Myopericytoma of the Maxillofacial Region: A Report of Two Cases
Abstract
Myopericytoma (MPC) was accepted as a separate entity by the World Health Organization in 2002 and it describes a lesion which comprises of myoid-like oval to spindle-shaped cells with a concentric perivascular type of growth. We present two unusual cases of MPC in the maxillofacial region. In our case management included complete surgical excision under general anesthesia and regular post-operative follow up. Myopericytomas belong to one of the three categories comprising a broader and heterogeneous group of lesions called Hemangiopericytoma (HPC)-like neoplasm's. These include glomangiopericytoma, infantile myofibromatosis (formerly known as infantile HPC), and portion of sinonasal HPC. A clinically relevant characteristic of hemangiopericytoma is its unpredictable clinical course which is often not related to its histopathologic characteristics. The latter course is in contrast to the benign course of MPC, thus it is essential for practitioners to differentiate between those two diagnoses.
Keywords
Myopericytoma, Hemangiopericytoma, Myofibromatosis, Sinonasal, Maxillofacial
Introduction
Myopericytoma (MPC) is a recently described, benign, soft-tissue tumor [1]. The term was endorsed by the World Health Organization in 2002 and it describes a lesion which comprises of myoid-like oval to spindle-shaped cells with a concentric perivascular type of growth [2]. The tumor has been reported to manifest with heterogeneous morphology and to feature a broad histopathological spectrum [3]. Reports of MPC are very rare [4], the tumor has been reported to appear more frequently in the dermis and subcutaneous layers in the extremities of middle-aged adults [5]. Myopericytomas (MPC) belong to one of the three categories comprising a broader and heterogeneous group of lesions called Hemangiopericytoma (HPC)-like neoplasms. Lesions belonging to this category show a benign clinical course, and also include glomangiopericytoma, infantile myofibromatosis (formerly known as infantile HPC), and a portion of the HPCs that appear in the sinuses and nasal cavity [6]. MPC usually arise in the superficial and subcutaneous tissues of the