A Possible Association between Arnold-Chiari Type I Malformation and Epilepsy: Case Series and Review
Introduction
Hans Chiari is the one credited with diagnosing Chiari malformations, dating back to 1890 [1]. Chiari malformations consists of four types of hindbrain abnormalities with the majority being a type I or type II CM-which is characterized by displacement of part of the cerebellum and brain stem into the foramen magnum, usually accompanied by myelomeningocele [2]. In this paper, the authors discuss the incidence and prevalence of epilepsy in a subset of pediatric patients harboring Chiari malformations type I (CM-I).
The displacement of the cerebellar tonsils is a classic hallmark of CM-I. Some authors suggest the definition of a cerebellar tonsil position ≥ 5 mm below the foramen magnum [3]. CM-I is a rare disease characterized by herniation of cerebellar tonsils below foramen magnum with associated anomalies of the posterior fossa [4]. It also has a wide array of symptoms, some of which are nonspecific such as headache and neck pain [3]. Of notice, 15%-20% of CM-I patients may develop hydrocephalus [5].
Epileptic seizures have been previously reported in patients with CM-I as an incidental finding [6-8]. Yet the widespread use of MRI in the diagnostic work up for children with epilepsy has shown incidental findings of CM-I in some children [9], thus de facto increasing the number of patients seen harboring both pathologies.
To date, no agreed-upon management exists in the literature. The authors report on 4 cases of epilepsy in CM-I patients which completely resolved without need for surgical correction of their cerebellar tonsillar herniation as well as a review of existing literature.
Methods
30 pediatric patients at a single institution which underwent MRI of the brain due to either headaches or other reasons and diagnosed with CM-I radiographically were reported. Patients underwent C spine MRIs as well. In patients with suspected epilepsy, electroencephalograms were done demonstrating epileptiform abnormalities. All patients underwent at least one more brain MRI imaging and were followed up by a treating pediatric neurologist.
Results
In our cohort we had a total of 30 patients. 18 were males and 12 were females, the average age was 8.43 years at diagnosis of CM-I. The vast majority of patients in the cohort underwent MRI imaging due to headaches, (10 patients). Some underwent MRI imaging of the brain due to the following: Failure to thrive (1), platybasia (1), precocious puberty (1), photophobia (1), dizziness (1), growth hormone deficiency workup (1) and 4 due to epilepsy. 10 other patients underwent brain imaging due to various unrelated reasons. Tonsillar herniation averaged at 10.2 millimeters upon diagnosis.
The mean follow-up was 22.3 months in all CM-I patients. Serial MRIs showed a consistent CM-I malformation without progression. One patient presented with ventricular dilatation. Only one patient had a cervical spine syrinx on imaging.
Patients were diagnosed first with an MRI and a follow up including a second MRI was performed at 6 or 12 months as part of a follow up. Timing of the second MRI was determined by the pediatric neurologist taking care of the patient.
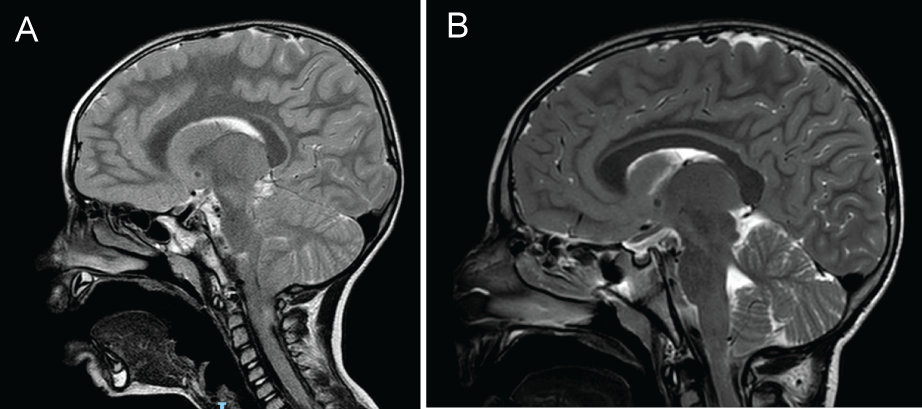
Of notice are the following patients without epilepsy in this cohort. One patient had a spontaneous resolution of his Chiari with no tonsillar herniation on a routine follow up imaging, (Figure 1A and Figure 1B), as well as disappearance of the syrinx. This patient presented with headaches and vomiting which resolved during follow up. The imaging which did not show tonsillar herniation was done 4 years after initial diagnosis. One patient had a bilateral choroid plexus papilloma. This patient presented with papilledema which resolved on follow up. He is currently asymptomatic. Another patient had initial diplopia which resolved after 6 weeks. Urinary and fecal incontinence spontaneously resolved in another patient.
