A Primary Gastric Neuroendocrine Tumour (GNET): A Rare Entity
Abstract
Gastric neuroendocrine tumour is one of the rare tumours of gastrointestinal tract with incidence ranging from 0.3% to 1.8% and more commonly seen in Caucasians [1]. Owing to growing incidence of tumour, we need to be updated about the knowledge and management guidelines as majority patients are asymptomatic and often present with diagnostic dilemma. We hereby present a case of 39-year-old male with complaints of pain abdomen which on USG and CT was suspected of having alternate diagnosis but ultimately radical surgical excision revealed the diagnosis. We need more such case reports and meta-analysis to keep our understanding of such rare tumour and its management, prognosis to look for availability of novel therapeutic options in future.
Keywords
Gastric, Neuroendocrine, Somatostatin, Endoscopy
Introduction
Gastric neuroendocrine tumour is the rare tumour which originates from Kulchitsky cells, embryologically of neural crest origin [2]. Majority of patients are asymptomatic at presentation and majority are located in small intestine, rectum, appendix, etc. with few in stomach. Amongst those diagnosed most are localized, while metastatic GNET constitutes only less than one third of the cases [3]. Endoscopic biopsy is often required for diagnosis, although rarely radical surgical excision only confirms definitive diagnosis. CT is required for staging, MRI better delineates the liver metastasis. Surgical excision to negative margin remains the mainstay of treatment although, role of somatostatin analogues or surveillance for small tumours is increasingly being considered. Rising incidence will pave the way for novel diagnostic as well as therapeutic options as more and more information becomes available on this rare and variedly expressing tumour.
Case Report
