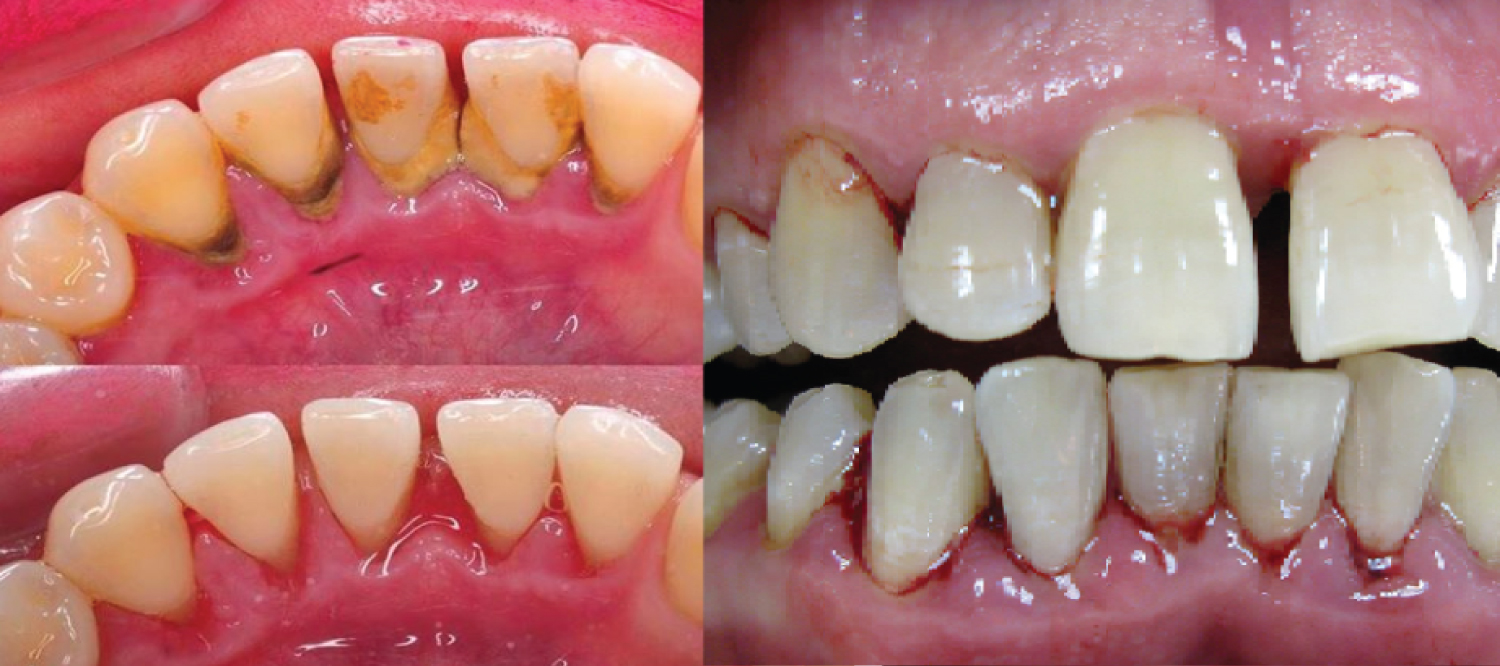
Monitoring the Effects of Combinatet Treatment Chronic Periodontitis Using Bone Metabolism Biomarker Osteocalcin
Abstract
Objectives
Evaluate the effect of combinatet treatment chronic periodontitis using bone metabolism biomarker osteocalcin.
Materials and Methods
84 patients with mild chronic periodontitis were participated in this study who received initial periodontal therapy. All patients underwent a thorough clinical and laborator examination including a determination of the serum osteocalcin content, at the beginning of the study and 1, 3 months after therapy.
Results
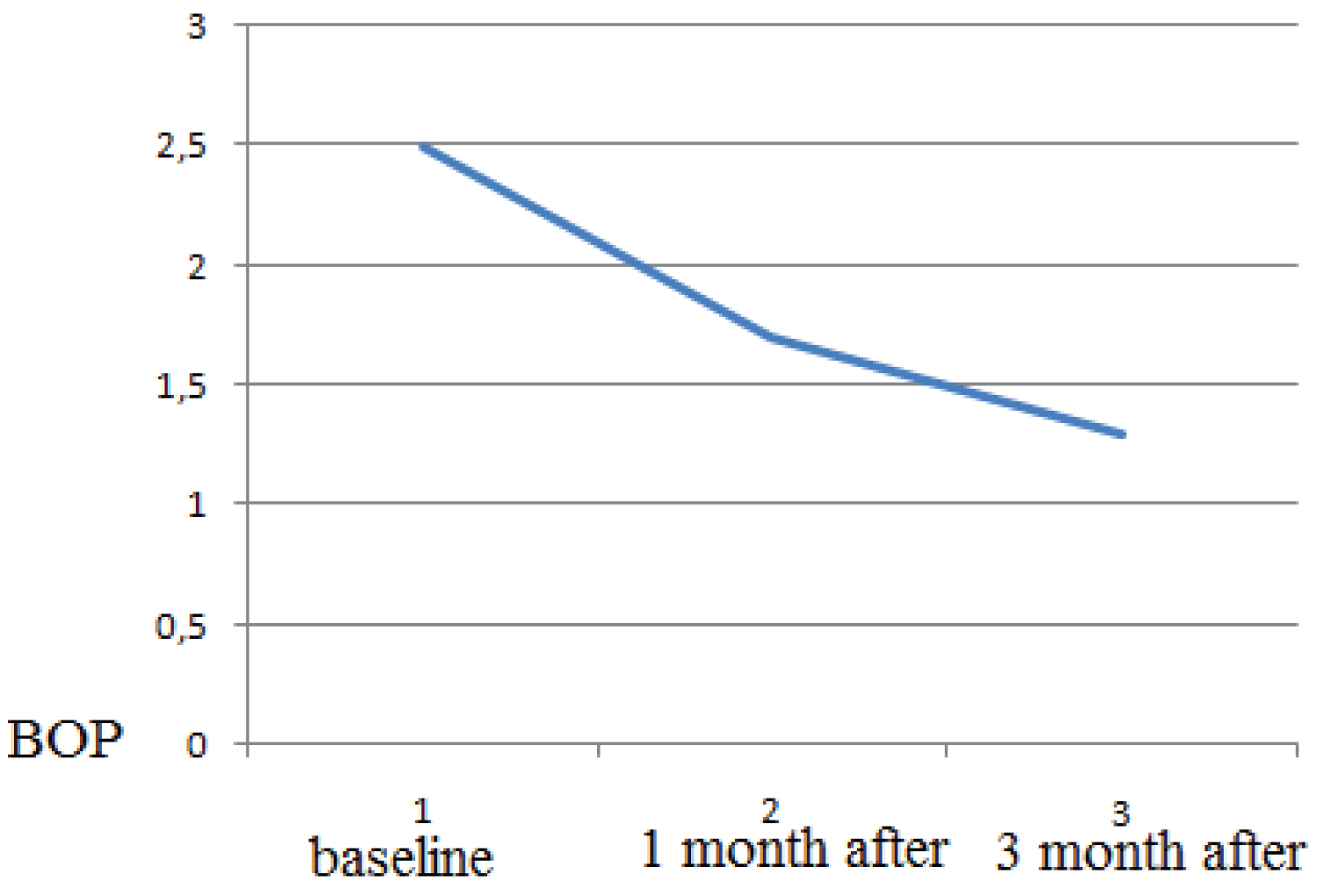
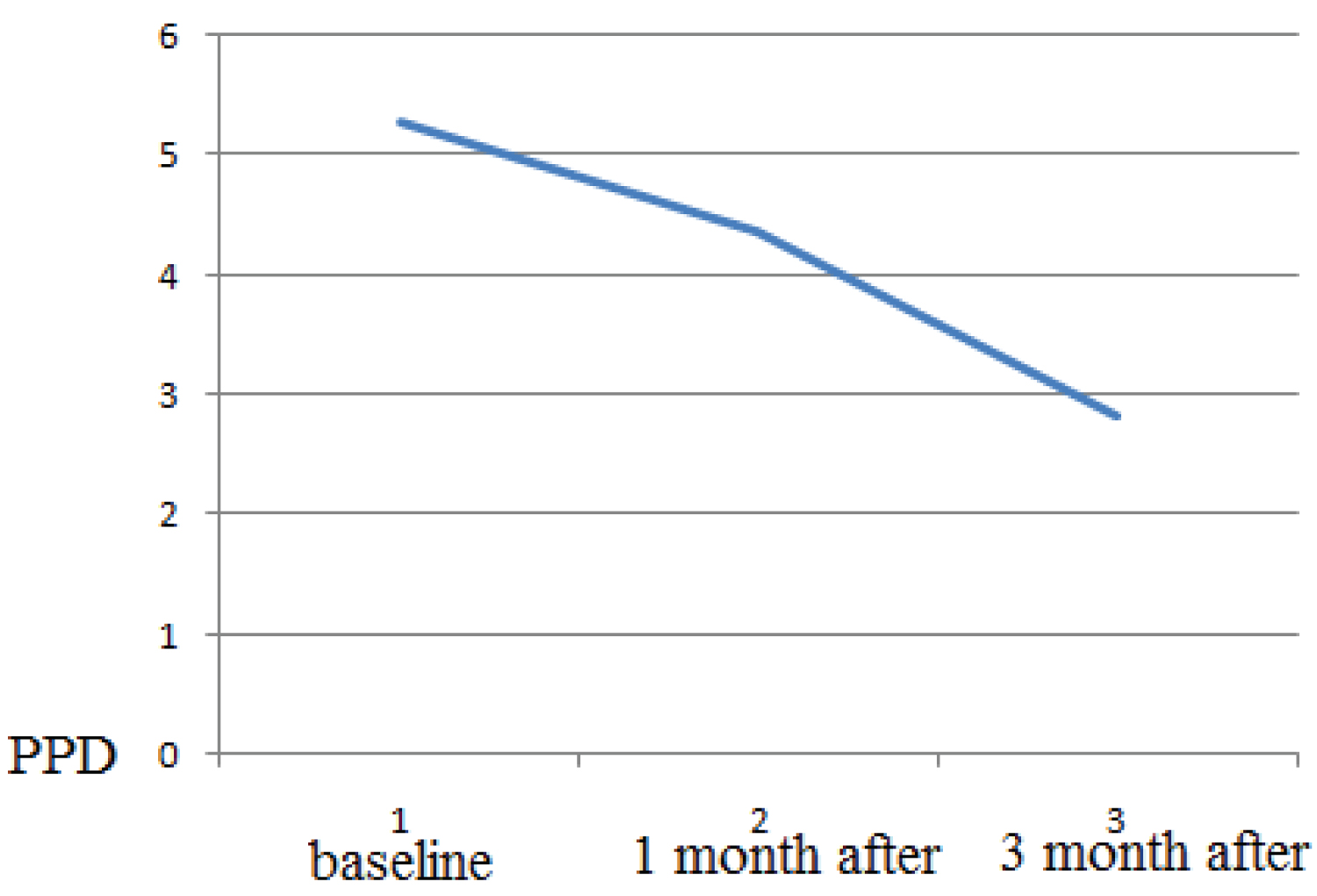
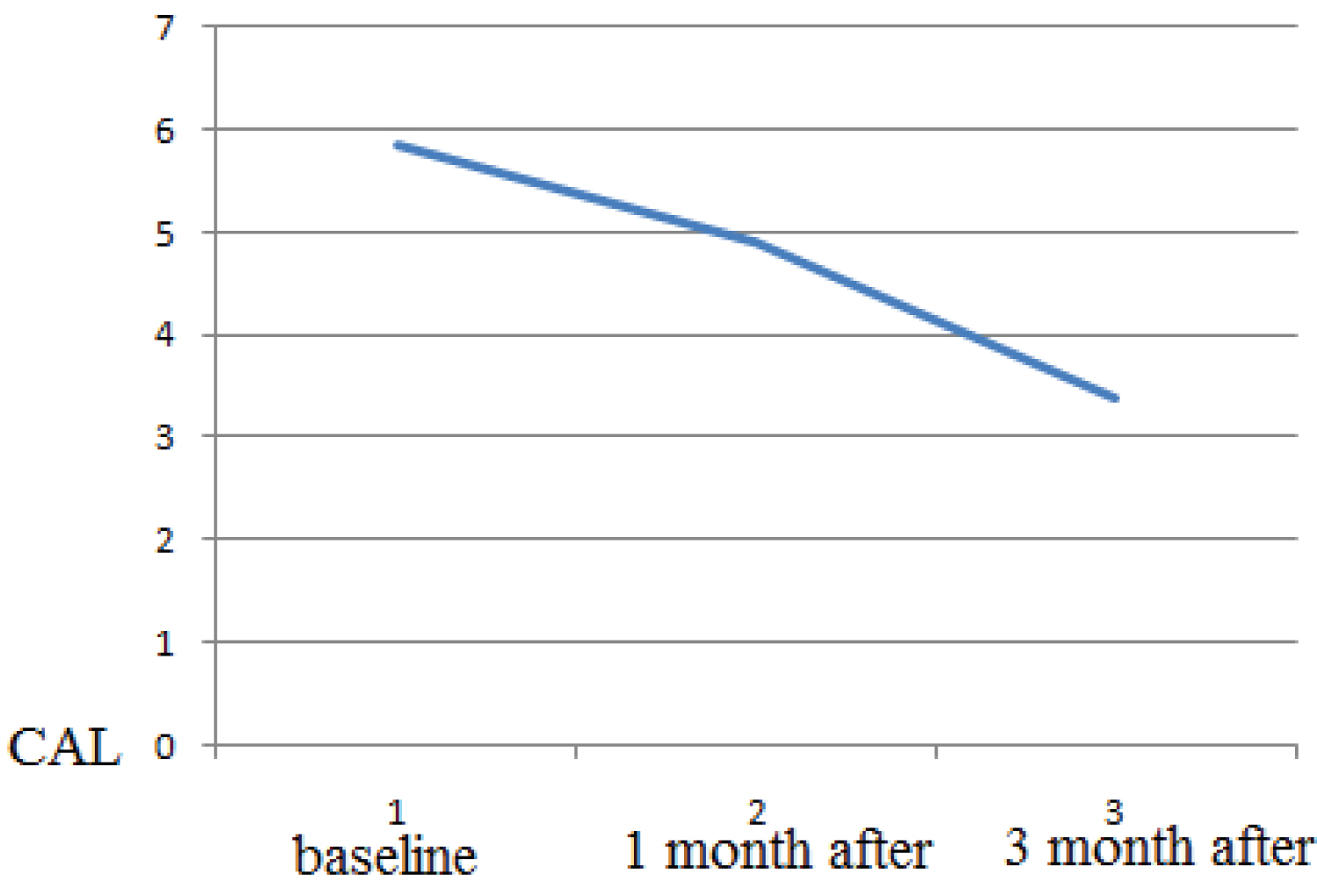
