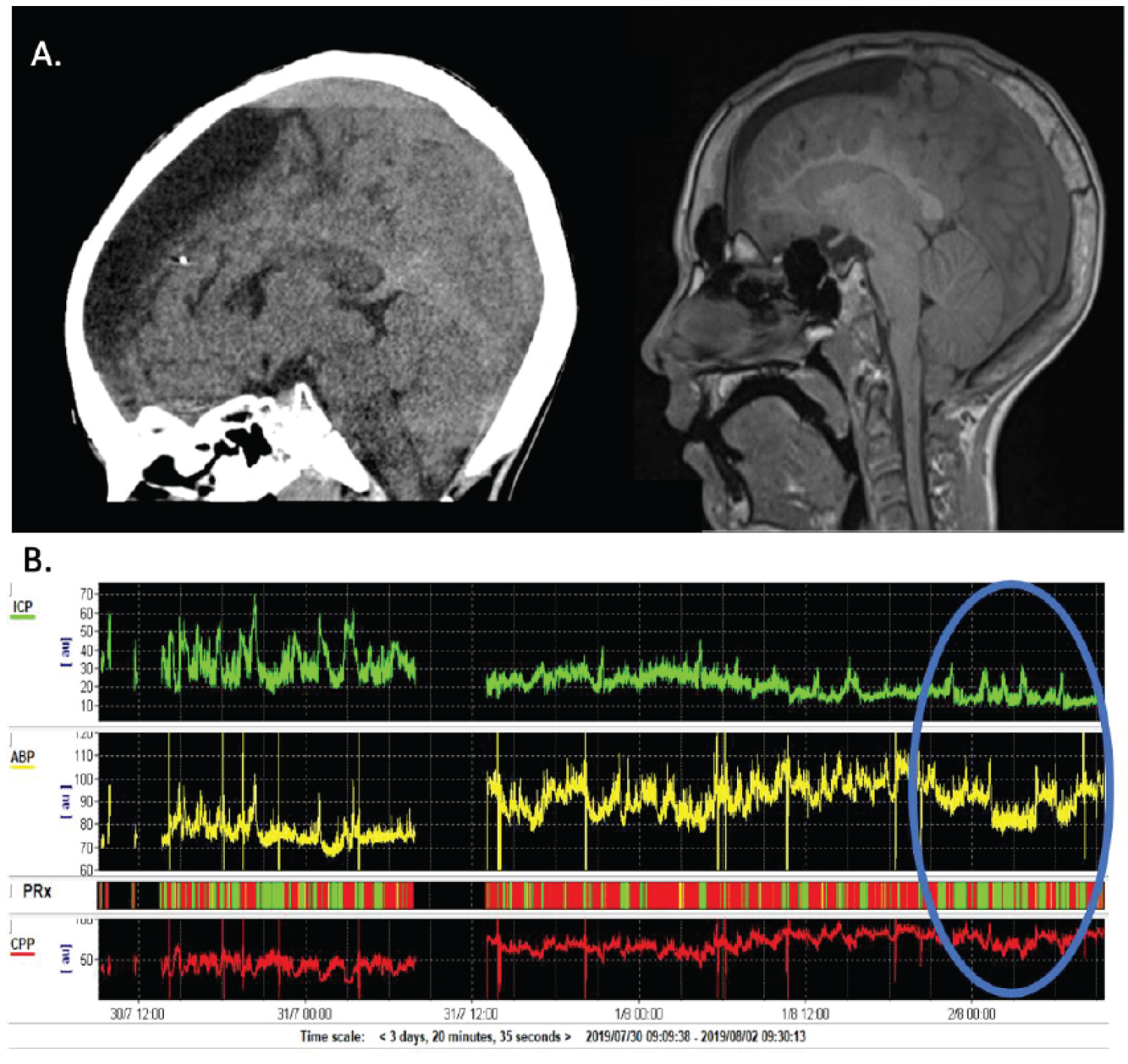
A 10-year-old boy presented to the emergency department with vomiting and impairment of multiple cranial nerves. He had a history of infantile spasms and prior workup revealed a left frontal cortical dysplasia. This was resected at 1-year of age and he subsequently developed an arachnoid cyst requiring shunting. The last VP shunt revision was 3-years prior to presentation, and his epilepsy was well controlled on carbamazepine and levetiracetam. The patient presented with a three-day history of vomiting, fatigue, and 'dysconjugate gaze' noted by his parents. On examination he had restricted bilateral upgaze more than downgaze. He had minimal adduction and no abduction of his left eye, and his right eye had no horizontal movement. He had a mild left facial droop including his forehead, and mild weakness with jaw clenching. CT and MR showed an unchanged left frontal arachnoid cyst, VP shunt in place, as well as significant skull thickening and crowding of the posterior fossa (shown in Figure 1A). Lateral ventricle size was small and stable compared to prior scans indicating intact shunt functioning. He was taken to the OR due to persistent altered level of consciousness for a sub occipital decompressive craniectomy, C1 arch excision, and insertion of a right frontal intraparenchymal intracranial pressure monitor.