A 39-year-old male presented with on and off complaints of pain epigastric region with no significant findings on physical examination. Ultrasound abdomen revealed paraaortic solid mass measuring 85 × 53 mm with few pelvic carcinomatosis. CT contrast revealed neoplastic etiology of stomach with background of Menetriers disease and second differential to be gastric lymphoma with multiple regional lymphadenopathy. Endoscopic biopsy revealed submucosal chronic inflammatory lesion while colonoscopy was normal. Patient underwent exploration and palliative total gastrectomy with regional lymphadenectomy along with oesophagojejumostomy and Roux en Y jejunojejunostomy. Biopsy revealed final diagnosis of Gastric neuroendocrine tumour with positive staining for Synaptophysin and chromagranin as IHC markers. During postoperative period patient developed Right subclavian vein, axillary vein and distal internal jugular vein DVT. Central line was removed, patient managed with heparin and Vitamin K antagonists and improved with no fresh complaints on discharge.
Histopathological Report
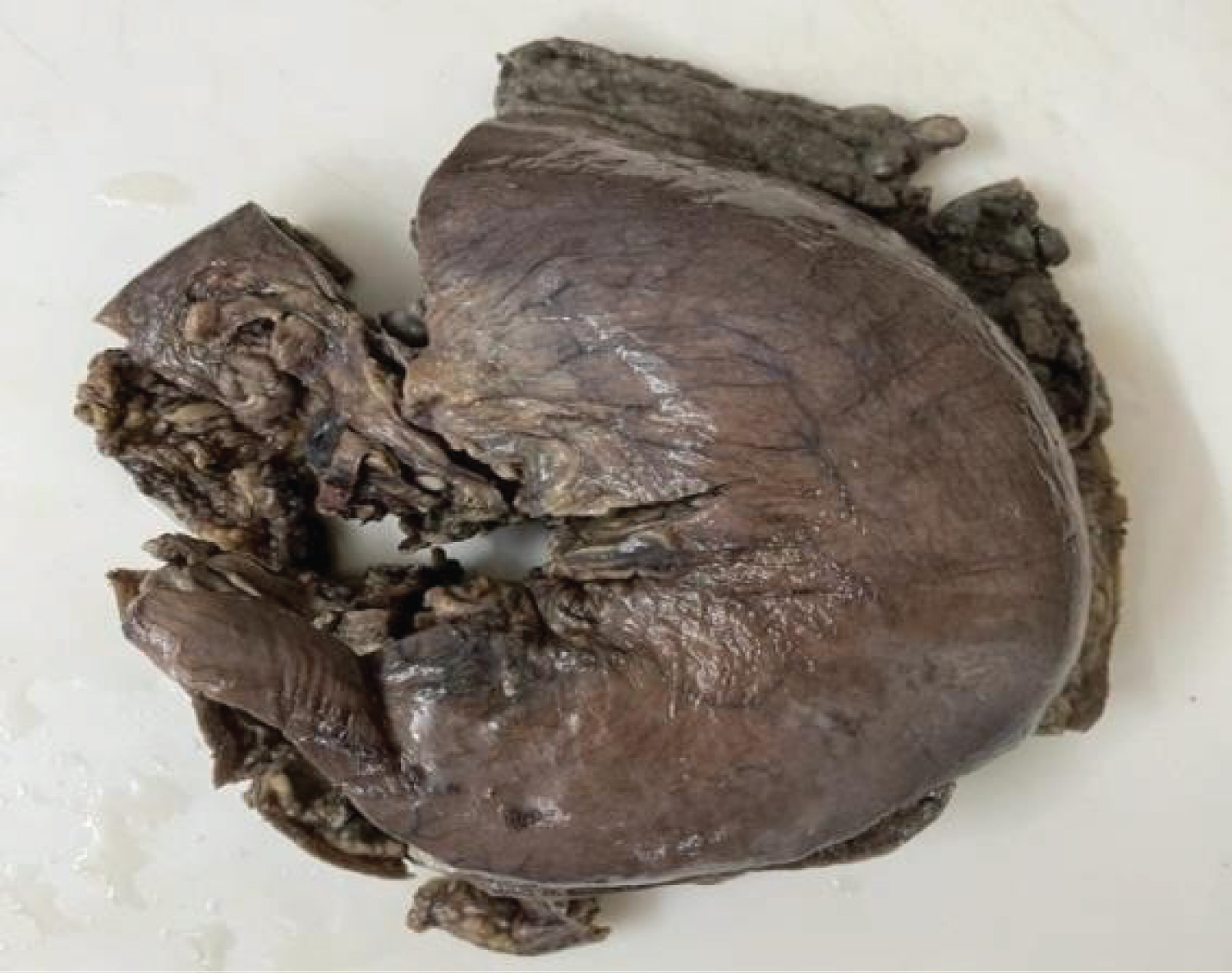
Gross: Gastrectomy specimen of size measuring 27 cm along greater curvature and 13 cm from lesser curvature was received with smooth external surface without perforation. Entire mucosa was replaced by numerous polypoidal growth of varying sizes ranging from 0.8 cm × 0.8 cm × 0.5 cm to 3 cm × 3.5 cm × 2.5 cm. Oesophageal part of 6 cm in length noted with external serosal surface free of tumour deposits. Large lymph node 9 × 6 × 4 cm noted (paraaortic) capsulated and congested. Cut surface reveals white, hemorrhagic and necrotic tissue. Oesophageal and jejunal cut ends measuring 3 cm and 2.5 cm in length received and were free of tumours grossly. Excised omentum reveals metastatic tumour deposits (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6).