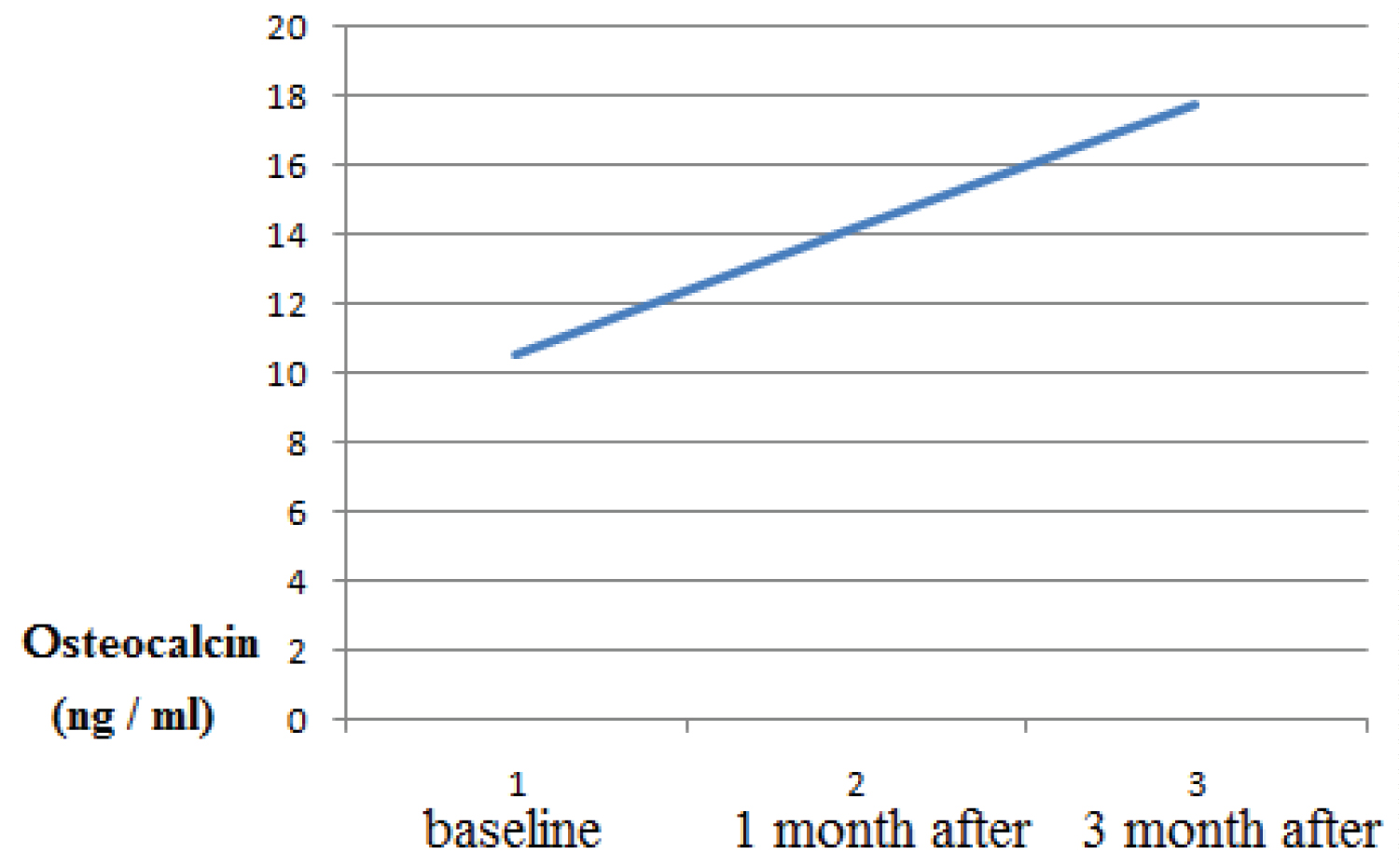
Healing period occurred without complications, and with minimal postoperative discomfort. The mean BOP before treatment was 2.5 ± 0.31, after 1 month treatment the mean BOP 1.7 ± 0.1, after 3 months treatment the mean BOP 1.3 ± 0.12 (p > 0.05). The probing depth according to the mean value (PD) was 5.27 ± 0.77 mm at the beginning of the study and, respectively, 4.35 ± 0.73 mm 1 month after therapy and 2.82 ± 0.43 (p > 0.05 ) 3 months after therapy. The clinical attachment level (CAL) mean value was 5.27 ± 0.77 mm at baseline and,1 month after therapy it was respectively, 4.35 ± 0.73 mm and 3 month after therapy it was 2.82 0.43 (p > 0.05 ). The osteocalcin in serum was 5.27 ± 0.77 mm at baseline 10.58 ng/ml, 1 month after periodontal therapy it was 14.23, 3 month after and, 3 month after periodontal therapy it was 17.74 ng/ml. As a result, there is a statistically significant increase in mean osteocalcin postoperatively (p > 0.05).
Conclusions
The study results showed that elevated serum osteocalcin concentration in patients with chronic periodontitis after periodontal therapy correlates with PPD and CAL, suggesting that its role could be used as a diagnostic test for patient and to complement other diagnostic tests.
Clinical relevance
The results of this study showed that a combination treatment with the use of bone graft materials, hyaluronic acid and magnetic laser therapy is an effective therapy for the treatment of periodontal disease.
