Reactivation of Dormant Tbial Methicillin-Resistant Staphylococcus Aureus Osteomyelitis after 17 Years: a Case Report
Abstract
Introduction
Staphylococcus aureus is the most common cause of osteomyelitis with significant morbidity and mortality outcomes. Methicillin-susceptible Staphylococcus aureus osteomyelitis reactivation years after the primary episode is a well-known phenomenon. However, there are few case reports documenting dormant Methicillin-Resistant Staphylococcus aureus osteomyelitis recurrence years later. Our case report of dormant Methicillin-Resistant Staphylococcus aureus tibial osteomyelitis reactivation after 17-years, represents one of the longest documented recurrence intervals from initial bout of Methicillin-Resistant Staphylococcus aureus, as well as the diagnostic and therapeutic challenges involved.
Case Report
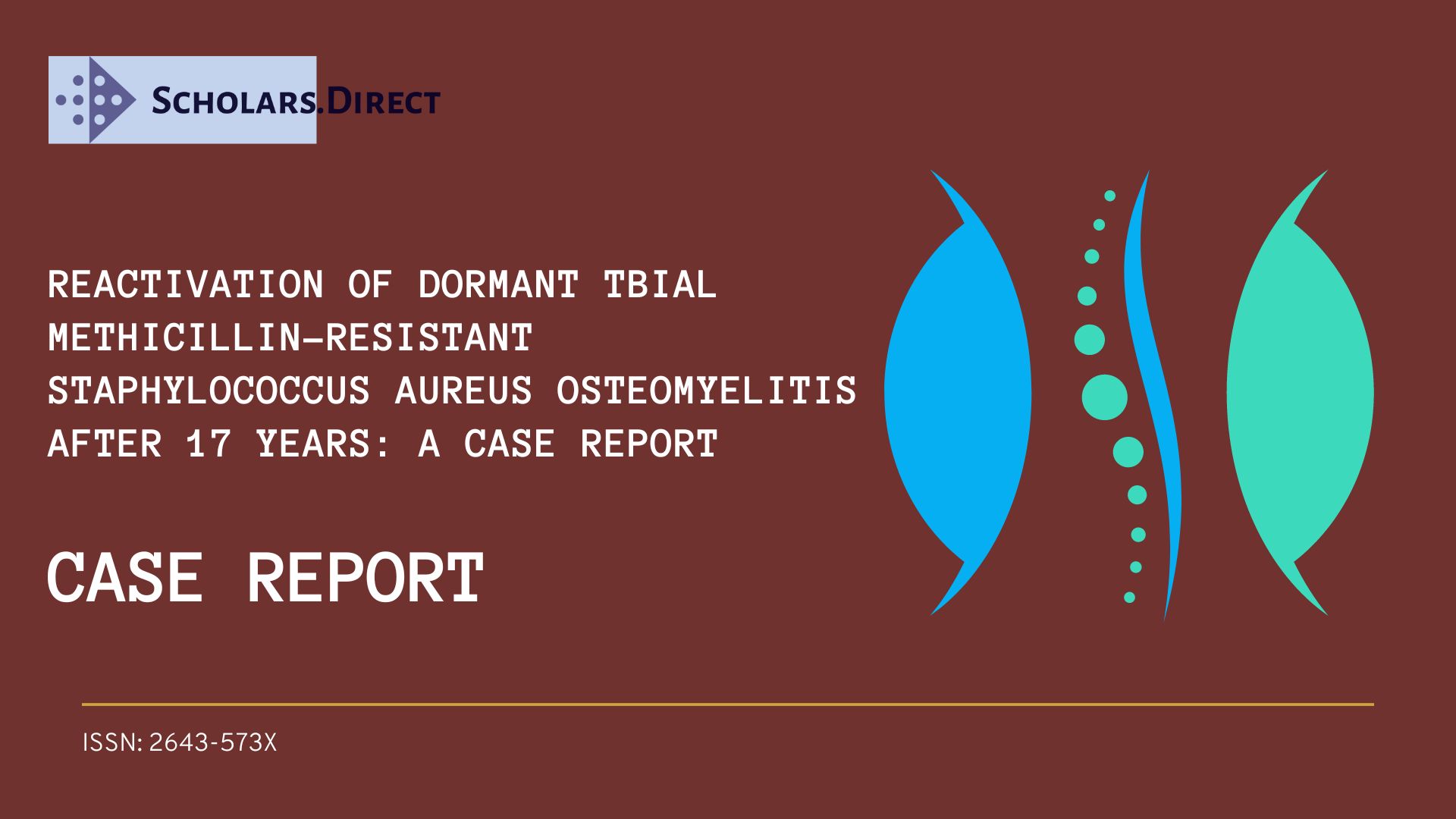
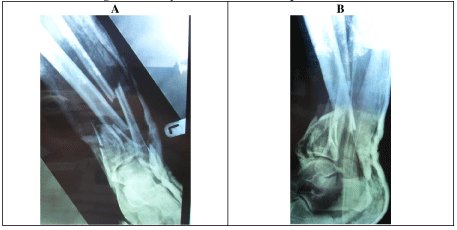
A 55-year-old male reports suffering a Gustilo & Anderson grade 1 open left tibial plafond fracture in 2004 that was managed with wound debridement and Taylor Spatial Frame. Initial management was with debridement and primary closure of the wound and application of a Taylor spatial frame (TSF) as definitive management of the fracture. One month following application of the frame, the patient reports he suffered a pin site infection. This was found to be infected osteomyelitis MRSA. The wound underwent surgical debridement and once the fracture was consolidated enough at three months, removal of external frame and intramedullary reaming and irrigation was performed. No further soft tissue coverage was required. The patient was treated with a prolonged course of intravenous vancomycin, followed by oral clindamycin. Since this episode, the patient has been asymptomatic and denied any further hospitalisations and the patient returned to full function without any limitations. The patient does not report any recurrent wounds, discharge, sinus or pain.
