Extranodal NK/T-Cell Lymphoma, Nasal Type with Thrombocytopenia
Abstract
Extranodal NK/T-cell lymphoma, nasal type (ENKTL) is a highly malignant and aggressive lymphoma. A 48-year-old woman with nasal obstruction, facial swelling, fever, and headache was found to have nasal tumor. The bilateral nasal cavity was occupied with hemorrhagic granulomatous tissue. The pathology by biopsy was diagnosed with exudate and necrotic tissue. Blood tests showed moderate leukopenia; anemia; thrombocytopenia; hypoalbuminemia; and decreased liver function. For a complete biopsy and treatment for tentatively diagnosed chronic sinusitis, she underwent bilateral endoscopic sinus surgery under general anesthesia. Platelet transfusion was performed preoperatively because of thrombocytopenia. The patient was finally diagnosed with ENKTL. Postoperative intermittent epistaxis lasted for 2 weeks. A blood transfusion was performed several times. A CHOP chemotherapy regimen was started. However, thrombocytopenia did not recovered and gradually deteriorated. After 2 courses of chemotherapy, massive bleeding from the gastrointestinal tract caused death. ENKTL show an angiocentric and angiodestructive growth pattern with fibrinoid changes within blood vessels. ENKTL may cause angioinvasion with tumor necrosis due to its angiocentric growth. In the case of surgery for biopsy, control of bleeding is very important.
Keywords
NK/T cell lymphoma, Thrombocytopenia, Nasal-type, Extranodal
Introduction
Extranodal NK/T-cell lymphoma, nasal type (ENKTL) is a highly malignant and aggressive lymphoma. The prognosis is poor, e.g. overall 5-year survival rate, 42% [1]. The major initial symptoms are relatively silent and include nasal obstruction, rhinorrhea, sinusitis, and epistaxis. Therefore, there is long delay, e.g. 11-14 months, from the appearance of initial symptoms to diagnosis [2]. ENKTL is rare in Europe and more frequent in Asia. Epstein-Barr virus (EBV) is present in nearly all ENKTL cases and ENKTL is defined in the 2008 World Health Organization classification as a predominantly extranodal lymphoma characterized by vascular damage and destruction, prominent necrosis, cytotoxic phenotype and association with EBV [3-5]. ENKTL is a disease derived from activated NK cells and occasionally from cytotoxic T cells latently infected by EBV [3-5]. EBV shapes the tumor microenvironment by promoting Th2-skewed T cell responses and by increasing the expression of the immune checkpoint ligand PD-L1 [6]. ENKTL is angioinvasion with tumor necrosis and causes nasal bleeding. Diagnosis requires a biopsy for histopathological confirmation demonstrating NK/T cell markers and EBV. However, patients experience massive bleeding after surgical resection for biopsy. In this report, we present a case study and discuss the problems.
Case
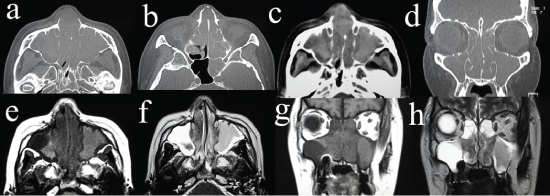
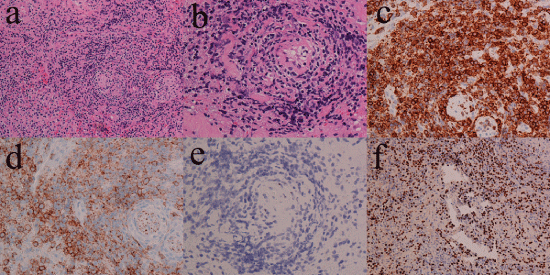
A 48-year-old woman presented with a 2.5-year history of right chronic sinusitis, a 4-month history of nasal obstruction, facial swelling, fever, and headache. She consulted an otolaryngological medical practitioner and was given oral antibiotics for 2 months, which did not resolve the fever and facial swelling. Her clinical history was epilepsy for 2 years. She consulted a doctor of neurology for epilepsy, and MRI suggested nasal disease. She was referred to our hospital. She suffered from nasal obstruction, occasional nasal bleeding, headache, fever, loss of appetite, and fatigue. Her face was slightly edematous around the eye lids and nose. The bilateral nasal cavity was occupied with hemorrhagic granulomatous tissue. The nasopharynx could not be observed by fiberscopy. The larynx was normal by observation using a laryngoscope. Cervical lymph was not swollen. A CT scan revealed bilateral sinus inflammatory changes without bony destruction and a slightly elevated density of the skin of the dorsum nasi (Figure 1a and Figure 1d). T1-weighted MRI demonstrated a low intensity area in the bilateral nasal cavities and an intermediate intensity area in the maxillary sinus, ethmoid sinus, frontal sinus, and sphenoid sinus (Figure 1e and Figure 1g). The lateral part of right maxillary sinus showed a low intensity area in T-1 weighted MRI. T2-weighted MRI demonstrated low intensity areas in the bilateral nasal cavity and in a part of the left maxillary sinus; and high intensity area in the right maxillary sinus, part of the left maxillary sinus, bilateral ethmoid sinus, frontal sinus, and sphenoid sinus (Figure 1f and Figure 1h). At the time of consultation at our institute, blood tests showed moderate leukopenia (white blood cell count, 2900/μL; metamyelocyte, 2%; band cell, 4%; segmental neutrophil, 59%; monocyte, 5%; lymphocyte, 30%); anemia (hemoglobin, 9.6 g/dL); thrombocytopenia (platelet count, 114 k/μL); hypoalbuminemia (albumin, 3.5 g/dL); normal renal function (creatinine, 0.56 mg/dL; urea nitrogen, 10 mg/dL); elevated