Keywords
Threatment periodontitis, Bone metabolism markers, Osteocalcin
Abbreviations
PPD: Probing Pocket Depth; BOP: Bleeding On Probing; MBL: Marginal Bone Level; CP: Chronic periodontitis; OC: Osteocalcin; GCF: Gingival Crevicular Fluid; Er:YAG: Erbium-Doped Yttrium Aluminium Garnet
Introduction
Chronic periodontitis is a very common pathology and is characterized by progressive bone destruction. Chronic periodontitis is regarded as the leading cause of tooth loss in the adult population and is a multifactorial immune-inflammatory disease of the oral cavity [1]. That results periodontitis leading gingival bleeding, formation of a pocket, alveolar bone loss and complete destruction and loss of teeth. Periodontitis occurs due to a violation of the symbiotic relationship between the flora of the oral cavity and the host's immune system, which are characterized by successive periods of exacerbation of microbes with subsequent periods of remission [2].
Periodontitis affects nearly 5-15% of the adult population worldwide and its severity increases with age, with a peak incidence of around 30-45 years, the most common form of periodontal disease [3]. Many pathogenic mechanisms have been suggested and included alteration of oral microbial flora, vascular changes, abnormal defense mechanisms and altered collagen metabolism [4,5]. The bacterial biofilm plays an important role in the development of the etiology of periodontitis, microbial colonization is played by colonizing on the surface of the tooth and in the gum groove. Commonly found microorganisms are Gram-negative anaerobes, Prevotella intermedia, Tannerella forsythia, Porphyromonasgingivalis, Aggregatibacteractino mycetemcomitans, Bacterioides forsythus, Prevotellanigrescens, Peptostreptococcus micros, Fusobacterium nucleatum [6,7]. Poor oral hygiene, inadequate dietary, alcohol and tobacco consumption, smoking, and genetic factors also plays an important role in the etiology periodontitis [8]. Chronic periodontitis is also involved in various systemic diseases, such as diabetes mellitus cardiovascular disorders, renal impairment and osteoporosis [9,10].
The pathogenesis of chronic periodontitis involves immunomodulatory patient responses triggered by toxic by-products secreted by bacterial microbes. Bacterial byproducts activate various cytokines, chemokines, pro-inflammatory mediators and macrophages that are responsible for the progressive destruction of the underlying gum tissue and subsequent tooth loss [11]. The European Federation of Period ontology (EFP) and the American Academy of Period ontology (AAP) have completed a new classification of diseases and conditions of the periodontal and peri-implant [12].
Periodontitis is clinically manifested with the formation of deep periodontal pockets, loss of periodontal ligament and cementum attachment, and resorption of the alveolar bone, resulting in complete tooth loss [3].
The commonly used diagnostic methods for assessing periodontitis include clinical pocket probe depth for sounding (PPD), bleeding on probing (BOP), loss of clinical attachment (CAL) and radiological measurements. The clinical nature of periodontitis is proposed based on for CAL ≥ 6 mm in two or more teeth and one or more sites with PD ≥ 5 mm. Clinical methods BOP and PPD are invasive, painful and not acceptable to patients; they can only detect past disease activity, but not current periodontal disease activity. Based on this, it is necessary to introduce clinical practice of non-invasive, highly sensitive diagnostic methods for early detection of periodontal disorders. The increasing prevalence of periodontitis requires the development of new innovative diagnostic methods.
Since one of the clinical features of periodontal disease is tissue destruction, including bone, our interest was focused on whether bone metabolism and, in particular, bone remodeling change during the development and progression of periodontal disease. Various factors are involved in the bone remodeling process, such as hormones, growth factors and cytokines. The process includes osteocytes, osteoblasts, and osteoclasts. Based on this, various components of serum and saliva, such as immunoglobulins, proteins, proinflammatory cytokines and enzymes, are currently being studied as biomarkers for screening cases of changes in soft tissues and hard tissues due to periodontitis [13,14].
Biomarkers are substances that are evaluated as indicators of normal biological and pathogenic processes and can be used to monitor health status, disease onset, response to treatment, and outcome. Over the past two decades, for the diagnosis of oral diseases, there has been a steady trend towards developing tests for monitoring periodontitis. These studies range from physical measurements to genetic susceptibility analysis and molecular analyzes. Significant results have been achieved in understanding the mediators that affect the different stages of periodontal disease.
The process of bone remodeling is a dynamic process that takes place in the bone, in which the rate of bone formation exceeds the rate of bone resorption and this process is balanced in healthy people. In periodontitis, the balance of bone remodeling changes and the rate of bone resorption exceeds the rate of bone formation of the alveolar process [15]. The role of biomarker tests is to provide earlier detection of periodontal disease and more reliable measurements of therapy efficacy [16]. To determine the degree of destruction of the alveolar bone and the risk of bone loss in periodontitis, biomarkers of bone metabolism have valuable diagnostic potential [17].
Bone markers are subdivided into markers of bone formation and markers of bone resorption. Biochemical markers of bone formation include phosphatase, osteocalcin, procollagen I, and plasma peptides and markers of bone resorption include calcium, hydroxyproline, pyridioline and deoxypyridine, cathepsin, crosspaws resistant to acids and tetrates phosphatase, and plasma telopeptides [18]. In light of this fact, in patients with periodontitis, various studies have quantified biomarkers of bone metabolism in curved gingival fluid (GCF) and saliva [19-21]. In patients with periodontitis, important studies have been carried out in the field of saliva and curved gingival fluid (GCF) diagnostics over the past 2 decades, in which various biomarkers have been found [22,23].
In periodontal disease, there has recently been interest in osteocalcin as a potential marker of bone metabolism. Osteocalcin is a marker of bone formation, increased levels of osteocalcin are associated with rapid bone remodel in gland due to its role in recruiting osteoclasts to the site of bone resorption, it is now widely accepted as a bone turnover marker. In serum/plasma, it moves freely in the form of a decarboxylated form, while in bone it is present in the form of an inactive carboxylated form [24,25]. It is the most abundant protein found in the extracellular matrix of bone. In serum/plasma, it moves freely in the form of a decarboxylated form, while in bone it is present in the form of an inactive carboxylated form It’s concentration in blood reflects the metabolic activity of osteoblasts, which is related to the rate of bone formation. Osteocalcin an indicator of the level of bone metabolism in general, as measurement of the content of OC in blood serum allows monitoring of bone metabolism [26,27].
A high level of Osteocalcin in the blood indicates an increased rate of bone remodeling. Elevated of osteocalcin levels in serum are associated with metabolic diseases such as multiple myeloma, rheumatoid arthritis, and bone regeneration. Levels of osteocalcin examined in three different fluids i.e. saliva, GCF and serum [28,29]. In patients with periodontal disease with salivary markers of bone turnover are useful tools for screening [30]. Over the past 20 years treatment of periodontal diseases has undergone a series of changes. Various treatment options are available periodontitis including non-surgical therapy (scaling, root planning, and antibiotics), surgical tissue engineering, photodynamic therapy etc. Since periodontitis is associated with chronic infection of periodontal pathogens, a large body of research has been conducted to develop effective antimicrobial regimens for the treatment of periodontitis. The main goal of periodontal therapy is the regeneration of periodontal tissues and, in particular, the loss of the alveolar ridge. However, it must be recognized that current treatments, including both surgical and non-surgical approaches, still need to be considered important and beneficial. Guided tissue regeneration techniques have been popular, but regeneration of a new attachment device after bone grafting, including new bone, cementum and functionally oriented periodontal ligament, usually does not occur, except at the base of the periodontal defect.