extremities [7], but there have been also reports involving rare locations such as the intracranial area [8], the head and neck region [9] and the thoracic spine [10]. Clinically, the MPC is generally considered a painless, slow growing, well demarcated, benign tumor with a diameter less than 2 cm [3], a description similar to the presenting case. We present two unusual cases of MPC in the maxillofacial region.
Case A
A 9-year-old female patient presented to the outpatient department of our clinic with a subcutaneous swelling at the region of the left mandibular mental foramen. The lesion appeared 6 months ago and, as reported, had a tendency to increase in size slightly over the last couple of weeks. Upon palpation, the swelling was painless, mobile, of firm to rubbery consistency and measuring 1.5 cm approximately. The patient also brought with her imaging examinations which included an orthopantomogram, a gray-scale sonography of the lesion's region and a MRI of the viscerocranium.
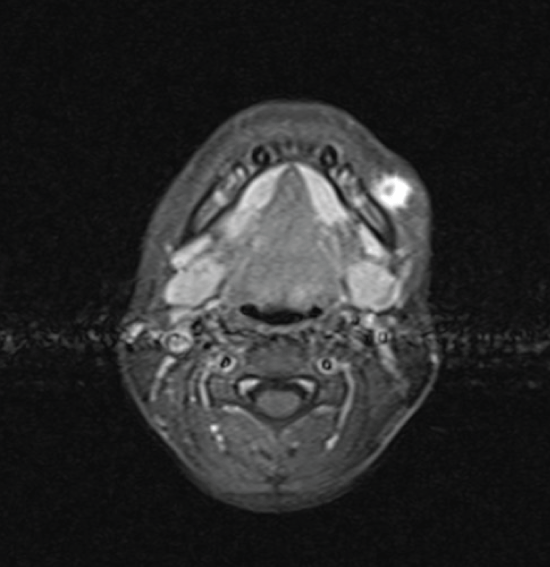
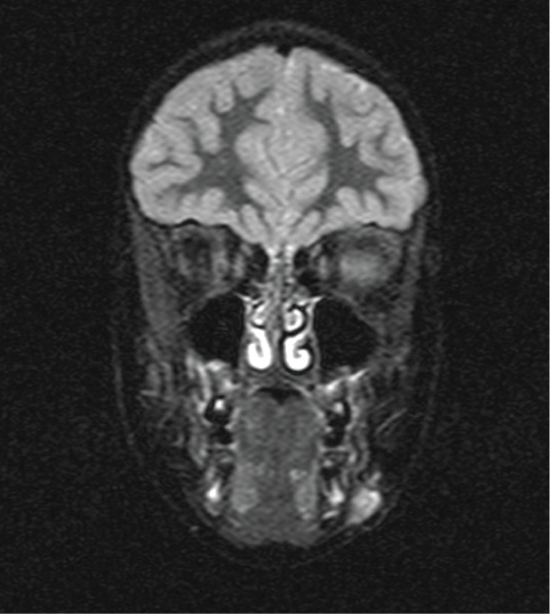
The panoramic X-ray showed normal findings of a mixed denture with no evidence that the lesion was of odontogenic etiology. The ultrasonographic exam of the neck revealed a 1.4 × 0.7 cm hypoechoic mass, with smooth and well-demarcated borders, whereas in the center of it there are some echogenic elements. Thin axial and coronal visceral scull MRI revealed a solid, subcutaneous lesion 1.5 × 1 × 1.7 cm in size on the left side of the mandible, laterally. The mass showed high signal in T2 and T2 FS sequences (Figure 1a) and demonstrated increased enhancement after intravenous administration of paramagnetic agent (Figure 1b). There were no signs of mandibular involvement. The lesion appeared to be closely attached to the facial muscles beneath it, while the skin above it did not show any signs of traction or thickening.
