Pre-Operative Simultaneous Fractionated Cisplatin and Radiation Therapy in the Treatment of Advanced Stage III and IV Squamous Cell Carcinoma of the Base of Tongue and Hypopharynx
Abstract
Background
The role of multi-modality pre-operative treatment of advanced, resectable squamous cell carcinoma of the head and neck is not standardized. Low-dose, fractionated cisplatin with simultaneous radiation therapy is one therapeutic approach.
Objective
To evaluate simultaneous fractionated cisplatin and radiation therapy for advanced operable Stage III and IV squamous cell carcinoma (SCC) of the base of tongue (BOT) and hypopharynx.
Design
Retrospective chart review.
Setting
Local hospitals.
Patients
57 patients with SCC of the BOT and hypopharynx received either CTRT (cisplatin, 20 mg/M2 for 4 days during weeks 1, 4, and 7 of radiotherapy; n = 42) or CONTROL (other cisplatin dosages or carboplatin and/or Taxol during radiotherapy; or CTRT following surgery; n = 15).
Main outcome measures
Toxicity, clinical (CCR) and histologic (HCR) complete response, surgery, recurrence, survival.
Results
Versus CONTROL, CTRT high-grade toxicity was lower (14% vs 40%, P < 0.05), and CCR (63% vs 33%, P < 0.05) and HCR (59% vs 23%, P < 0.05), were higher. Among CTRT HCR patients, 17% required surgery versus 65% with residual disease (P < 0.01). 4% CTRT recurred versus 82% with residual disease (P < 0.0001). CTRT had less distant metastasis compared to CONTROL (0% vs 42%, P < 0.01), and less death with disease (50% vs 100%, P < 0.01). Kaplan-Meier analysis suggested increased survival CTRT compared to CONTROL (P > 0.05).
Conclusion
CTRT had reduced toxicity, higher CCR and HCR, and lower distant metastases and death with disease versus CONTROL. CTRT is a good first line treatment for Stage III and IV BOT/hypopharynx SCC.
Introduction
Squamous cell carcinomas of the head and neck (SCCHN) make up approximately 3 percent of all cancer cases in the United States [1]. SCCHN are most common in the oral cavity, pharynx, and larynx [2]. These cancers often are curable at any stage of localized disease, but most patients present with locally advanced Stage III or IV disease, where multi-disciplinary management is more complicated. Traditional treatment for such cancers involved surgery and/or post-operative radiotherapy [3]. More recently, multi-modality therapies have become useful for improving locoregional control and organ preservation; although survival is still poor [2]. Multi-modality therapies involve a combination of surgery, nontraditional radiation therapy, and chemotherapy integration. However, the roles of each technique are not yet standardized.
While no single treatment regimen has been defined as most effective in treating SCCHN, several studies have identified certain multi-modality combinations that produce greater success in terms of organ preservation, survival, locoregional control, and toxicity to treatment. Common multi-modality treatments include docetaxel plus cisplatin followed by fluorouracil infusion for 4 days every 3 weeks; high-dose cisplatin given on days 1, 22, and 43 of radiotherapy; daily low-dose concomitant cisplatin; and a weekly combination of carboplatin and taxol [2,4-6]. These regimens are just a sample of the variety of SCCHN treatments that have difficult to determine which treatment is best for the patients.
In recent years, investigators have found that concurrent chemotherapy and radiation prior to surgery show synergistic effects in tumor treatment, improving overall disease control and survival [3]. Organ preservation, which is highly valued by most patients, is also improved due to less post-chemo-radiotherapy surgery. Several pilot investigations have suggested that low-dose, fractionated cisplatin administered simultaneously with concomitant high-dose radiotherapy may be effective in curing cancer while preserving head and neck function [7-9]. We described the results of this regimen among SCCHN patients overall [10]. The objective of the present study was to evaluate patients with advanced operable Stage III and IV SCCHN who were treated with 20 mg/M2 IV cisplatin given on 4 consecutive days every 3 weeks during high-dose irradiation therapy (CTRT), reserving extirpative surgery for biopsy-proven residual disease after CTRT.