Good supportive periodontal therapy can support periodontal health, alternative approaches to this gold standard patient-friendly, should be easy to use, affordable. Various systemic and topical antibiotics and antimicrobial medicines are used to treat periodontal disease. Surgical treatments include soft tissue grafts, pocket reduction surgery, bone grafts, targeted tissue regeneration, proteins, and stimulating tissues. Based on this, the determination of biochemical markers of bone metabolism of osteocalcin (OS) in parallel with other diagnostic tests plays an important role in order to facilitate early detection, accurate diagnosis and development of effective therapies leading to optimal clinical management of periodontitis. Therefore, the aim of this study was to evaluate the effect of the combined treatment of chronic periodontitis using the bone metabolic biomarker osteocalcin.
Materials and Methods
The present study included a total of 84 patients (39 women and 45 men, aged 28-56) years without any systemic diseases with moderate chronic periodontitis.
Inclusion criteria
1. Patients with generalized chronic periodontitis;
2. Age group between 35 and 56 years;
3. Periodontal pocket depth and loss of clinical attachment of about 5 mm or more,
4. Systemically healthy individuals/no underlying medical conditions.
Exclusion criteria
1. Patients with any systemic diseases which are known to affect any of the considered parameters in any way;
2. Patient on any protein supplements;
3. Any periodontal therapy in the previous 6 months;
4. Use of any antimicrobials in the previous 3 months.
Clinical parameters
The following parameters were recorded at the beginning of the study (before any treatment), after 1 month (after nonsurgical therapy), at the end of the 3-month postoperative surgery (flap surgery).
1. Probing pocket depth (PPD)
2. Clinical attachment level (CAL)
3. Bleeding On Probing (BOP
4. Blood samples were collected for serum osteocalcin.
Indications for Marginal Bone Level (MBL) were evaluated by periapical radiographs (taken at the baseline diagnostic appointment).
Each patient was measured from the cement enamel junction to the alveolar crest and expressed as a proportion of root length. Before measuring clinical indices, the gums and teeth were blown dry. PPD was measured from the gingival margin to the most apical penetration of the probe. BOP was measured according to gingival bleeding index. Bleeding On Probing (BOP) is assessed as present if bleeding was evident within 30 seconds after the study or was not present if bleeding was not observed within 30 seconds after the study.
BOP indices were evaluated by the following criteria
0 - No bleeding,
1 - Bleeding occurs no earlier than 30 seconds,
2 - Bleeding occurs in less than 30 seconds,
3 - Bleeding occurs when eating or brushing your teeth.
The degree of bleeding was assessed by the criteria
0.1 - 1.0 - Mild inflammation,
1.1 - 2.0 - Medium inflammation,
2.1 - 3.0 - Severe inflammation.
Serum sample were collected for study group were measured using enzyme linked immunosorbent assay kits at baseline and 3 months after therapy.
The serum was blood for the determination of osteocalcin concentration in serum was taken, samples were centrifuged to obtained plasma, and Quantification of serum osteocalcin concentration samples was examined by the ELISA.
Phase I Periodontal Therapy
The treatment plan consisted of the following items: Trauma from tooth occlusion if detected was relieved. Extraction poor prognosis tooth, Initial periodontal treatment, Scaling and root planning, Supportive periodontal therapy.
Antibacterial therapy
Orally with duration of 7-10 days, patients were prescribed systemic antibiotics (amoxicillin 500 mg and metronidazole 200 mg or augment in 875 mg or ciprofloxacin 250 mg). For the selection of the most effective antibiotic for each case, microbial testing was performed.
Patients were given detailed instructions on self-administered plaque control measures. After 4-6 weeks, only those patients underwent surgery who maintained optimal oral hygiene.
Surgical Procedure
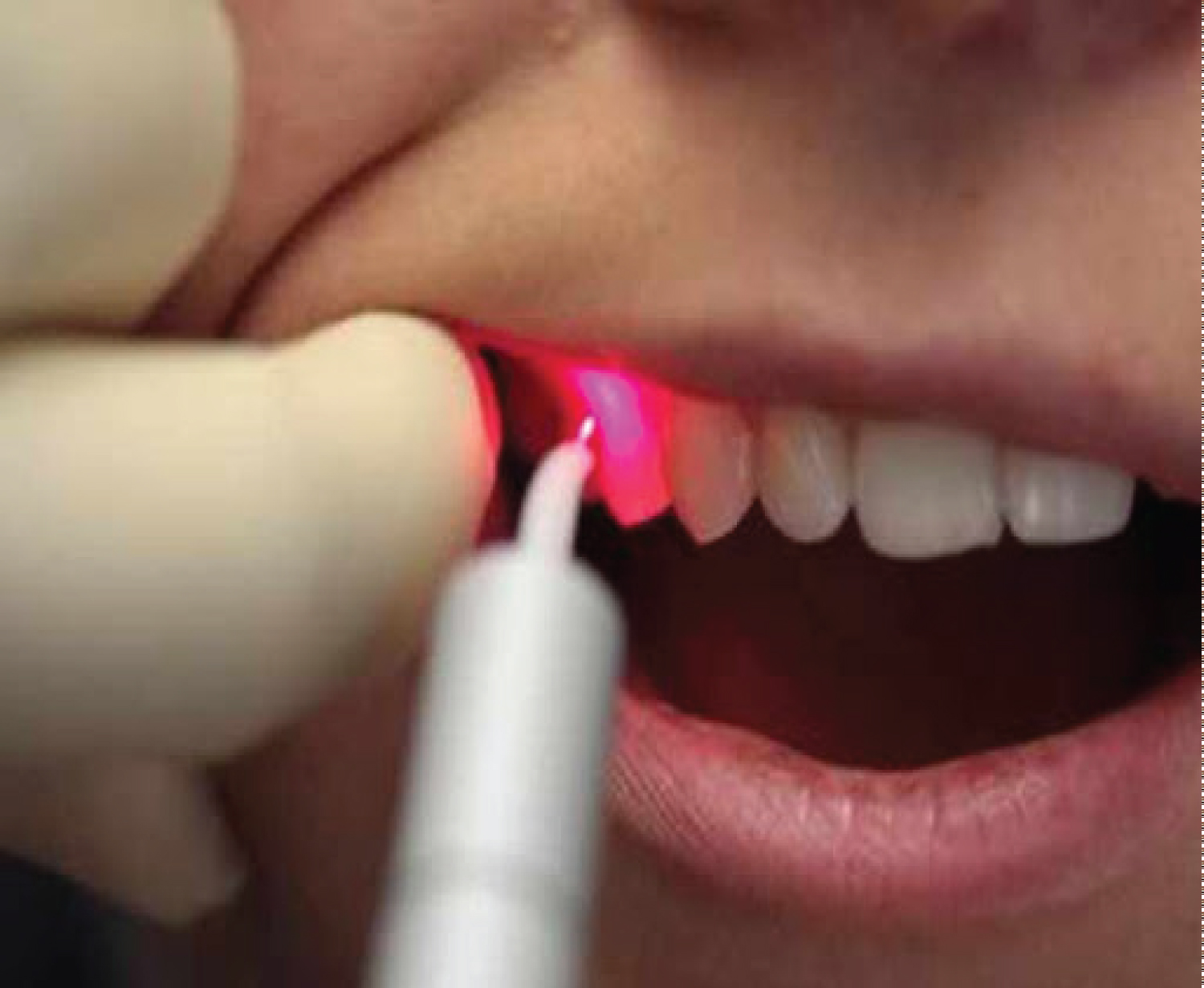
Perioral preparation with povidone-iodine was followed by rinsing of the mouth with 10 ml of 0.2% chlorhexidine The operative site was anesthetized with 2% lignocaine hydrochloride with adrenaline (1:100,000). After achieving adequate anesthesia indicated flap surgery was done. Bone loss was evaluated intrasurgically; granulation tissue was carefully removed in the infrabone defect around a tooth root, successive topical applications of 0.12% chlorhexidine, sterile physiological saline. Operation area underwent magnetic laser irradiation with a wavelength of 810 nm and a density of 100 mW for 3 min was subjected to magnetic laser irradiation with a wavelength of 810 nm and a density of 100 mW for 3 min in the area of the affected area.