CM-I and Epilepsy
There were 4 patients who presented with epilepsy. Three patients experienced absence epilepsy and one had focal epilepsy as witnessed by their parents and reported to the treating neurologist on scheduled follow ups. These epileptic fits were later-on confirmed with an EEG recording device displaying abnormal activity. All four patients were epilepsy free on follow up (12, 12, 23 and 31 months after initial diagnosis was made), and the median follow-up was 21 months per patient. All 4 patients were put on antiepileptic drugs in which 2 of them epilepsy drugs were stopped during follow up as these patients were seizure free for several months. The seizures did not recur in any of the four patients.
Of the four epilepsy patients, one presented with headaches with epilepsy appearing later during follow up, and 3 presented with an epileptic attack upon presentation. Tonsillar herniation was 9, 6, 6 and 6 millimeters for these 4 patients. None had any other CNS abnormalities (syrinx or ventricular dilatation), aside for one patient with cortical dysplasia seen on brain MRI imaging. These four patients are depicted in Table 1.
Discussion
The authors present 4 cases out of a cohort of 30 cases with CM-I which diagnosed with epilepsy between the years 2010-2017 in one institution. Meaning that 13% of the cohort had epilepsy. All patients in this series did not go any neurosurgical treatment but were followed up for a period of 23 months.
There is wide accepted agreement that symptomatic CM-I patients presenting with brainstem dysfunction, cranial nerves deficits, syringomyelia or kyphosis should be operated on. Whereas asymptomatic CM-I patients should not be operated on [10]. This may present as a challenge to the treating physician as he/she must bear in mind that other, coexistent disease might appear with CM-I which will not be amended by surgery alone. Several studies exist describing outcome in CM-I patients undergoing surgery whereas fewer studies exist describing nonsurgical CM-I patients with epilepsy. Buoni, et al. described disappearance of EEG abnormalities after posterior fossa decompression in CM-I patients, however this report takes into account 3 cases with asymptomatic children with EEG pathologies [11]. Granata, et al. describes 6 patients out of 99 CM-I patients with epilepsy [9]. All 99 patients underwent surgery with 4 patients being seizure free. 2 patients however presented with seizures after surgery was performed. In addition, Pandey, et al. described cerebellar fits in 13 patients out of a group of 47 patients with CM-I [12]. All 13 underwent surgery with symptom resolution. However, indication for surgery due to cerebellar fits is still not indicated in CM-I patients, and our cohort did not contain patients with abnormal EEGs which would have raised suspicion of cerebellar abnormalities. Therefore, the question of conservative management and a “wait and watch” strategy is at present a more acceptable approach.
Several theories have been proposed to connect between CM-I and epilepsy. Iannetti, et al. have used a type of photo emission CT study to demonstrate that in CM-I patients, hypoperfusion areas correlated to pathologic areas on EEG in CM-I patients with epilepsy [13]. However, presently no statistically significant studies exist to corroborate these assumptions. As such, current coexistence of both a CM-I and epilepsy patient is considered merely an incidental association.
In a study of 7 patients harboring CM-I with epilepsy, Elia, et al. described complete control of seizures in 4 patients in the long term as well as reduction in seizure rate in 3 other patients [14]. All did not undergo surgery for their CM-I [14]. Granata, et al. indeed mentions that epilepsy with CM-I may be an incidental finding and that prognosis is usually good [9]. The authors of this paper agree with this view and recommend a watch and wait strategy in CM-I patients presenting with epilepsy after a complete workout containing brain and cervical spine imaging, EEG and close follow up.