PRx monitoring showed relatively intact cerebrovascular reactivity with few periods of poor cerebrovascular reactivity (shown in Figure 1B). ICP was persistently high and fluctuated between 20-60 mmHg, therefore a cranial expansion procedure was performed with a left craniotomy and bone flap thinning (drilled to approximately 3-4 mm thickness from 6-7 mm), with subsequent replacement of the flap. Following this procedure he had an instant decrease in his ICP to 15-30 mmHg, with a worsening of his pressure reactivity index, therefore suggesting poorer cerebrovascular reactivity (shown in Figure 1B). Over the next day, he had slow normalization of his ICP to 10-20 mmHg, improvement of his PRx, and clinical improvement with near resolution of his multiple cranial neuropathies at 3 weeks follow up.
This case highlights the utility of PRx in studying ICP dynamics, particularly the phenomena of cerebrovascular compensation over time and re-normalization after iatrogenic disruption. The patient likely had craniocerebral disproportion secondary to a combination of chronic over shunting and long-term anti-seizure medications [2,3]. After cranial vault expansion, ICP quickly stabilized and decreased to mean values of approximately 20 mmHg.
Discussion
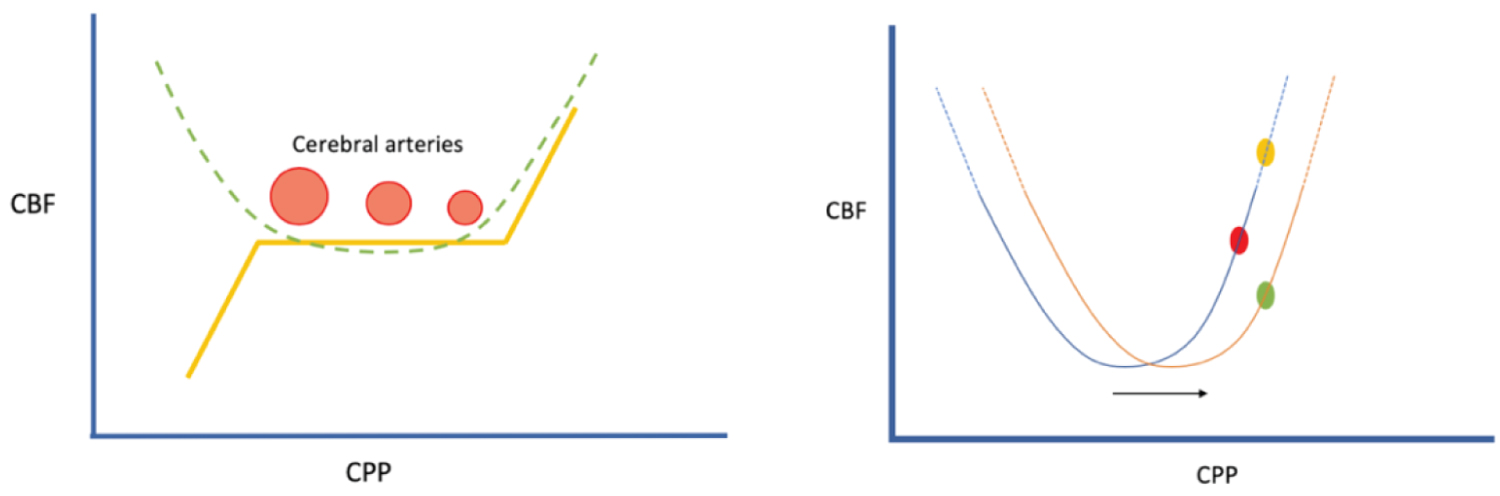
In healthy individuals, constant cerebral blood flow is maintained by autoregulation of ABP and ICP. Cerebral perfusion can be derived by subtracting ICP from ABP (CPP = ABP-ICP). In order to maintain CBF, if ICP increases, blood pressure should also increase to accommodate for the increase in end resistance, thereby ensuring a relatively constant CPP. This is achieved by regulating the diameter of the cerebral arterioles (shown in Figure 2). Cerebral blood vessels dilate in response to low systemic blood pressure and constrict in response to high blood pressure (shown in Figure 2). If ICP is lowered by increasing the size of the intracranial vault, blood pressure should decrease. However, analysis of the PRx immediately after surgery in our case shows that cerebral autoregulation was poor (red). ICP decreased due to intervention, but ABP increased thereby causing higher CPP values, i.e. ABP and CPP were moving passively in parallel. Thus, prior to surgery, there was better cerebrovascular reactivity despite chronically high ICP, and by disrupting this equilibrium we induced an initial period of poor cerebrovascular reactivity. It took approximately 24 hours for the system to recalibrate and resume good cerebrovascular control on post-operative day 2. This can be seen as the PRx values shift to green and ABP decreases with ICP (shown in Figure 1B).