Methods
With the approval of the Inspira Health Network Institutional Review Board, medical records of 42 patients with Stage III and IV squamous cell carcinoma of the base of tongue retrospectively and compared with a CONTROL group of 15 patients who underwent other regimens at the discretion of their treating physicians. The CONTROL group, thus, was not a homogeneous treatment population but, rather, a heterogeneous cohort, reflective of the practices of their radiation and medical oncologists and surgeons. CTRT chemotherapy consisted of cisplatin, 20 mg/M2 administered as a continuous intravenous infusion daily for 4 consecutive days during weeks 1, 4, and 7 of radiotherapy. Conversely, CONTROL chemotherapy consisted of several regimens: cisplatin, 75 mg/M2 intravenously on days 1, 22, and 43 of radiotherapy; carboplatin, 100 mg/M2 and taxol, 45 mg/M2 once per week during radiotherapy; or CTRT regimen following surgery. Both CTRT and CONTROL patients were treated within the same time period. The treatment chosen was at the discretion of the treating physicians at Inspira Health Network.
Over the course of the study, the radiation therapy technique varied as the technology changed. In the earlier portion of the study, patients were generally treated with a regimen consisting of single daily fractionation with 6 MV photons and 3D treatment planning followed by a boost, in which they were treated with a hyper fractionated (two fractions/day) regimen with concurrent chemotherapy. In 2006, patients were treated with normal fractionation to a higher total dose, between 70-74 Gy. In the latter part of the treatment study, patients were treated with a field-within-a-field technique utilizing head and neck IMRT. PTVs were treated between 70-74 Gy. Most treatment regimens were delivered with 6 MV photons with either customized blocks or multi-leaf collimator generated blocks. Verification was performed using port films and later changed to stereoscopic imaging followed by cone beam CT.
The study variables included age, sex, race, vital status, alcohol use, tobacco use, tumor site, tumor grade, clinical stage, surgery, chemoradiotherapy regimen, clinical response, post-CTRT biopsy result, recurrence, and toxicity to treatment. Clinical stage was determined according to the classification of the American Joint Committee on Cancer Staging, third through sixth Editions (1992 through 2011). Post-chemoradiotherapy biopsy of the primary tumor site determined patients as having either a histologically complete response (HCR) or residual tumor. Planned biopsy of the primary tumor site was carried out between four weeks and eight weeks after the last day of CTRT, and at the discretion of treating physicians for CONTROL. During the first decade of this study, neck dissection was performed if any residual masses that were palpated following CTRT. However, when the primary tumor was HCR, nearly all of such neck dissections contained no viable cancer. Thus, thereafter, if neck disease was still palpable after CTRT, then the palpable mass was excised to identify residual disease or HCR. Modified neck dissections then could be performed for residual disease. When all clinical neck metastases had resolved, with CTRT, potential residual neck disease was evaluated by PET scan, with a completely negative PET counted as HCR. Patients with residual disease were recommended for curative surgery. Toxicity to treatment was determined according to the NCI Common Terminology Criteria for Adverse Events.
Statistical analysis was performed using the chi-square test to compare CTRT and CONTROL. Analysis of variance was used to compare age. Overall survival and disease-specific survival were statistically analyzed by Kaplan-Meier logarithmic rank test. Median follow-up was 20 months, with a range of 1 to 141 months. The level of significance was set as p < 0.05 [11].
Results
Of the 57 patients evaluated in this study, 21 patients had squamous cell carcinoma of the base of tongue (BOT) and 36 of the hypopharynx. Patient demographics and tumor characteristics for CTRT and CONTROL are displayed in Table 1. No significant differences between CTRT and CONTROL regarding age, sex, race, alcohol/tobacco use, tumor site, clinical stage, or tumor grade were found. In the CTRT group, 6 patients had T3 N0 cancers and 2 patients had T4 N0 tumors, compared to the CONTROL group which had no patients with T3 N0 tumors and 3 patients with T4 N0 tumors. The remaining patients had nodal disease: CTRT had 10 N1 tumors, 16 N2 tumors, and 8 N3 tumors; CONTROL had 4 N1 tumors, 7 N2 tumors, and 1 N3 tumor.