Seventeen years later, after a closed blow to the knee, the patient present febrile with knee pain. He was found to have Methicillin-Resistant Staphylococcus aureus tibial osteomyelitis, most concentrated in the proximal medial tibial cortex. This was treated with targeted surgical debridement, an antibiotic nail and prolonged antibiotics. The patient has remained asymptomatic with apparent resolution of infection 6-months post.
Conclusion
Our case report represents one of rare and longest document recurrence intervals of Methicillin-Resistant Staphylococcus aureus. Patients with Methicillin-Resistant Staphylococcus Aureus osteomyelitis should have appropriate treatment and follow-up with orthopaedic surgeons and infectious disease physicians to monitor disease progression and to ensure resolution of the infection.
Abbreviations
CRP: C-Reactive Protein; CT: Computerised Tomography; MRI: Magnetic Resonance Image; MRSA: Methicillin-Resistant Staphylococcus Aureus; MSSA: Methicillin-Sensitive Staphylococcus Aureus; MCS: Microscopy, Culture, and Sensitivity; OM: Osteomyelitis; TSF: Taylor Spatial Frame; WCC: White Cell Count; XR: X-ray
Introduction
Optimal treatment of methicillin-resistant Staphylococcus Aureus (MRSA) osteomyelitis has continued to elude clinicians. Osteomyelitis remains a healthcare burden with studies showing an overall incidence of 21.8 cases per 100,000 person-years [1]. Methicillin- Sensitive Staphylococcus aureus (MSSA) osteomyelitis reactivation many years after the primary episode is a well-known phenomenon [2,3]. However, there are few case reports documenting dormant MRSA osteomyelitis recurrence years later. This is postulated to be due to the aggressive nature of MRSA causing chronic symptomatic infection. On review of the literature, two rare case reports include lumbar MRSA osteomyelitis [4] and femur MRSA osteomyelitis [5] recurrence after 12 and 16 years of quiescent, respectively. Our case report of dormant MRSA tibial osteomyelitis reactivation represents one of the longest document recurrence intervals. This case demonstrates the diagnostic and therapeutic challenges involved in MRSA osteomyelitis.