Based on the above clinical and imaging findings the patient was scheduled for excisional biopsy under general anesthesia. An intraoral approach was selected for best aesthetic result. The incision was made to the mandibular gingivobuccal fold at the premolar region on the left. The facial muscles below were exposed and dissected. At this stage, the mental nerve as well as the marginal branch of the facial nerve were identified and carefully preserved. The excision presented a certain amount of difficulty due to the lesion's close anatomic relation to the muscles as well as to the thin subcutaneous tissue and the skin. Three surgical specimens were obtained and the excision was complete. The patient's postoperative course was uneventful.
Histology (case-A)
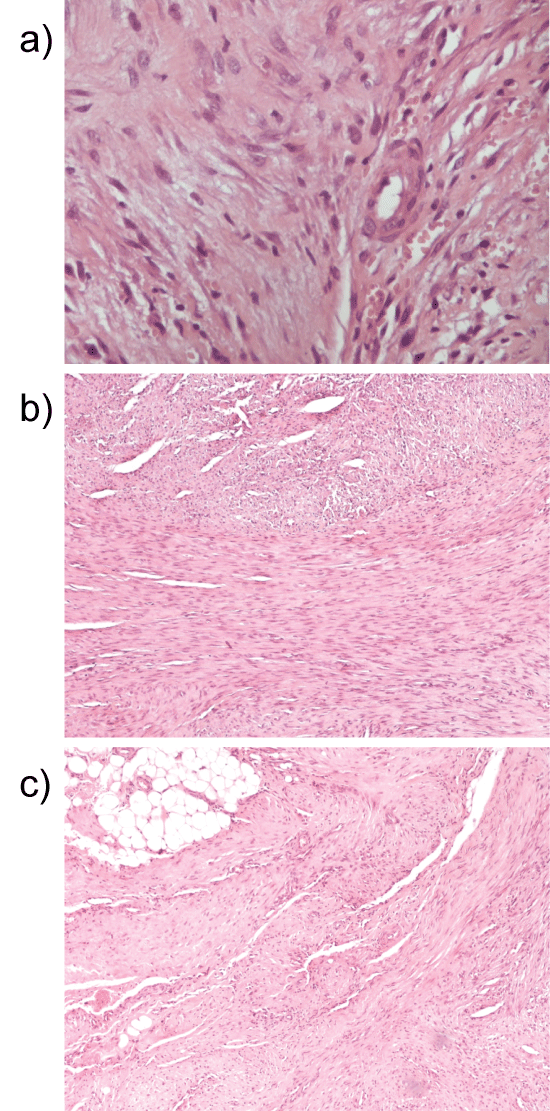
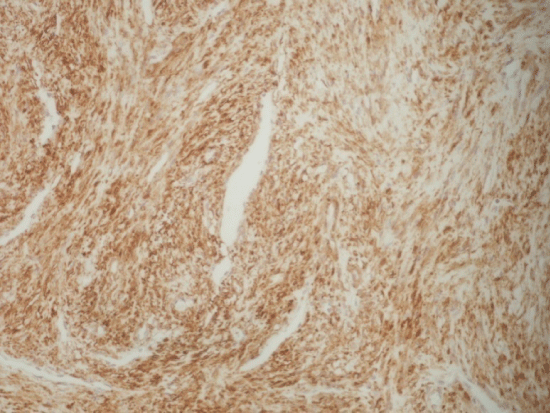
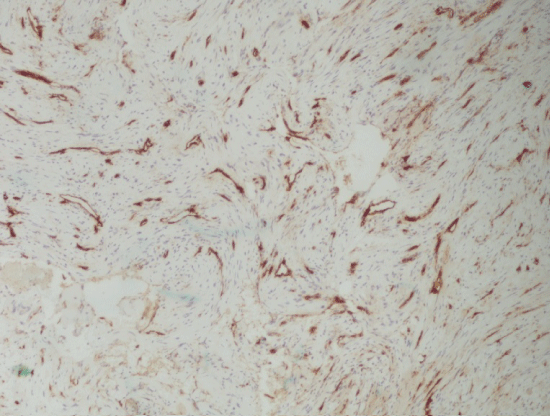
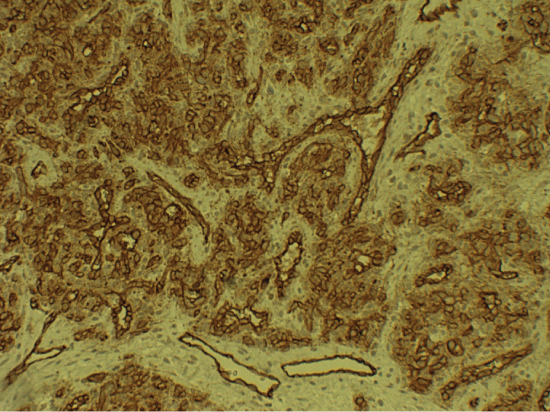
The microscopic study revealed the lesions' high cellular density. Myoid or spindle-shaped cells with mild hyperchromatic nuclei as well as round or oval-shaped cells with hypochromatic nuclei were present (Figure 2). The cells' architecture was diffuse or concentric especially at perivascular spaces. Necrosis, atypia or mitoses were not present. There was focal hyalinization of the stroma Immunohistochemically, the tumor cells were diffusely positive for Smooth Muscle Actin (SMA) (Figure 3). CD34 positivity existed in normal endothelia while tumor cells were negative (Figure 4). Staining for CD99 and Bcl-2 was negative. The Ki-67 labeling was 1% approximately in the Case A. At the specimen periphery muscle fibers and adipose tissue were present without any pathological findings.
Case B
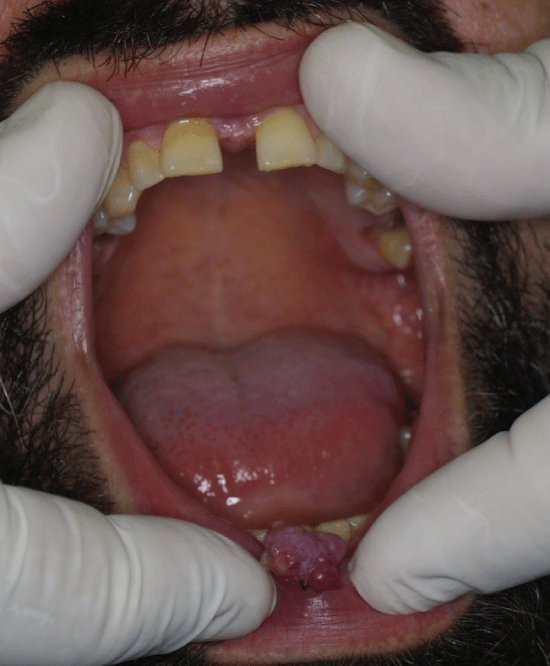
A 37-year-old male patient presented to the private practice of one of the authors with a protruding lesion in the vermillion of the lower lip. The lesion appeared 4 months ago it was reported to have increased substantially in the past three weeks. The lesion was painless, mobile, of firm to rubbery consistency and measuring 0.9 cm approximately. The lesion appeared to be closely attached to the orbicularis oris muscle beneath it, while the remainder vermilion did not show any signs of traction or thickening (Figure 5). The patient was scheduled for excisional biopsy under topical anesthesia. The excision left a wedge shaped defect that was immediately closed. The patient's postoperative course was uneventful. Macroscopically, the surgical specimen was 1.6 × 1.1 × 0.5 cm.