A collapan (Intermediapatit RF) was mixed with a Gengigel hyaluronic acid preparation and the bone defect was filled, a Bio-Gide biodegradable collagen membrane was placed over the filled defect. After bone grafting, the flaps were transferred and sutured. Patients were instructed to flush twice a day for 1 minute for 2-3 weeks with 0.12% chlorhexidine. After surgery the patients received magnetic laser irradiation 7 days with a wavelength of 810 nm and a density of 100 mW during 5 min. After 7-10 days the surgery, the sutures were removed.
Result
Healing periods occurred without complications, and with minimal postoperative discomfort. Clinical measurements and sampling were repeated at before periodontal treatment, 1, 3 months after treatment. The diagnostic parameters were comparable at baseline and after treatment. Radiologically increased or stable levels of the marginal bone compared with the baseline periapical x-rays is considered to be a treatment success. Reduction BOP, PPD and MBL was observed in comparison with basic clinical measurements. Clinical evaluation of the results of treatment after 1, 3 months showed reduction in BOP, CAL and PPD were as compared with the baseline clinical measurements.
The mean BOP before treatment was 2.5 ± 0.31, after 1 month treatment the mean BOP of patients had mean 1.7 ± 0.1, after 3 months treatment the mean BOP 1.3 ± 0.12 (p > 0.05) (Figure 1).
The mean value of probing pocket depth (PPD) at baseline in the study group was 5.27 ± 0.77 mm, 1 month after therapy it was 4.35 ±0.73 mm and 3 month after therapy it was 2.82 ± 0.43. As a result, there was a statistically significant decrease in mean PPD post-operatively (at P < 0.001) (Figure 2).
The mean value of clinical attachment level (CAL) at baseline in the study group was 5.84 ± 0.79 mm, 1 month after therapy it was was 4.92 ± 0.71 mm. and 3 month after therapy it was3,4 ± 0,7. Therefore, in periodontitis patients; there was a statistically significant decrease in mean CAL post-operatively (at P < 0.001) (Figure 3).
The mean value of osteocalcin in serum at baseline was 10.58 ng/ml, 1 month after periodontal therapy it was 14.23 ng/ml, 3 month after periodontal therapy it was was 17.74 ng/ml, As a result, there is a statistically significant increase in mean osteocalcin postoperatively (at P < 0.001) (Figure 4).
The results of this study indicated that a periodontol treatment, bone grafting with grafts materials Collapan L and hyaluronic acid Gengigel, magnetic-laser therapy accelerates was an effective therapy for treatment of periodontitis. Оver a long period of time, magnetic laser therapy has led to a positive effect on clinical and radiological parameters (Figure 5 and Figure 6).
Discussion
In periodontitis, there is a loss of connective tissue and bone around the teeth, combined with the formation of periodontal pockets due to apical migration of the connective epithelium. According to the World Health Organization, periodontitis leading to tooth loss is diagnosed in 5-15% of the world’s population [31].
Early diagnosis and treatment of periodontitis is required so that the disease does not become irreversible. Diagnosis of periodontitis is crucial for treatment planning and provides important data during the stages of periodontitis treatment and disease monitoring. Over the past two decades, in the field of diagnostics of diseases of the oral cavity, there has been a steady trend towards the development of periodontitis monitoring tools. Clinical traditional parameters for periodontal diagnosis include probing depth, probing bleeding, and levels of clinical attachment, plaque index, and radiographs assessing alveolar bone level [32].
And they are inherently limited to assessing only the history of the disease, not the current state of the disease. periodontal probe and radiographic assessments of alveolar bone loss measure damage from past episodes and require a 3 mm threshold change before the site can be identified as having significant anatomical shape [33], to determine the state of periodontal disease, this process must be repeated at regular interval recording multiple parameters at six sites per tooth, resulting in a laborious diagnostic process that is also dependent on the individual clinician performing the examination.
Today, one of the main problems in the field of periodontics is the determination of a fast, effective and objective method of diagnosis and monitoring with the possibility of screening for susceptibility to periodontal diseases, diagnosis, assessment of response to treatment, and prediction of future tissue. Destruction and identification of disease progression. An ideal diagnostic and monitoring method would be one that can control the disease at both the patient and site level.
Although there are many potential biomarkers, the gold standard for diagnosing periodontal disease is not available. Since an ideal diagnostic test should have high sensitivity, specificity and predictive value. There is still a lot of work to be done to fully validate the usefulness of systemic periodontal diagnostic tests, which are still in their early stages of development, to become an important and cost-effective aspect of clinical diagnosis, treatment planning or patient monitoring.
It is very important that the new diagnostic tests developed can detect the presence of the disease, predict its development and assess the response to periodontal therapy, thereby improving the clinical management of patients with periodontal disease. Progress in the diagnosis of oral and periodontal disease is moving towards methods by which the risk of periodontal disease can be determined and quantified using objective measures such as biomarkers, hence the ideal diagnostic test should be of high sensitivity, specificity and predictive value. The gold standard for diagnosing periodontal disease is not available although there are many potential biomarkers.
As defined by the Working Group on Biomarker Definitions, a biomarker is defined as “a parameter that is objectively measured and assessed as an indicator of normal biological or pathological processes.
In periodontitis of the affected tissues, periodontal disease, biochemical signaling, which includes three biological phases-inflammations, degradation of connective tissue and alveolar outflow of bones, contributes to clinical morbidity.