Case presentation
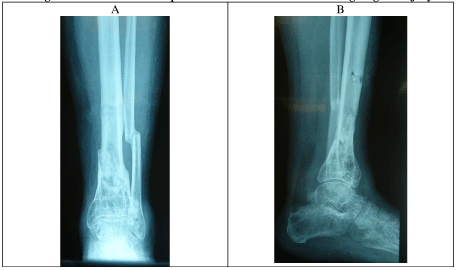
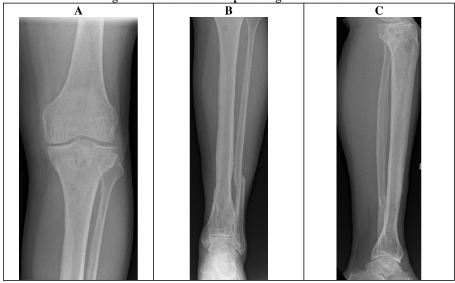
A 55-year-old male reports suffering a Gustilo & Anderson grade 1 open left tibial plafond fracture 17-years ago (Figure 1). This was managed by wound debridement and closure along with application of Taylor-Spatial Frame (TSF) (Figure 2). One month following initial management, this was complicated by a pine site infection which developed into MRSA osteomyelitis in the distal tibia.
The wound underwent surgical debridement and once the fracture was consolidated enough, removal of external frame and intramedullary reaming and irrigation (Figure 3). The wound did not require soft tissue coverage but required prolonged treatment with negative pressure dressing. The patient received a prolonged course of intravenous vancomycin, followed by oral clindamycin. Three months following this management, the patient was asymptomatic with evidence of radiographic and clinical union of the tibial plafond fracture (Figure 4).
Since this episode, the patient has been entirely asymptomatic and returned to full function. He denies any further treatment for his tibia and has had no hospitalisations. The patient reports no other medical history, is a non-smoker and returned to work as a carpenter.
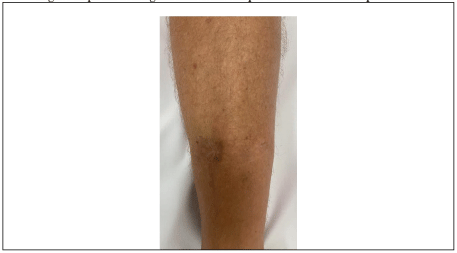
Seventeen years later, the patient presented with pyrexia (39 ℃) with knee pain. There was a history of blunt knee trauma following a fall 2-weeks prior with no skin breach. Examination of the knee joint demonstrated moderate knee joint effusion with a non-irritable knee joint range of motion from 5-100 degrees, and a healed distal tibia surgical scar (Figure 5). There was no tenderness over the proximal tibia.
Blood results included white cell count (WCC) 12 (10 * 9/L) and C-Reactive Protein (CRP) 119 (10 * 9/L). Knee aspirate demonstrated turbid yellow fluid, WCC of 6900 × 10 ^ 6.L, scant calcium pyrophosphate crystal and no organisms on gram stain.
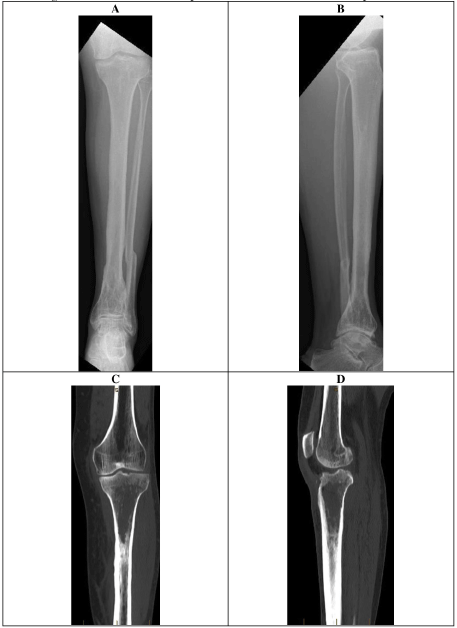
X- ray and CT scans demonstrated a united distal tibial fracture, however, the possibility of superimposed osteomyelitis could not be excluded (Figure 6).
Due to the complex clinical picture, it was impossible to rule out septic arthritis. Hence, the patient underwent a left knee arthroscopy, debridement, and irrigation acutely. Intra-operative findings were of significant synovitis in the suprapatellar pouch but otherwise unremarkable findings within the knee. Multiple samples were sent for microscopy and culture and histology, but all resulted in negative results for bacterial infection. Tissue culture showed leukocytes nil, epithelials nil and organism not seen with no growth on culture. Sterile
aspirate showed yellow appearance, WBC's 2633 × 10 * 6/L, polymorphs 90%, crystals not seen, no organisms on gram stain.