Histology (case-B)
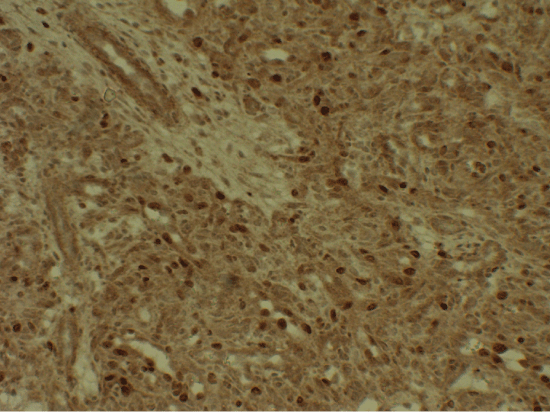
Histology was similar to the one of Case A. Ki-67 while it was approximately 20% in the Case B (Figure 6). CD34 staining of Case-B, again showed CD34 positivity in normal endothelia while tumor cells were negative (Figure 7).
Currently, both our patients are free of disease and show no signs of functional or aesthetic disturbance after at-least 44 months of post-operative follow up.
Discussion
The above morphological and immunohistochemical findings in both cases were consistent with the diagnosis of myopericytoma. Despite the reported male preponderance in the largest series by Mentzel, et al. [9], we hereby report a case in a young female and a male case.
Imaging methods used to assess such lesions include ultrasonography, CT and MRI, although there are not many reports describing the imaging findings of MPC. On gray-scale sonography the mass is well circumscribed, hypoechoic and demonstrates marked internal vascularity on color Doppler sonography [11]. On CT images the lesion demonstrates a sharply defined ovoid homogeneously enhancing (at the same intensity with vascular structures) mass [5] and MRI imaging reveals the lesion's intense enhancement on contrast-enhanced T1-weighted images [8]. US-guided fine-needle aspiration biopsy, despite the fact that it is a simple, safe, and inexpensive procedure, it has little diagnostic value for lesions like myopericytoma, the diagnosis of which requires immunohistochemical staining [12]. Preoperative differential diagnoses based on imaging findings, have been reported to include hyper vascular metastasis, particularly from papillary thyroid carcinoma, Castleman's disease of hyaline vascular type, and paraganglioma or schwannoma of the vagus nerve [5,13].
The usual clinical presentation of MPC is a slowly enlarging, painless mass. It most frequently affects the skin and superficial soft tissues of the distal extremities in adults [9], but with increased recognition, lesions in head and neck region and trunk have been described [3,9]. MPCs are usually solitary lesions although there have been patient case reports of multiple occurrences [2,7,9]. Most cases of myopericytoma behave in a benign manner, with recurrence being uncommon even when excision was incomplete. In the study by Mentzel, et al. [9], despite a reported 50% ratio of marginal or incomplete excision of these tumors, there were only two tumors that recurred (a malignant at year 1 and an intravascular myopericytoma at year 4).
Most authors agree that the diagnosis of MPC is obtained by histopathological and immunohistochemical examinations of the surgical specimen [9,14]. However, the patient's medical history, clinical examination and imaging exams are of paramount importance as well. Typical MPC has been reported to comprise from monomorphic-to some extent-oval to spindle-shaped myoid-like cells with striking multilayered concentric growth around lesion blood vessels [15]. Myopericytes are employed as cells of uncertain position in the morphologic spectrum between pericytes and vascular smooth muscle cells, and share many features with myofibroblasts [7,15]. The defining histologic feature of myopericytoma is a distinctive, concentric perivascular proliferation of myoid tumor cells. These cells show strong and diffuse immunoreactivity to SMA and are almost always negative for CD34, CD99, cytokeratin and S100 protein [3,16]. On the basis of histopathological findings, the differential diagnoses of MPC, has been reported to include myofibroma/myofibromatosis, glomus tumor, perivascular epithelioid cell tumor, angioleiomyoma, glomangiomyoma and hemangiopericytoma. Among them, distinguishing between hemangiopericytoma and MPC can be very challenging [16]. Hemangiopericytoma, originally described by Stout and Murray [17], is characterized by staghorn-shaped spaces with an intervening proliferation of cells, a feature that usually does not appear in MPC. Moreover it does show consistent positivity for CD34 and CD99, whereas immunoreactivity to SMA is only rarely seen [16]. A clinically relevant characteristic of hemangiopericytoma is its unpredictable clinical course which is often not related to its histopathologic characteristics. The latter course is in contrast to the benign course of MPC, thus it is essential for practitioners to differentiate between those two diagnoses [15,16].