To date, the complex mechanism of cerebral autoregulation is not entirely understood. Multiple theories exist to explain the dynamic interaction between metabolic demand and pressure differentials. Hypotheses include the ability of vasculature to sense hypoxia and ischemia though chemoreceptors within the neurovascular unit, and additionally, the capability of sensing ICP directly. Mechano-sensitive ion channels in the vascular smooth muscle of arterioles are postulated to be able to sense incoming pressure and flow, and may respond to both stretch and shear stress [4]. Such baroreceptors were postulated to exist based on early work in animals, and to be located within the 4th ventricle in the rostral ventrolateral medulla [5]. New theories have expanded upon this, and suggest that there is a CNS ABP set point that functions independently of the renal ABP set point, circulating around the idea that the kidneys are not solely responsible for setting blood pressure targets [6]. The specific mechanisms surrounding this model have yet to be elucidated. One point arguing for the later hypothesis in the case presentation above, is that there was no acute trauma or metabolic stress that would trigger chemoreceptors given the controlled change in ICP via surgical intervention. However, all of these theories operate on immediate responses to the local environment, and do not explain why it took 24 hours to re-equilibrate pressures in our case.
This case highlights the importance of monitoring individualized PRx curves over time. Importantly, research has shown that auto regulatory curves are not constant between individuals, or within the same individual over time [7]. While theories exist, the exact mechanisms behind cerebral autoregulation are not fully understood. The vast differences that occur in individualized settings highlight the importance of monitoring these changes, especially prior to undergoing surgical and pharmacological interventions. Acute iatrogenic changes could lead to poor neurological consequences, especially if a drastic change is made and interferes with a chronically compensated state.
Statement of Ethics
The patient's guardian gave written informed consent for the publication of this medical case and images. The study was exempt from ethical approval according to the Institutional Review Board of the University of Calgary in accordance with national guidelines (Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS2), Article 2.1).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There was no funding provided for this manuscript.
Author Contributions
All authors equally contributed towards data acquisition, analysis and interpretation, drafting and revising the manuscript, final approval of the version to be published, and are accountable for all aspects of the work in ensuring accuracy and integrity.
References
- Sandler AL, Daniels LB, Staffenberg DA, et al. (2013) Successful treatment of post-shunt craniocerebral disproportion by coupling gradual external cranial vault distraction with continuous intracranial pressure monitoring. J Neurosurg Pediatr 11: 653-657.
- Martínez-Lage JF, Ruíz-Espejo AM, Almagro MJ, et al. (2009) CSF overdrainage in shunted intracranial arachnoid cysts: a series and review. Childs Nerv Syst 25: 1061-1069.
- Siddiqui Z, Singh K (2017) Calvarial thickening due to anticonvulsants other than phenytoin. J R Coll Physicians Edinb 47: 359.
- McBryde FD, Malpas SC, Paton JF (2017) Intracranial mechanisms for preserving brain blood flow in health and disease. Acta physiologica 219: 274-287.
- Doba N, Reis DJ (1972) Localization within the lower brainstem of a receptive area mediating the pressor response to increased intracranial pressure (the Cushing response). Brain Res 47: 487-491.
- Osborn JW (2005) Hypothesis: Set-points and long-term control of arterial pressure. A theoretical argument for long-term arterial pressure control system in the brain rather than the kidney. Clin Exp Pharmacol Physiol 32: 384-393.
- Tzeng YC, Ainslie PN (2014) Blood pressure regulation IX: Cerebral autoregulation under blood pressure challenges. Eur J Appl Physiol 144: 454-559.
Corresponding Author
Kristine Elizabeth Woodward, MD/MSc, Division of Clinical Neuroscience, Department of Pediatrics, Alberta Children's Hospital, University of Calgary, 28 Oki Drive NW T3B6A8, Canada, Tel: 4039902724.
Copyright
© 2022 Woodward KE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.