Biomarkers of saliva and curved fluid and gums (GCF can potentially provide information at the site level; and blood, serum, or plasma contains systemic biomarkers and can potentially provide information at the patient level; and saliva contains both local and systemic markers and also provides information on patient level. Any of these diagnostic fluids have advantages and disadvantages. The concentration of markers of bone metabolism in patients with periodontal disease changed in the gingival fluid, in serum and in all saliva, which makes them putative biomarkers of the disease [34].
The advantage of using GCF as a diagnostic fluid is that the GCF collection method is non-invasive, since the fluid gives the site a specific diagnosis of periodontal disease. Serum or plasma provides valuable information about the inflammatory stimulus and/or response generated in the circulation to periodontal pathogens that colonize in the sub gingival stomach [35].
Blood sampling is usually more invasive than saliva; it is easy, fast and can be performed outside the dental office and as part of a routine general diagnostic check up blood tests can be used to diagnose and monitor periodontal disease due to a simple, fast and relatively non-invasive sampling method.
In this context, in patients with periodontal disease, quantitative and qualitative diagnostics of bone biomarkers provide information on the extent of alveolar ridge involvement. However, it is clear that studies are needed to determine the prognostic value and diagnostic accuracy of these markers of bone turnover in periodontitis.
The aim of this study was to monitor the effects of periodontitis (CP) treatment using osteocalcin (OS) biomarkers of bone metabolism.
To accomplish the tasks 84 patients free of any systemic conditions with moderate chronic periodontitis (study group) were included in this study. MBL, BOP, PPD, CAL was measured. Serum sample were collected at baseline and 1, 3 months after therapy for study group, serum osteocalcin were measured using enzyme-linked immunosorbent assay kits. The diseased patients received initial periodontal therapy and after 4-6 weeks, only those patients maintaining optimum oral hygiene were subjected to the surgical procedure.
Bone loss was evaluated intrasurgically, granulation tissue was carefully removed in the infrabone defect around a root operation area underwent magnetic-laser irradiation with a wavelength of 810 nm and a density of 100 mW for 30 seconds. After surgery the patients received magnetic laser irradiation 7 days with a wavelength of 810 nm and a density of 100 mW during 5 min. Clinical measurements and sampling were repeated at 1, 3 after treatment. Clinical evaluation of the results of treatment after 1,3 months showed reduction in both BOP CAL and PPD were as compared with the baseline clinical measurements.
The baseline mean value of osteocalcin in serum after periodontal therapy increased in parallel with the clinical tests. An elevated levels osteocalcin in blood serum in patients after periodontal therapy shows the activity of the remodeling process the bone, which is favorable for periodontal tissues. Laser therapy is a modern therapeutic technique that can be effectively used as a complement to traditional mechanical therapy for the treatment of periodontitis. Diode lasers have been shown to have potent bactericidal effects based on this diode laser, carbon dioxide (CO2) and Erbium Yttrium, Aluminum, Garnet (Er: YAG) lasers are suitable for irradiating [35-38].
Magnetic-laser therapy has shown promising therapeutic effect in treatment of periodontitis.
Curative effect of magneto-laser therapy is determined by the Biostimulation and mobilization of the existing energetic potential and is manifested as immune-modulating, anti-inflammatory, antispastic, regenerative, normalizing blood Rheology and hemodynamic [39]. The use of magneto-laser therapy in our study for decontamination of the affected surface of the implant has demonstrated promising results treating periodontitis.
Due to its anti-inflammatory and antibacterial action, hyaluronic acid can be used as an adjunct to mechanical therapy in the treatment of periodontitis. Hyaluronic acid, due to its protective effect and slow absorption, ensures reliable and predictable regeneration of the augmentate, which is very important in the process of wound healing. Our results suggest that the preparation of hyaluronic acid Gengigel is used in combination with Collapan represents a good adjunctive treatment to conventional therapy of periodontitis.
The higher efficiency and osteoconductive properties of Collapan L for repairing intrabony defects are demonstrated in this study. The medical effect of Collapan L is caused by its Osseo genial antibacterial properties: The preparation contains high-purity collagen and HA, which is a matrix for re-formed bone fabric, Lincomycin possesses anti-inflammatory action, and creates an antiseptic background in a wound with prolonged allocation of an antibiotic within 20 days [40]. Our results indicate that Gengigel hyaluronic acid represents a reliable adjunct treatment to conventional therapy. This barrier function of hyaluronic acid is very important in the wound healing process, and it is a very promising material for improving the results of periodontitis treatment [41-45].
Our results show that Gengigel hyaluronic acid preparation used in combination with collapan is a good adjunct to conventional periodontitis therapy.
This study demonstrates the higher efficiency and osteoconductive properties of collapan L for the repair of intracellular defects. The therapeutic effect of collapan L is due to its osteogenic antibacterial properties: The drug contains high-purity collagen and HA, which is a matrix for reformed bone tissue, lincomycin has an anti-inflammatory effect and creates an antiseptic background in the wound.
The results of this study indicated that surgical protocol described in this article gives positive results, therefore it is recommended as effective method therapy periodontitis.
A study of osteocalcin (OC) as markers of bone metabolism showed that the assessment of highly specific biochemical parameters of bone metabolism is useful for assessing the clinical status of this metabolism. Knowing the correlation between levels of bone metabolism markers and clinical status may help in making an appropriate decision.
Conclusions
Treatment of periodontal diseases was effective in improving clinical parameters in patients with chronic periodontitis. Magnetic-laser supportive therapy may be considered an adjunct to the conventional surgical treatments of periodontitis. Osteocalcin could be used as a potential diagnostic marker for periodontal disease activity in serum.
Conflict of Interest and Financial Disclosure
The author declares that he has no conflict of interest and there was no external source of funding for the present study.None of the authors has any relevant financial relationship(s) with a commercial interest.
Source of Funding
No funding.
Ethical Approval
This study was reviewed and approved by the Ethics Committee of the of the Yerevan State Medical University after M. Heratsi (protocolN16,5.10.17) and in accordance with those of the World Medical Association and the Helsinki Declaration. Informed consent Patients were informed verbally and in writing about the study and gave written informed consent.
References
- Eija Könönen, Mervi Gursoy, Ulvi Kahraman Gursoy, et al. (2019) Periodontitis: A multifaceted disease of tooth-supporting tissues. J Clin Med 8: 1135.
- Kinane DF, Stathopoulou PG, Papapanou PN, et al. (2017) Periodontal diseases. Nat Rev Dis Primers 22: 17038.
- Papapanou PN, Susin C (2017) Periodontitis epidemiology: Is periodontitis under-recognized, over-diagnosed, or both? Periodontology 75: 45-51.
- Salvi GE, Lawrence HP, Offenbacher S, et al. (2000) Influence of risk factors on the pathogenesis of periodontitis. Periodontol 14: 173-201.
- Wilton JM, Griffiths GS, Curtis MA, et al. (1988) Detection of high risk groups and individuals for periodontal diseases. Systemic predisposition and markers of general health. J Clin Periodontol 15: 339-346.