Conclusion
Myopericytoma is a rare, recently delineated, benign, soft-tissue neoplasm. Even though the diagnosis is set based on histopathological and immunohistochemical findings, medical anamnesis, clinical examination and imaging studies are of outmost importance as well. Our management included complete surgical excision under general anesthesia and regular post-operative follow up. Although there are some cases of malignant MPC reported [18,19], the low Ki-67 labeling (1%) and the clinical course indicate the benign nature of the presented case.
Conflict of Interest
None declared.
References
- Maheshwari V, Alam K, Jain A, et al. (2008) Myopericytoma of neck region - A case report. Indian J Otolaryngol Head Neck Surg 60: 179-180.
- Folpe AL, McMenamin ME (2002) Pericytic (perivascular) tumours. In: Fletcher CDM, Unni KK, Mertens F, World Health Organisation Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press, Lyon, France, 135-139.
- Jung YI, Chung YK, Chung S (2012) Multiple myopericytoma of the face and parotid gland. Arch Plast Surg 39: 158-161.
- Terada T (2010) Minute myopericytoma of the neck: a case report with literature review and differential diagnosis. Pathol Oncol Res 16: 613-616.
- Lee SK, Kwon SY (2009) Gray-scale and power Doppler sonography and CT findings of myopericytoma of the posterior cervical space. AJNR Am J Neuroradiol 30: 1604-1606.
- Gengler C, Guillou L (2006) Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology 48: 63-74.
- Dray MS, McCarthy SW, Palmer AA, et al. (2006) Myopericytoma: a unifying term for a spectrum of tumours that show overlapping features with myofibroma. A review of 14 cases. J Clin Pathol 59: 67-73.
- Rousseau A, Kujas M, van Effenterre R, et al. (2005) Primary intracranial myopericytoma: report of three cases and review of the literature. Neuropathol Appl Neurobiol 31: 641-648.
- Mentzel T, Dei Tos AP, Sapi Z, et al. (2006) Myopericytoma of skin and soft tissues: clinicopathologic and immuno histochemical study of 54 cases. Am J Surg Pathol 30: 104-113.
- Cox DP, Giltman L (2003) Myopericytoma of the thoracic spine: a case report. Spine 28: E30-E32.
- Harish S, O'Donnell P, Briggs TW, et al. (2007) Myopericytoma in Kager's fat pad. Skeletal Radiol 36: 165-169.
- Nyquist GG, Tom WD, Mui S (2008) Automatic core needle biopsy: a diagnostic option for head and neck masses. Arch Otolaryngol Head Neck Surg 134: 184-189.
- Rho BH, Lee SK, Kwon SY (2011) Myopericytoma of the neck: sonographic appearance and sonographically guided needle biopsy. J Clin Ultrasound 39: 469-472.
- Chu ZG, Yu JQ, Yang ZG, et al. (2009) Myopericytoma involving the parotid gland as depicted on multidetector CT. Korean J Radiol 10: 398-401.
- Granter SR, Badizadegan K, Fletcher CD (1998) Myofibromatosis in adults, glomangiopericytoma, and myopericytoma: a spectrum of tumors showing perivascular myoid differentiation. Am J Surg Pathol 22: 513-525.
- Xia L, Chen Y, Geng N, et al. (2010) Multifocal myopericytoma in the maxillofacial region: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109: e59-e62.
- Stout AP, Murray MR (1942) Hemangiopericytoma. A vascular tumor featuring Zimmerman's pericytes. Ann Surg 116: 26-33.
- McMenamin ME, Fletcher CD (2002) Malignant myopericytoma: expanding the spectrum of tumours with myopericytic differentiation. Histopathology 41: 450-460.
- Terada T (2012) Myopericytoma of low grade malignancy in the oral cavity. Rare Tumors 4: e9.
Corresponding Author
Kyrgidis Athanassios, MD, MSc, PhD, Departments of Clinical Pharmacology and Oral & Maxillofacial Surgery, Aristotle University of Thessaloniki, 18 Iasonidou St, 55236, Panorama, Thessaloniki, Greece, Tel: +302315551062; +306947566727.
Copyright
© 2017 Valasidis A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.