concentrations of total bilirubin (0.7 mg/dL), transaminates (aspartate aminotransferase, 272 U/L; alanine aminotransferase, 132 U/L), lactate dehydrogenase (689 U/L), alkaline phosphatase (694 U/L), and γ-glutamyl transpeptidase (84 U/L); and slightly elevated concentrations of C-reactive protein (1.1 mg/dL). Prothrombin time was 11.9 s, and the concentrations of total IgE and interleukin-2 receptor were 44 IU/mL and 1290 U/mL, respectively. We consulted a hepatologist because of hepatic dysfunction. Hepatitis B and Hepatitis C were not detected. An abdominal ultrasound examination showed a normal-sized liver with no infiltration of tumor. After 3 weeks, thrombocytopenia worsened. At first, we suspected drug hepatopathy due to a long-term administration of antibiotics, so the prescription for antibiotics was stopped. However, after 2 weeks, transaminates were still high, and thrombocytopenia worsened. The nasal cavity was completely obstructed with granulomatous tissue, which was biopsied. The pathology was diagnosed with exudate and necrotic tissue. The patient was tentatively diagnosed with bilateral chronic rhinosinusitis with a suspected rhinosinusitis tumor. For a complete biopsy and treatment for tentatively diagnosed chronic sinusitis, she underwent bilateral endoscopic sinus surgery under general anesthesia. Preoperative blood tests showed pancytopenia (white blood cell count, 2300/μL; hemoglobin, 9.3 g/dL; mean cell volume, 68.1 fL; platelet count, 69 k/μL); hypoalbuminemia (albumin, 2 g/dL); elevated concentrations of total bilirubin (1.3 mg/dL), transaminates (aspartate aminotransferase, 324 U/L; alanine aminotransferase, 121 U/L), lactate dehydrogenase (723 U/L), alkaline phosphatase (1652 U/L), and γ-glutamyl transpeptidase (180 U/L); and slightly prolonged prothrombin time of 15.7 s. Platelet transfusion was performed preoperatively because of thrombocytopenia. The bilateral nasal cavities were completely obstructed with hypertrophic granulomatous inferior turbinates and nasal septum. The middle turbinates were also hypertrophic and granulomatous. The membrane of the bilateral ethmoid sinus was composed of granulomatous tissue. The bilateral maxillary sinus was occupied with mucous discharge, and the mucous membrane around the natural ostium was hypertrophic and granulomatous, but the membrane inside was slightly edematous but neither hypertrophic nor granulomatous. The bilateral ethmoid sinus had an edematous mucous membrane but no granulomatous tissue. The frontal and sphenoid sinuses were not approached because of poor vision. The hypertrophic granulomatous tissue was hemorrhagic. The hypertrophic tissue in the common nasal meatus was resected to keep the nasal airway open. The operative time was 60 min, and bleeding was 600 ml. Postoperative intermittent epistaxis lasted for 2 weeks although the nasal cavities were tightly packed by absorbable cotton and gauze containing antibiotics. A blood transfusion was performed several times. Due to disturbance of consciousness and repeated epileptic seizure, endotracheal intubation and artificial respiration were required. Postoperative pathology showed diffuse infiltration of lymphoid cells with angiocentric and angioinvasive patterns (Figure 2a and Figure 2b). By immunohistochemistry, the neoplastic cells express CD3, CD56, TIA-1, perforin, Granzyme-B, CD30-HQ (slightly), Ki-67 LI (70%), and c-MYC (20-30%) but not CD20, CKAE1/3, CD4, and CD8 (Figure 2c, Figure 2d and Figure 2e). They are positive for EBV-encoded RNA (EBER) by in situ hybridization (Figure 2f). The patient was diagnosed with ENKTL. A bone marrow biopsy could not be performed because of the patient’s severe general condition. Disturbance of consciousness was suspected to be caused by probable infiltration of lymphoma into the central nerve system. Three weeks after endotracheal intubation, tracheostomy was performed for long-term artificial respiration. Although slight bleeding occurred during the tracheostomy, the patient undertook preoperative platelet transfusion. Chemotherapy using DeVIC regimen (dexamethasone, etoposide, ifosfamide, and carboplatin) was considered. However, patient’s performance status was too poor to be treated with DeVIC regimen. A CHOP chemotherapy regimen (cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone) was started. The transaminates were gradually decreased, but total bilirubin and thrombocytopenia gradually deteriorated. After 2 courses of chemotherapy, the blood tests showed severe pancytopenia (white blood cell count, 200/μL; hemoglobin, 4.8 g/dL; mean cell volume, 84.5 fL; platelet count, 12 k/μL) and massive bleeding from the gastrointestinal tract caused death. This patient has very advanced ENKTL, and the bone marrow, the gastrointestinal tract was presumably infiltrated by ENKTL, and she finally died of gastrointestinal bleeding, which may probably due to necrosis of tumor induced by CHOP chemotherapy.