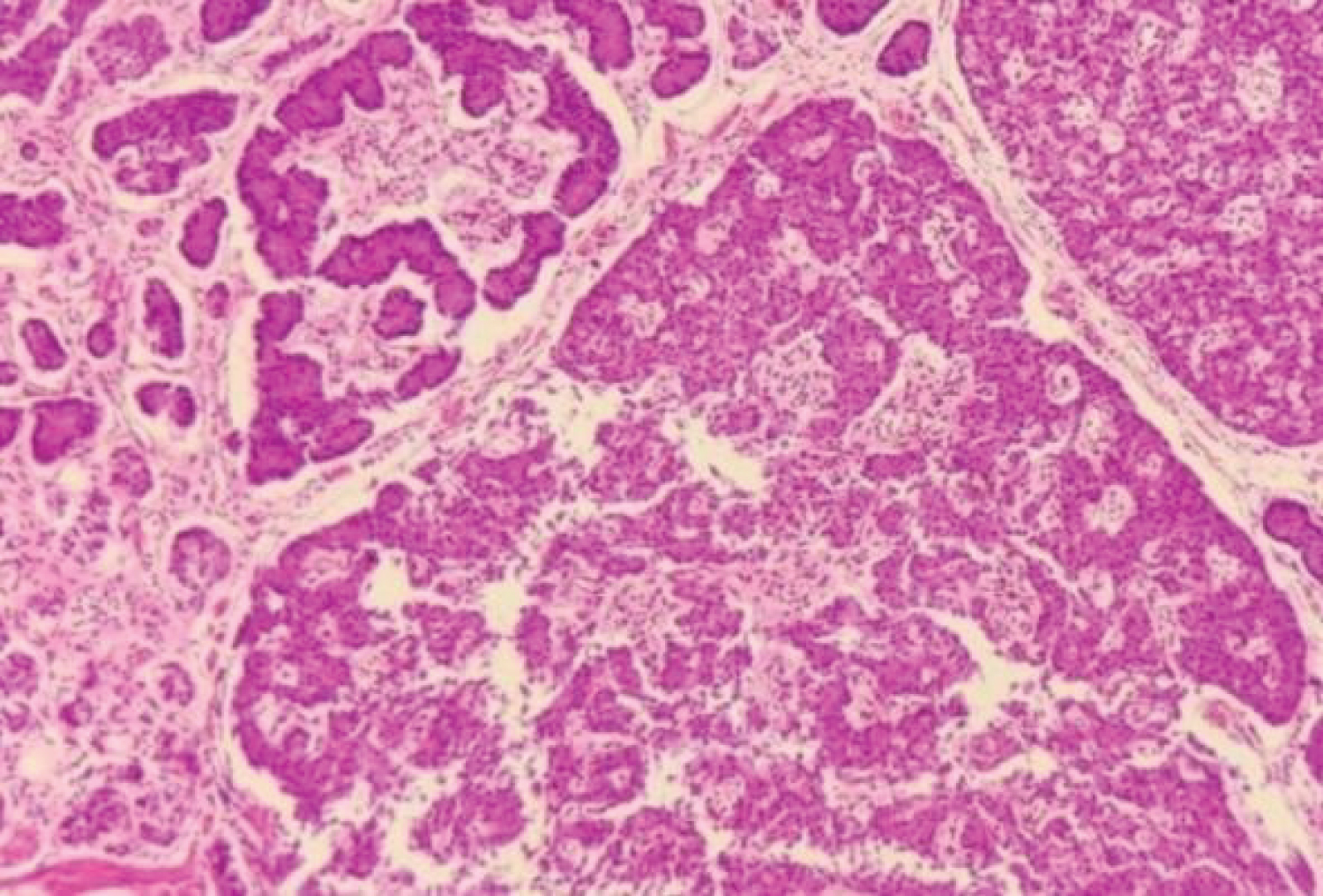
Microscopy: Well differentiated Neuroendocrine tumour which is grade I (G1), multifocal with mitotic rate of < 2/HPF, Ki-67 index is < 3%. Tumour involves muscularis propria with negative margin and lymphovascular invasion present but no perineural invasion. Out of the total 17 lymph nodes received, 12 had metastatic tumour deposits. Tumour is positive for both Synaptophysin and chromagranin but negative for cytokeratin 7 (CK-7) IHC markers.
Pathological staging as per AJCC 8 th classification is pT 2 N 1 .
Discussion
• Gastric neuroendocrine tumour is increasingly being diagnosed due to more and more use of routine endoscopy. Despite this fact, the diagnosis of neuroendocrine tumours remains a diagnostic dilemma as majority remain asymptomatic and also lack of specific markers, varied presentations and mimicking various GI tumours as differential.
