Oral, Pharyngeal and Laryngeal Lesions in Mucous Membrane Pemphigoid and Usefulness of Narrow Band Imaging
Abstract
Mucous membrane pemphigoid is a relatively rare disorder involving mucosal lesions. Here, we report two cases of mucous membrane pemphigoid. This report is the first to discuss narrow band imaging of the mucosal features of mucous membrane pemphigoid. The two patients presented with sore throat and multiple stomatitis. Optic fiberscopy revealed mucosal lesions on the gingiva, palate, epiglottis, and arytenoid. The appearance showed both erosion and erosion with a white coating. Narrow band imaging clearly showed a white area at the site of the sub epithelial blisters and a mixed blue and brownish area at the site of the erosive area in which the mucosa was exfoliated without blistering. Narrow band imaging was therefore also useful for detecting blister lesions in mucous membrane pemphigoid because lesions were more clearly visible. Pharyngolaryngeal examination using optic fiberscopy should be performed in all mucous membrane pemphigoid patients.
Keywords
Autoimmune disease, BP180, BP230, Pharyngeal, Laryngeal, Narrow band imaging
Introduction
Pemphigoid was first described by Lever in 1953 as a sub epidermal blistering disease [1]. This autoimmune bullous disorder of the skin targets the basement membrane zone [2,3]. Blisters are formed due to the destruction of the basement membrane zone by autoantibodies. The basement membrane zone plays an adhesive role as the junction between the epidermis and dermis [3,4]. In this zone, the hemidesmosomes are located at the bottoms of the basal cells. Hemidesmosomes consist of plectin, the 230-kDa bullous pemphigoid antigen 1 (BPAG1; BP230), the 180 kDa bullous pemphigoid antigen 2 (BPAG2; BP180, collagen XVII), and integrin α6β4 [3,4]. Both plectin and BP230 are connected with keratin (tonofilament), which is a part of the cytoarchitecture in the basal cells. The anchoring filaments, consisting of laminin 5 (laminin 332; α3, β3, γ2 chains), exit the hemidesmosomes through the lamina lucida into the lamina densa [3,5]. The anchoring fibrils from the lamina densa loop toward the dermis to form attachments with dermal collagen fibers [5]. The identified target antigens are BP230, BP180, laminin 5, laminin 6, type VII collagen, and integrin β4 subunit [6]. The IgG autoantibodies attached to the basement membrane zone activate complement and disturb the junction at the site of the basement membrane zone [7].
Pemphigoid is classified into several subtypes: bullous pemphigoid, mucous membrane pemphigoid, and anti-laminin 5 pemphigoid. Bullous pemphigoid is the most common form, and its common antigens are BP230 and BP180, which are hemidesmosome components. Mucous membrane pemphigoid is relatively rare. Here, we report the features of oral, pharyngeal, and laryngeal lesions in two cases of mucous membrane pemphigoid. Additionally, we report the first mucosal findings using narrow band imaging with central wavelengths of 415 and 540 nm [8] of mucous membrane pemphigoid using a flexible nasopharyngolaryngeal endoscope (Olympus 3.2 mm ENF Type V2, Olympus Corporation, Tokyo, Japan) with a xenon lamp light source (Olympus VISERA Pro video system; Olympus Corporation, Tokyo, Japan).