- Hajishengallis G (2015) Periodontitis: From microbial immune subversion to systemic inflammation. Nat Rev Immunol 15: 30-44.
- Bartold PM, Van Dyke TE (2019) An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J Clin Periodontol 46: 6-11.
- Bouchard P, Carra MC, Boillot A, et al. (2017) Risk factors in periodontology: A conceptual framework. J Clin Periodontol 44: 125-131.
- Nazir MA (2017) Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim) 11: 72-80.
- Zeng XT, Leng WD, Lam YY, et al. (2016) Periodontal disease and carotid atherosclerosis: A meta-analysis of 17,330 participants. Int J Cardiol 15: 1044-1051.
- Meyle J, Chapple I (2015) Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000 69: 7-17.
- Mealey BL, Van Dyke TE, Bartold PM, et al. (2018) Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol 1: 74-84.
- Hamda Shazam, Fouzia Shaikh, Zaheer Hussain, et al. (2020) Bone turnover markers in chronic periodontitis: A Literature Review. Cureus 12: e6699.
- Joseph Betsy, Javali MA, Amanullah M, et al. (2019) Diagnostic accuracy of salivary biomarkers of bone turnover in identifying patients with periodontitis in a saudi arabian population. Journal of Dental Sciences 14: 269-276.
- Kanjevac T, Bijelic B, Vasovic R, et al. (2018) Stolicimpact of chronic kidney disease mineral and bone disorder on jaw and alveolar bone metabolism: A narrative review. Oral Health Prev Dent 16: 79-85.
- Colburn WA (2003) Biomarkers in drug discovery and development: From target identification through drug marketing. J Clin Pharmacol 43: 329-341.
- UKGursoy, Könönen E, Pradhan-Palikhe P, et al. (2010) Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J Clin Periodontol 37: 487-493.
- Geiser AG, Hale LV, Sells Galvin RJ, et al. (2007) PINP: A serum biomarker of bone formation in the rat. J Bone Miner Res 40: 1103-1109.
- Pamela Maffioli, Giuseppe Derosa (2017) Overview of biochemical markers of bone metabolism Biomarkers in Bone Disease, 3-21.
- Ulrike Schulze-Späte, Ryan Turner, Ying Wang, et al. (2015) Relationship of bone metabolism biomarkers and periodontal disease: The osteoporotic fractures in men (MrOs) study. J Clin Endocrinol Metab 100: 2425-2433.
- Barros SP, Williams R, Offenbacher S, et al. (2016) Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol 70: 53-64.
- Korte DL, Kinney J (2016) Personalized medicine: An update of salivary biomarkers for periodontal diseases. Periodontol 2000 70: 26-37.
- Lee AJ, Walsh TF, Hodges SJ, et al. (1999) Gingival crevicular fluid osteocalcin in adult periodontitis. J Clin Periodonto 26: 252-256.
- Giannobile W, Al-Shammari KF, Sarment DP, et al. (2003) Matrix molecules and growth factors as indicators of periodontal disease activity. Periodontol 2000 31: 125-134.
- Rodan GA (1992) Introduction to bone biology. Bone 1: S3-S6.
- Becerik S, Afacan B, Oztürk VÖ, et al. (2011) Gingival crevicular fluid calprotectin, osteocalcin and cross-linked n-terminal telopeptid levels in health and different periodontal diseases. Dis Markers 31: 343-352.
- Bullon P, Chandler L, Segura Egea JJ, et al. (2007) Osteocalcin in serum, saliva and gingival crevicular fluid: Their relation with periodontal treatment outcome in postmenopausal women. Med Oral Patol Oral Cir Bucal 12: E193-E197.
- Ram VS, Parthiban, Sudhakar U, et al. (2015) Bonebiomarkers in periodontal disease: A review article. J Clin Diagn Res 9: 7-10.
- Miricescu D, Totan A, Calenic B, et al. (2014) salivary biomarkers: Relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol Scand 72: 42-47.
- Kunimatsu K, Mataki S, Tanaka H, et al. (1993) A cross-sectional study on osteocalcin levels in gingival crevicular fluid from periodontal patients. J Periodontol 64: 865-869.
- Axelsson P, Nyström B, Lindhe J, et al. (2004) The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol 31: 749-757.
- Armitage GC (2004) Analysis of gingival crevice fluid and risk of progression of periodontitis. Periodontology 2000 34: 109 -119.
- Goodson JM (1992) Conduct of multicenter trials to test agents for treatment of periodontitis. J Periodontol 63: 1058-1063.
- Yoshihara A, Deguchi T, Hanada N, et al. (2009) Relation of bone turnover markers to periodontal disease and jaw bone morphology in elderly japanese subjects. Oral Dis 15: 176-181.
- Pussinen PJ, Susanna Paju, Paivi Mantyla, et al. (2007) Serum microbial- and host-derived markers of periodontal diseases: A review. Curr Med Chem 14: 2407-2412.
- Behdin S, Monje A, Lin GH (2015) Effectiveness of laser application for periodontal surgical therapy: Systematic review and meta-analysis. J Periodontol 86: 1352-1363.
- Maria Emília de AC, Angélica Rodrigues de Araújo, Pinotti M, et al. (2014) Effects of low-power light therapy on wound healing: LASER x LED*. An Bras Dermatol 89: 616-623.
- Vartika Kathuria, Jatinder Kaur Dhillon, Gauri Kalra, et al. (2015) Low level laser therapy: A panacea for oral maladies. Laser Ther 24: 215-223.
- Darendeliler MA, Darendeliler A, Sinclair PM, et al. (1997) Effects of static magnetic and pulsed electromagnetic fields on bone healing. Int J Adult Orthodon Orthognath Surg 12: 43-53.
- Reet Kamal, Parveen Dahiya (2013) Hyaluronic acid: A boon in periodontal therapy. N Am J Med Sci 5: 309-315.
- Hakobyan G (2005) Bone grafting procedures for osseous defects associated with dental implants. J Oral Implantol 31: 145-152.
- Sujith Sukumar, Ivo Drízhal (2007) Hyaluronic acid and periodontitis. Acta medica (Hradec Králové) 50: 225-228.
- Pagnacco A, Vangelisti R, Erra C, et al. (1997) Double-blind clinical trial versus placebo of a new sodium-hyaluronate-based gingival gel. Attual Ter In 15: 1-7.
- Ballini A, Cantore S, Capodiferro S, et al. (2009) Esterified hyaluronic acid and autologous bone in the surgical correction of the infra-bone defects. Int J Med Sci 6: 65-71.
- Hakobyan G, Seyranyan A, Khachatryan, et al. (2018) Regenerative therapy for the treatment of peri-implantitis. Austin Head Neck Oncol 2: 1007.
Corresponding Author
Gagik Hakobyan DMSc, PhD, Department of Oral and Maxillofacial Surgery, Yerevan State Medical University, 0028 Kievyan str. 10 ap. 65 Yerevan, Armenia, Tel: +37410-271146.
Copyright
© 2020 Hakobyan G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.