• It is of 3 types- Type I, II and III. Type I is most commonly seen (~70%) and usually associated with atrophic gastritis or PPI use, Type II is seen in 5-10% cases and its occurrence is linked with Zollinger Ellison syndrome or hypergastrinemia. Type III is seen in 15-20% cases and is prognostically more aggressive and malignant [4].
• Tumour can be functional or non-functional but foregut NET usually secrete high 5- hydroxy tryptophan or ACTH but low serotonin, although symptoms may range from being asymptomatic to malignant carcinoid syndrome presenting with flushing, etc.
• Diagnosis is often by endoscopic biopsy. However, staging is done by Contrast CT and liver metastasis is better seen in MRI. If CT fails to delineate metastasis, then somatostatin receptor scintigraphy using 68Ga DOTATATE or 64Cu DOTATATE reveals metastasis in better way [5].
• Usually large cell NET’s stain positive for both Synaptophysin and chromagranin while rarely small cell variety may be negative for both (< 5%). Punctuate necrosis is one of the characteristics of NET commonly seen.
• Treatment options include surgical resection with negative margin with regional lymphadenectomy if involved. However, treatment depends on many factors including size, mitotic rate, metastasis, Ki 67 index, Grade of the tumour, WHO classification, involvement of muscularis propria, lymphovascular invasion, etc. Metastatic functional or surgically unfit patients respond to somatostatin analogues while novel therapy like mTOR inhibitors, radionuclide linked peptide therapy is ongoing in many European or US centres [6].
• Median survival after treatment is variable ranging from a year to even 10 years owing to varied presentations, multiple factors deciding therapy, effectiveness of each therapy, etc.
• Due to rare occurrence, asymptomatic and varied presentations, diagnostic dilemma, prognostic variability it becomes necessary to report such cases for case series, systemic reviews and encourage more trials with novel therapies on GNET. For instance, role of microRNA as marker for sub analysis for differentiation of non-endocrine NET from endocrine NET is important as it will make us to detect non-functional tumours early which in turn will predict prognosis and make available therapeutic options, making chances of cure at its best. This will also help to predict outcomes in a better way [7].
Conclusion
As the incidence and prevalence of Gastric neuroendocrine tumours is on rise we need to be more specified in diagnostic criteria, defining therapies of choice and selection criteria with better prognostic information availability. However, due to different subtypes prevalent, currently multidisciplinary analysis based on clinipathological features, histopathological characteristics like mitotic rate, degree of differentiation, metastasis, lymphovascular invasion, IHC staining profile, Ki 67 index, etc. in combination best predicts prognosis and therapeutic measures for GNET. Presently, suitable therapy should be individualised based on above mentioned factors as per ENETS or NCCN guides for treatment or follow-up.
References
- Modlin IM, Lye KD, Kidd M (2004) A 50-year analysis of 562 gastric carcinoids: Small tumor or larger problem? Am J Gastroenterol 99: 23-32.
- Frilling A, Akerstrom G, Falconi M, et al. (2012) Neuroendocrine tumor disease: An evolving landscape. Endocr Relat Cancer 19: R163-R185.
- Dasari A, Shen C, Halperin D, et al. (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3: 1335-1342.
- Corey B, Chen H (2017) Neuroendocrine tumors of the stomach. Surg Clin North Am 97: 333-343.
- Johnbeck CB, Knigge U, Loft A, et al. (2017) Head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/ CT: A prospective study of 59 patients with neuroendocrine tumors. J Nucl Med 58: 451-457.
- Breeman WA, de Jong M, Kwekkeboom DJ, et al. (2001) Somatostatin receptor-mediated imaging and therapy: Basic science, current knowledge, limitations, and future perspectives. Eur J Nucl Med 28: 1421-1429.
- Roldo C, Missiaglia E, Hagan JP, et al. (2006) MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol 24: 4677-4684.
Corresponding Author
Ankita Purohit, Academic Junior Resident, Department of Pathology, Dr S N Medical College, Jodhpur, India.
Copyright
© 2024 Purohit A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.