Case 1
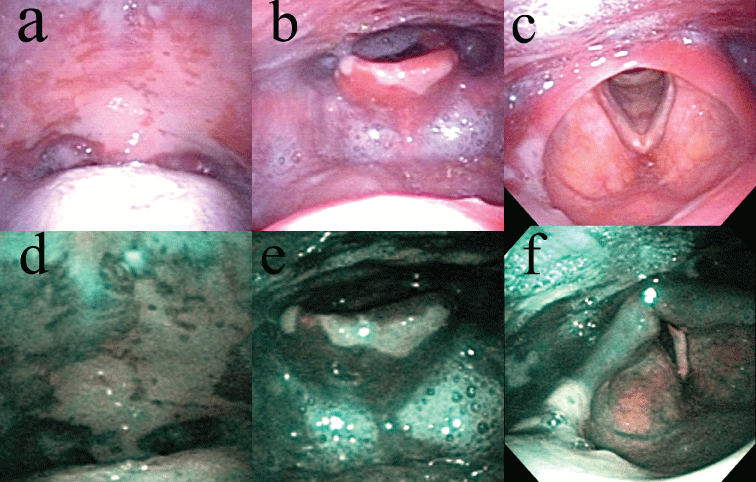
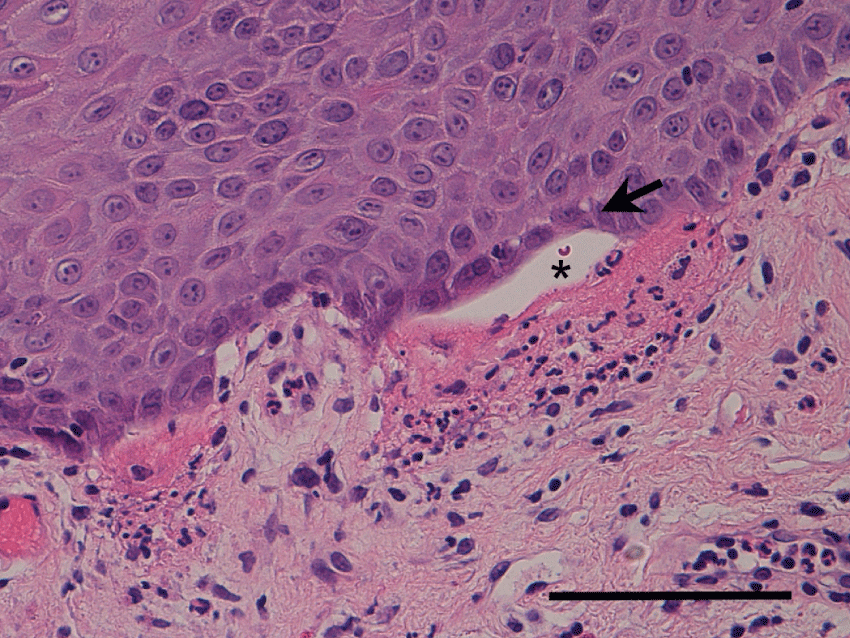
A 77-year-old man presented with sore throat and recurrent multiple stomatitis lasting 18 months. He had erosive membranous lesions in the gingiva, soft palate, epiglottis, and arytenoid (Figure 1a, Figure 1b and Figure 1c). The gingiva revealed erosion. The palate, epiglottis, and arytenoid all showed erosion coated with a whitish membrane (Figure 1a, Figure 1b and Figure 1c). Narrow band imaging clearly showed a white area at the site of the sub epithelial blisters and a mixed blue and brownish area at the site of the erosive area in which the mucosae were exfoliated without blistering (Figure 1d, Figure 1e and Figure 1f). The patient temporarily showed skin lesions of crusty erosion on the right shoulder. The white blood cell count was 6600 per µL, and C-reactive protein level was 0.7 mg/dL. Serum level of BP180 autoantibodies was less than 7 index and BP180 autoantibodies was undetected (cutoff value: BP180, 9 index). Serum levels of antibodies against desmoglein (Dsg) 1 and 3 were both less than 5 index (cutoff values: Dsg 1, 13 index; Dsg 3, 6 index). Histopathology from the buccal mucosae revealed sub epithelial blisters under the basal cell layer with eosinophil infiltration (Figure 2). Direct immunofluorescence (DIF) revealed linear deposits of IgG and deposits of C3 along the basement membrane zone. Therefore, the patient was diagnosed with mucous membrane pemphigoid using the criteria of the presence of mucous membranous lesions; sub epithelial blistering as seen on hematoxylin-eosin examination of biopsy specimens; and the presence of linear IgG, IgA and/or C3 deposits along the basement membrane zone of perilesional skin as detected by DIF. He was treated with minomycin (200 mg), and nicotinic acid amide (1200 mg). The membranous lesions slightly improved, and his symptoms were reduced.
Case 2
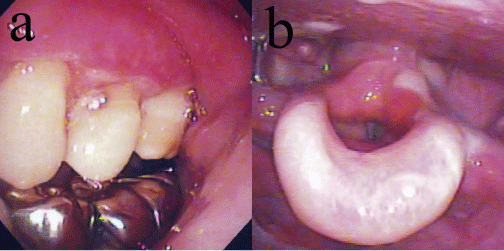
A 57-year-old woman presented with multiple stomatitis and sore throat lasting 3 weeks. She had erosive membranous lesions in the gingiva, soft palate, and epiglottis (Figure 3). The epiglottis showed erosion coated with a whitish membrane (Figure 3). She did not show any skin lesions. The white blood cell counts was 8700 per µL and C-reactive protein level was 0.1 mg/dL. Serum levels of BP180 and BP230 autoantibodies were 36 index and less than 5, respectively (cutoff value: BP230, 9 index). Serum levels of antibodies against desmoglein (Dsg) 1 and 3 were both less than 5. Histopathology revealed sub epithelial blisters, and DIF revealed linear deposits of IgG along the basement membrane zone. Therefore, the patient was diagnosed with mucous membrane pemphigoid. She was treated with predonisolone at an initial dose of 20 mg. The membranous lesions gradually improved, and the erosive lesions disappeared. After the lesions were controlled, the prednisolone dose was tapered.