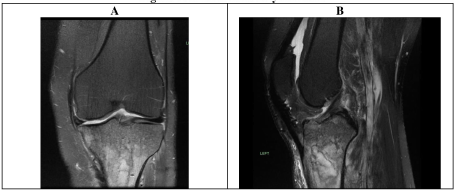
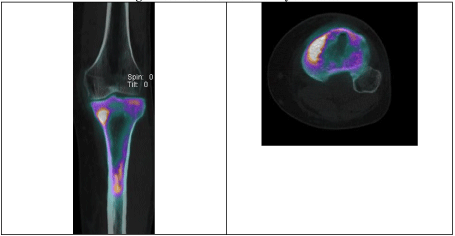
Due to ongoing symptoms 1 week post arthroscopy, an MRI scan was performed of the whole tibia, demonstrating features of proximal tibial osteomyelitis (reported as acute on chronic changes evident) (Figure 7). A triple phase white cell bone scan showed proximal tibia increased blood flow, hyperaemia and osteoblastic activity (Figure 8).
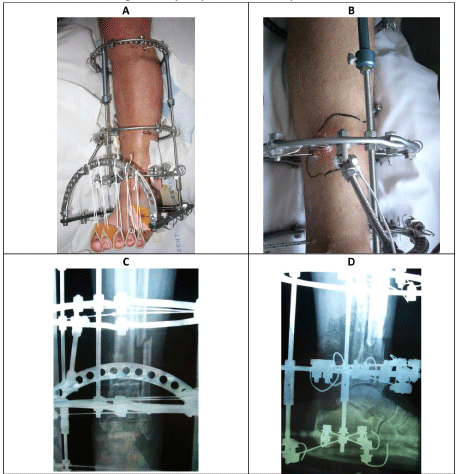
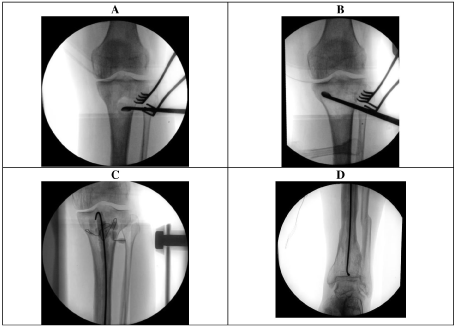
The patient underwent tibial canal reaming, irrigation, extensive proximal tibial bone debridement and vancomycin impregnated cement nail and beads (Figure 9). Frank pus was noted in the proximal tibial metaphysis at the time. Multiple tissue samples grew MRSA. Antibiotic treatment included 2 weeks of intravenous vancomycin and 4 weeks of oral clindamycin. Three months following the initial surgery, the patient underwent removal of antibiotic nail, insertion of antibiotic stimulant to the proximal tibia, with tissue sampling at the time showing no growth.
Since the debridement and antibiotics, the wounds have fully healed, and the patient has been asymptomatic and has returned to work. He has had follow-up for over 6 months with normal CRP and an XR showing interval healing of the proximal tibial debridement site (Figure 10).
Discussion
Derived from Greek staphyle (bunch of grapes) and kokkos (berry), Staphylococcus aureus has become one of the most common organisms associated with osteomyelitis [6]. It was first described as a "masses that looked like a bunch of grapes" from a surgical knee joint abscess by the Scotch surgeon Sir Alexander Ogston in 1880. Later in 1984, the German physician Rosenback further highlighted it as a gram-positive coccus, golden in colour, from the Latin aurum for gold [6]. It has been over 200 years since Sir Benjamin Brodie first described the "brodies" bacterial abscess [7] and 40 years since William Costerton's biofilm hypothesis, which explained the pathogenic mode of existence by which sessile bacteria adhere to implants and necrotic tissue during chronic infection [8]. Nevertheless, the treatment for osteomyelitis remains complex requiring a multidisciplinary approach including infectious diseases specialists, orthopaedic & plastic surgeons, radiologists, and physiotherapists with treatment including surgical debridement, bone reconstruction, soft tissue coverage and antibiotics therapy [3].