Discussion
ENKTL is an aggressive type of lymphoma [1]. Its major symptoms are nasal obstruction and epistaxis. Pathologically, lesions show a diffuse, permeative lymphomatous infiltration. An angiocentric and angiodestructive growth pattern [7] is common with fibrinoid changes within blood vessels. ENKTL may cause angioinvasion with tumor necrosis due to its angiocentric growth.
If the function of the liver or bone marrow is deteriorated, a decrease of coagulability and thrombocytopenia occurs. With respect to bleeding, nasal surgery for ENKTL should be considered as a high risk of bleeding. In general, a nasal biopsy can be taken by forceps and is often sufficient for pathological diagnosis. However, nasal biopsy of ENKTL is often difficult, and re-biopsy may be required because ENKTL is rich in fibrinoid changes, necrotic tissue, and infectious mucosa, e.g. chronic sinusitis, fungal sinusitis, or necrosis, often co-exist with lymphoma. Infectious tissue around the tumor is often biopsied instead of the objective tumor. It commonly takes a long time to diagnose ENKTL. Therefore, biopsy needs to take enough tissue to detect ENKTL, and sometimes surgery is needed. On the other hand, massive resection can induce bleeding, such as in our case. This is a controversial and very worrisome problem. In the case of surgery, the preoperative transfusion of platelets may be considerable.
Treatment for ENKTL
In limited stage ENKTL, chemotherapy using CHOP regimen (cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone) is unsatisfactory [8], because ENKTL expresses P-glycoprotein resulting in tumor multidrug resistance [9]. Radiotherapy is effective but cannot prevent recurrence [10]. Radiotherapy combination chemotherapy is a standard therapy for limited stage ENKTL. Acceptable combination chemotherapy regimen to add to radiotherapy is DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) [11] or combination of weekly cisplatin followed by VIDL (etoposide, ifosfamide, dexamethasone, and L-asparaginase) [12]. The best treatment for advanced stage ENKTL is still unknown. Anthracycline-based chemotherapy showed poor results [13]. The several reports suggest the efficacy of regimens incorporating L-asparaginase [14]. A phase II study of SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) had been highly successful in treatment [14]. Further study is ongoing.
Financial Disclosure
The authors received no funding for this study.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Vose J, Armitage J, Weisenburger D, et al. (2008) International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 26: 4124-4130.
- Coha B, Vucinic I, Mahovne I, et al. (2013) Extranodal lymphomas of head and neck with emphasis on NK/T-cell lymphoma, nasal type. J Craniomaxillofac Surg 42: 149-152.
- Kim WY, Jung HY, Nam SJ, et al. (2016) Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch 469: 581-590.
- Asano N, Kato S, Nakamura S (2013) Epstein-Barr virus-associated natural killer/T-cell lymphomas. Best Pract Res Clin Haematol 26: 15-21.
- Chan JK, Quintanilla-Martinez L, Ferry JA, et al. (2008) Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, World Health Organization Classfication of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France, IARC Press, 285-288.
- Gru AA, Haverkos BH, Freud AG, et al. (2015) The Epstein-Barr Virus (EBV) in T Cell and NK Cell Lymphomas: Time for a Reassessment. Curr Hematol Malig Rep 10: 456-467.
- Fu YS, Perzin KH (2001) Pathology of the nasal cavity, paranasal sinuses, and nasopharynx. In: Fu YS, Wenig BM, Head and Neck Pathology with clinical correlations. Churchill livingstone, Philadelphia, 137-230.
- Kim WS, Song SY, Ahn YC, et al. (2001) CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol 12: 349-352.
- Yamaguchi M, Kita K, Miwa H, et al. (1995) Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 76: 2351-2356.
- Isobe K, Uno T, Tamaru J, et al. (2006) Extranodal natural killer/T-cell lymphoma, nasal type: the significance of radiotherapeutic parameters. Cancer 106: 609-615.
- Yamaguchi M, Tobinai K, Oguchi M, et al. (2009) Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 27: 5594-5600.
- Kim SJ, Yang DH, Kim JS, et al. (2014) Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann Hematol 93: 1895-1901.
- Au WY, Weisenburger DD, Intragumtornchai T, et al. (2009) Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 113: 3931-3937.
- Yamaguchi M, Kwong YL, Kim WS, et al. (2011) Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 29: 4410-4416.
Corresponding Author
Masafumi Ohki, MD, Department of Otolaryngology, Saitama Medical Center 1981 Kamoda, Kawagoe-shi, Saitama 350-8550, Japan, Tel: +81-49-228-3685, Fax: +81-49-225-6312.
Copyright
© 2017 Ohki M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.