Discussion
Both patients in our study presented with oral lesions (on the gingiva and palate) and laryngeal lesions (on the epiglottis). Arytenoid lesions were presented in one case. Skin lesions were also observed in one case. The mucous lesions appeared as erosions coated with a whitish membrane.
Mucous membrane pemphigoid primarily features mucosal lesions but occasionally develops scar formation, leading to severe complications such as blindness due to ocular scarring, airway obstruction, and dysphagia. Previously, the disorder was known as cicatricial pemphigoid. Mucous membrane pemphigoid and bullous pemphigoid are sometimes difficult to discriminate when skin lesions and mucosal lesions co-exist. Mucous membrane pemphigoid with skin lesions clinically resembles bullous pemphigoid with mucosal lesions and without scar formation. Prospective research by Alexandre, et al. showed that cutaneous lesions were present in 63% patients with mucous membrane pemphigoid [9].
The diagnostic criteria of these disorders are based on clinical features, optical microscopy, and immunohisto staining results. However, only clinical features can be used to distinguish between bullous pemphigoid and mucous membrane pemphigoid. According to the present criteria, discrimination between the two disorders depends on whether mucosal or skin lesions are more clinically important [6]. The sensitivity of serum antibodies is low or moderate for diagnosis; these antibodies are detectable at a rate of 70%-75% in bullous pemphigoid and at 20% in mucous membrane pemphigoid [10,11]. Bullous pemphigoid and mucous membrane pemphigoid overlap with respect to detectable antibodies because BP180 is detectable in 19%-50% of patients with bullous pemphigoid and 40%-70% of patients with mucous membrane pemphigoid [10,12].
A new methodology using immunoelectron microscopy can differentiate between the two disorders [10]. The diagnosis of bullous pemphigoid is based on the detection of immune deposits in the upper lamina lucida by immunoelectron microscopy, whereas mucous membrane pemphigoid is diagnosed by the detection of immune deposits in the lamina densa or in the sublamina densa and the anchoring fibril zone. Patients diagnosed with bullous pemphigoid by the standard criteria are sometimes diagnosed differently by other criteria based on immunoelectron microscopy features [10,13]. In previous studies, immunoelectron microscopy reconfirmed 68% of patients as having bullous pemphigoid and differently diagnosed 10% of patients with mucous membrane pemphigoid [10,13]. Despite this accuracy, routine immunoelectron microscopy is impractical due to time and expense concerns.
Epidermolysis bullosa acquisita, and vesiculobullous systemic lupus erythematosus are also subepidermal autoimmune bullous dermatoses with a feature of linear deposits predominantly of IgG and complement component C3 in the cutaneous basement membrane zone [10]. The clinical criteria are mechanical bullae with milia and scarring on extensor or acral surfaces for epidermolysis bullosa acquisita; and vesicles or blisters and erythematous plaques, without scarring and associated with systemic lupus erythematosus for vesiculobullous systemic lupus erythematosus [10]. These disorders are discriminated by the predominance of mucosal involvement and no association with systemic lupus erythematosus in our cases.
Mucosal lesions appear as erosions, erosions with white coating, erythematous patches, and blisters. The most prominent site of these lesions is the oral cavity (i.e., the palate, gingiva, or buccal mucosae) [9]. In addition, nasopharyngolaryngeal symptoms are present in 35% of cases [9]. The appearance and sites of mucosal lesions in mucous membrane pemphigoid are similar to those of mucosal lesions in pemphigus vulgaris or bullous pemphigoid [14]. In pemphigus vulgaris, mucosal lesions appear as erosions, erosions with white coating, and erythematous lesions that are most prominent in the oral cavity, as well as in the laryngopharynx, esophagus, and nasal cavity [15]. It is therefore impossible to differentiate mucous membrane pemphigoid from other autoimmune bullous diseases by visual examination of membranous lesions alone.
The symptoms of mucosal lesions in mucous membrane pemphigoid are relatively mild compared to those of pemphigus vulgaris [16-18]. If mucosal lesions exist, they may be asymptomatic. Pharyngolaryngeal lesions cannot be sufficiently visualized by direct visual examination without optic fiberscopy. Bullous pemphigoid patients generally do not consult an otolaryngologist unless they are symptomatic. If optic fiberscopy is not performed by a dermatologist or patients do not consult an otorhinolaryngologist, pharyngolaryngeal lesions will likely be overlooked. We therefore believe that it is necessary to check all mucous membrane pemphigoid patients using optic fiberscopy to avoid overlooking these lesions.