Staphylococcus aureus is an extremely versatile and opportunistic pathogen. This can be attributed to its arsenal of virulence factors and resistance mechanisms. These include toxin secretion, biofilm formation, small colony variant (SCV) subpopulations, and antimicrobial resistance strains. Due to these highly evolved persistence mechanisms, clinical Methicillin- Sensitive Staphylococcus Aureus (MSSA) osteomyelitis recurrence after decades of quiescence remains a prevalent problem [3].
While MSSA osteomyelitis recurrence is well documented, there are few case reports in the literature associated with MRSA reactivation. A rare case report, by Steven's et al, reported lumbar MRSA osteomyelitis walled off in the disc space and subsequently inoculated the soft tissues with ensuing bacteraemia after 12-years of dormancy [4]. Similarly, Townsend et al reported a unique case of MRSA osteomyelitis recurrent of the femur after being quiescent for 16-years [5]. In both these reports, the patient was not in the hospital setting after the initial bout of osteomyelitis, however, no information of patient comorbidities or treatment were provided in the latter case. Our case report represents the longest documented recurrence intervals of 17-years from initial bout of MRSA.
Inferiority of vancomycin to other antistaphylococcal antibiotics, MRSA has been associated with worse outcomes than MSSA for OM. A meta-analysis found that, in invasive infections, patients with MRSA had a significantly higher mortality than those with MSSA (OR, 1.93; 95% CI, 1.54-2.42; P < 0.001) [9]. MRSA infections have also been associated with longer hospital stays and increased costs to the health care system than MSSA infections (7 vs. 9 days, P = 0.045; 19,212 dollars vs 26,424 dollars, P = 0.008) [10].
In the complex panorama of osteomyelitis, MRSA infections are often more invasive and therefore treatment is more challenging. MRSA more frequently involves abscess formation than MSSA, thus aggressive, targeted surgical debridement during the index procedure is paramount to prevent the need for a return to the operating room [11]. Compared to MSSA, MRSA is known to require surgical intervention more frequently and require more procedures to treat the condition [12]. In our case report, aggressive surgical debridement of the proximal medial tibial was essential to remove all infected tissue (Figure 9).
The diagnosis of chronic osteomyelitis (OM) is based on clinical, laboratory, imaging, microbiological, and pathohistological features [13]. Often, symptoms of chronic osteomyelitis are subtle, and the classical signs of infection are absent. While inflammatory markers can be normal in osteomyelitis, persistently non elevated erythrocyte sedimentation rate and CRP level virtually rule out the pathology. Imaging is vital for investigation with plain x-rays to rule out other pathologies such a fractures and neoplasia. Chronic osteomyelitis displays radiological features of osteolysis and bony destruction with sclerotic zones and periosteal bone apposition. CT is helpful in defining necrotic bone fragments. MRI in chronic OM can detect necrotic bone, sinus tracts, or abscesses. Routine bone scintigraphy can have limited specificity and false-positive results may be due to diabetic arthropathy, gout, trauma, and recent surgery. However, leucocyte scintigraphy accurately diagnoses chronic osteomyelitis in the peripheral skeleton (sensitivity 84% and specificity 80%).
Before antibiotic therapy is started, direct deep tissue samples must be obtained. This will confirm the diagnosis and guide antibiotic therapy. The gold standard diagnostic criteria for osteomyelitis involves a positive culture from bone biopsy and histopathology with necrosis. Interestingly, CRP correlates with clinical response to therapy and can be used to monitor for recurrence.
Goals of treatment for MRSA osteomyelitis include eradication of the infection, pain reduction as well as retained limb function [3,6]. In all treatment algorithms, management principles include radical sequestrectomy, dead space management, soft tissue reconstruction, restoration of bone stability, appropriate antibiotic coverage and mitigating an array of possible patient risk factors. Management of the bony issues may involve bone resection, debridement of devitalised tissue, removal of hardware, marrow reaming and irrigation, implantation of antibiotic cement beads. Soft tissue management may involve prolonged vacuum dressing as well as soft tissue coverage with skin grafts and muscle flaps. Healthy, vascularised overlying soft tissue is vital for management of this complex problem.
Interestingly, Pozo, et al. found independent variables associated with osteomyelitis relapse were duration of the infection for more than 3 months at presentation, bone exposure, mostly after an open fracture, and treatment other than surgical debridement with flap coverage [14].
We present a case of recurrence of tibial MRSA osteomyelitis, 17 years after initial management, with apparently successful treatment with aggressive debridement and antibiotic therapy.