Study Limitations
In this cohort of 30 patients, patients with CM-I and a report of witnessed epilepsy underwent an EEG study. However not all parents were routinely asked whether or not epilepsy symptoms have occurred in their children. In addition, not all patients underwent an EEG as part of their work up for CM-I, which may have resulted in undetected instances of epilepsy in some patients and an underestimation of the actual number of children with CM-I malformations and epilepsy. In addition, the four children diagnosed with CM-I and epilepsy were diagnosed at a smaller age than the rest of the group. This may lead to the assumption that patients with epilepsy and CM-I are diagnosed at an earlier age. However, this number is too small to assume this assumption.
Conclusion
Epilepsy in CM-I is an unexplained entity. In this cohort of patients however, it appeared in higher frequency in patients harboring CM-I pathologies. Our study raises the possibility of the dual occurrence of epilepsy and CM-I in which epilepsy is transient after several years. The use of anti-epileptic drugs which are eventually tapered down to a minimum or stopped altogether is an option in this group of patients. The medical staff treating these patients must bear in mind that CM-I and epilepsy can coexist albeit for a short period of time in a CM-I patient's life when taking care of these patients. Further studies are needed in-order to clarify the association between CM-I and epilepsy.
The authors confirm the following statements are correct:
• The authors confirm this manuscript has not been published elsewhere.
• The authors report no financial disclosures.
References
- Abd-El-Barr MM, Strong CI, Groff MW (2014) Chiari malformations: Diagnosis, treatments and failures. J Neurosurg Sci 58: 215-221.
- Shen J, O'Keefe K, Webb LB, et al. (2015) Acute porphyria in a patient with Arnold Chiari malformation. Am J Case Rep 16: 99-103.
- Khalsa SSS, Geh N, Martin BA, et al. (2018) Morphometric and volumetric comparison of 102 children with symptomatic and asymptomatic Chiari malformation Type I. J Neurosurg Pediatr 21: 65-71.
- Giammattei L, Borsotti F, Parker F, et al. (2018) Chiari I malformation: Surgical technique, indications and limits. Acta Neurochir (Wien) 160: 213-217.
- Strayer A (2001) Chiari I malformation: Clinical presentation and management. J Neurosci Nurs 33: 90-96.
- Aitken LA, Lindan CE, Sidney S, et al. (2009) Chiari type I malformation in a pediatric population. Pediatr Neurol 40: 449-454.
- Elster AD, Chen MY (1992) Chiari I malformations: Clinical and radiologic reappraisal. Radiology 183: 347-353.
- Tubbs RS, McGirt MJ, Oakes WJ (2003) Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg 99: 291-296.
- Granata T, Valentini LG (2011) Epilepsy in type 1 Chiari malformation. Neurological Sciences 32: 303-306.
- Haines SJ, Berger M (1991) Current treatment of Chiari malformations types I and II: A survey of the Pediatric Section of the American Association of Neurological Surgeons. Neurosurgery 28: 353-357.
- Buoni S, Zannolli R, di Bartolo RM, et al. (2006) Surgery removes EEG abnormalities in patients with Chiari type I malformation and poor CSF flow. Clin Neurophysiol 117: 959-963.
- Pandey A, Robinson S, Cohen AR (2001) Cerebellar fits in children with Chiari I malformation. Neurosurg Focus 11: E4.
- Iannetti P, Spalice A, De Felice Ciccoli C, et al. (2002) Seizures in paediatric Chiari type I malformation: The role of single-photon emission computed tomography. Acta Paediatr 91: 313-317.
- Elia M, Biondi R, Sofia V, et al. (1999) Seizures in Chiari I Malformation: A Clinical and Electroencephalographic Study. J Child Neurol 14: 446-450.
Corresponding Author
Uri P Hadelsberg, Department of Neurosurgery, Shaare Zedek Medical Center, Shmuel Beit 12, Jerusalem, Israel, Tel: +972-547-804-594.
Copyright
© 2018 Hadelsberg UP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.