Narrow band imaging (NBI) is an image enhancement technology that uses light with central wavelengths of 415 (blue) and 540 nm (green). Light wavelength in hemoglobin absorption shows strong peaks at approximately 415 and 540 nm. When the mucosal epithelium is exposed by filtered, narrow wavelengths of 415 and 540 nm, these light wavelengths are absorbed by hemoglobin [8]. Then the intraepithelial blood vascular structures can be visualized as brown. In this report, blister lesions were revealed as white, and other areas were shown as mixed blue and brownish. Blister lesions showed poor vasculature and appeared white, while other areas, such as erosive areas in which the mucosae were exfoliated without blistering and normal mucosae, were vasculature-rich and appeared as mixed blue and brownish. Narrow band imaging was therefore also useful for detecting blister lesions in mucous membrane pemphigoid. However, the lesion detected by narrow band imaging is not distinct for mucous membrane pemphigoid. Therefore, narrow band imaging cannot discriminate mucous membrane pemphigoid from other blistering diseases.
Conclusion
In mucous membrane pemphigoid, mucosal lesions are present in the oral cavity, but pharyngolaryngeal and nasal mucosa can be affected as well. The pharyngolaryngeal areas should thus be examined by an otorhinolaryngologist in patients with mucous membrane pemphigoid.
Acknowledgments
None.
Financial Disclosure
None.
Conflict of Interest
None.
References
- Lever WF (1953) Pemohigus. Medicine (Baltimore) 32: 1-123.
- Jordon RE, Beutner EH, Witebsky E, et al. (1967) Basement zone antibodies in bullous pemphigoid. JAMA 29: 751-756.
- Kasperkiewicz M, Zillikens D (2007) The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol 33: 67-77.
- Shinkuma S, McMillan JR, Shimizu H (2011) Ultrastructure and molecular pathogenesis of epidermolysis bullosa. Clin Dermatol 29: 412-419.
- Masunaga T, Shimizu H, Yee C, et al. (1997) The extracellular domain of BPAG2 localizes to anchoring filaments and its carboxyl terminus extends to the lamina densa of normal human epidermal basement membrane. J Invest Dermatol 109: 200-206.
- Chan LS, Ahmed AR, Anhalt GJ, et al. (2002) The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol 138: 370-379.
- Jordon RE, Kawana S, Fritz KA (1985) Immunopathologic mechanisms in pemphigus and bullous pemphigoid. J Invest Dermatol 85: 72s-78s.
- Irjala H, Matar N, Remacle M, et al. (2011) Pharyngo-laryngeal examination with the narrow band imaging technology: early experience. Eur Arch Otorhinolaryngol 268: 801-806.
- Alexandre M, Brette MD, Pascal F, et al. (2006) A prospective study of upper aerodigestive tract manifestations of mucous membrane pemphigoid. Medicine (Baltimore) 85: 239-252.
- Vaillant L, Bernard P, Joly P, et al. (1998) Evaluation of clinical criteria for diagnosis of bullous pemphigoid. French Bullous Study Group. Arch Dermatol 134: 1075-1080.
- Ghohestani R, Kanitakis J, Nicolas JF, et al. (1996) Comparative sensitivity of indirect immunofluorescence to immunoblot assay for the detection of circulating antibodies to bullous pemphigoid antigens 1 and 2. Br J Dermatol 135: 74-79.
- Bernard P, Prost C, Durepaire N, et al. (1992) The major cicatricial pemphigoid antigen is a 180-kD protein that shows immunologic cross-reactivities with the bullous pemphigoid antigen. J Invest Dermatol 99: 174-179.
- Joly P, Courville P, Lok C, et al. (2004) Clinical criteria for the diagnosis of bullous pemphigoid: a reevaluation according to immunoblot analysis of patient sera. Dermatology 208: 16-20.
- Ohki M, Kikuchi S, Ohata A, et al. (2016) Features of oral, pharyngeal, and laryngeal lesions in bullous pemphigoid. Ear Nose Throat J 95: E1-E5.
- Amagai M (2010) Autoimmune and infectious skin diseases that target desmogleins. Proc Jpn Acad Ser B Phys Biol Sci 86: 524-537.
- Budimir J, Mihic LL, Situm M, et al. (2008) Oral lesions in patients with pemphigus vulgaris and bullous pemphigoid. Acta Clin Croat 47: 13-18.
- Korman NJ (1998) Bullous pemphigoid. The latest in diagnosis, prognosis, and therapy. Arch Dermatol 134: 1137-1141.
- Lever WF (1979) Pemphigus and pemphigoid. A review of the advances made since 1964. J Am Acad Dermatol 1: 2-31.
Corresponding Author
Masafumi Ohki, Department of Otolaryngology, Saitama Medical Center, Saitama Medical University, 1981 Kamoda, Kawagoe-shi, Saitama 350-8550, Japan, Tel: +81-49-228-3685, Fax: +81-49-225-6312.
Copyright
© 2017 Ohki M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.