Conclusion
There are few case reports documenting dormant MRSA osteomyelitis recurrence years later. MRSA osteomyelitis strains are particularly lethal pathogens that possess aggressive virulence mechanisms. We report a rare case of dormant MRSA tibial osteomyelitis reactivation 17-years later, in a fit and well 55-year-old male. Treatment of MRSA osteomyelitis require more frequent and more aggressive surgical debridement for complete removal of this highly persistent pathogen. This case demonstrates the diagnostic and therapeutic challenges involved in MRSA osteomyelitis and apparently successful treatment.
Clinical Message
Dormant MRSA osteomyelitis reactivation is a rare phenomenon documented in the literature. This is postulated to be due to the aggressive nature of MRSA, which more commonly causes chronic symptomatic infection. Treatment of MRSA osteomyelitis requires more frequent and more aggressive surgical debridement for complete removal of this highly persistent pathogen. Treatment principles include radical sequestrectomy, dead space management, soft tissue reconstruction, restoration of bone stability, appropriate antibiotic coverage and management of host factors. Although uncommon, we report on the ability of MRSA osteomyelitis to lie dormant before reactivation years later. This case demonstrates the diagnostic and therapeutic challenges involved in MRSA osteomyelitis.
Declarations of Conflicting Interest
The author Callum Fryer and other authors declare that there is no conflict of interests.
Ethics
This case study has ethics committee approval and informed consent.
Funding
This case report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Authors Contributions
This case report was collated and written by CF. SC and NJ guided data collation and case report writing. FT was the consultant surgeon who cared for the patient and supervised the case report.
References
- Kremers HM, Nwojo ME, Ransom JE, et al. (2015) Trends in the epidemiology of osteomyelitis: A population-based study, 1969 to 2009. J Bone Joint Surg Am 97: 837-845.
- Daniel H Libraty, Chinmay Patkar, Brenda Torres (2012) Staphyloccocus aureus reactivation osteomylitis after 75 years. N Eng J of Med 366: 481-482.
- Masters EA, Trombetta, RP de Mesy Bentley, et al. (2019) Evolving concepts in bone infection: Redefining "biofilm", "acute vs. chronic osteomyelitis", "the immune proteome" and "local antibiotic therapy". Bone Res 7: 20.
- Stevens QE, Seibly JM, Chen YH, et al. (2007) Reactivation of dormant lumbar methicillin-resistant Staphylococcus aureus osteomyelitis after 12 years. J Clin Neurosci 14: 585-589
- Townsend DE, Ashdown N, Pearman J, et al. (1985) Genetics and epidemiology of methicillin-resistant staphylococcus aureus isolated in a Western Australian hospital. Med J Aust 142: 108-111.
- Watkins R, Michael D, Salata R (2012) Current concepts on the virulence mechanisms of methicillin-resistant staphylococcus aureus. J Med Microbiol 61: 1179-1193.
- Brodie BC (1813) Pathological researches respecting the diseases of joints. Med Chir Trans 4: 210-280.
- Costerton JW, Geesey GG, Cheng KJ (1978) How bacteria stick. Sci Am 238: 86-95.
- Cosgrove S, Sakoulas E, Perencevich G, et al. (2003) Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin Infect Dis 36: 53-59.
- Cosgrove S, Qi Y, Kaye K, et al. (2005) The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: Mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 26: 166-174.
- Pendelton A, Kocher M (2015) Methicillin-resistant staphylococcus aureus bone and joint infections in. J Am Acad Orthop Surg 23: 29-37.
- Hawkshead J, Patel N, Steele R, Heinrich S, et al. (2009) Comparative severity of pediatric osteomyelitis attributable to methicillin-resistant versus methicillin- sensitive staphylococcus aureus. J Pediatr Orthop 29: 85-90.
- Teermat M, Raijmakers P, Scholten H, et al. (2005) The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: A systematic review and meta-analysis. J Bone Joint Surg 87: 2464-2471
- Pozo G, Collazos J, Camporro D, et al. (2018) Factors predictive of relapse in adult bacterial osteomyelitis of long bones. BMC Infect Dis 18: 635.
Corresponding Author
Callum Fryer, Department of Orthopaedics, Gold Coast University Hospital, Gold Coast, QLD, Australia.
Copyright
© 2022 Fryer C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.