Toxicity from chemotherapy and radiation therapy is listed in Table 2. Acute morbidity in CTRT included grade III bleeding and hospitalization in 6 patients. In CONTROL, morbidity included 3 patients with grade III toxicity, 2 patients with grade IV bleeding and hospitalization, and 1 patient with grade V bradycardia. No toxicity was noted in 19% (8) of CTRT patients and in 7% (1) of CONTROL patients. High-grade toxicity (grade 3-5) was significantly increased in CONTROL compared to CTRT (40% versus 14%; p = 0.035).
Response to pre-operative treatment is described in Table 3. A clinical complete response was seen in 63% (26/41) of CTRT patients versus 33% (5/15) in CONTROL (P < 0.05). One patient in CTRT was unavailable for post-treatment response analysis. Post-chemoradiotherapy biopsy revealed a histologically complete response in 24 out of 41 CTRT patients (59%) and in 3 out of 13 CONTROL patients (23%) (P < 0.05). Two patients in CONTROL did not receive post-treatment biopsies.
Curative cancer surgery results are seen in Table 4. CTRT and CONTROL did not differ in the number of patients who required curative surgery (15/42 versus 5/13; P = 0.8625). In CTRT, 7 of the surgeries were neck dissection only, compared to 3 in CONTROL (P = 0.7642). Thus, organ preservation was achieved in 81% of CTRT and 85% of CONTROL. In CTRT, 4/24 HCR patients underwent post-treatment curative surgery (neck dissection) versus 11/17 patients with residual disease (P < 0.01).
Cancer recurrence and survival data are tabulated in Table 5. Median follow-up time was 20 months, with a range from 1 to 141 months. Recurrences developed in 15 out of 40 (37.5%) CTRT, and in 7 out of 12 (58%) CONTROL (P = 0.2003). Of those 7 CONTROL recurrences, 4 were local and 3 were distant, whereas all CTRT recurrences were local (P < 0.01). Among patients who expired, 50% of CTRT patients died with disease compared to 100% in CONTROL (P < 0.01). CTRT patients who achieved a complete response had a 5-year survival rate of 36%, while none of the patients with a partial response reached 5-year survival (P < 0.01).
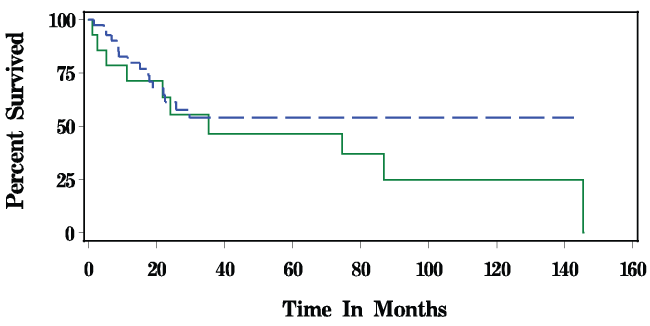
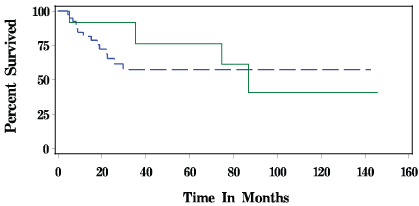
Figure 1 displays the overall Kaplan-Meier survival for patients with squamous cell carcinoma of the tongue and hypopharynx. With a median follow-up of 20 months, survival overall was 61.90% for CTRT and 28.57% in the CONTROL group (P < 0.39). Disease-free survival is depicted in Figure 2. For CTRT, disease-free survival was 66.67% for CTRT versus 71.43% in CONTROL patients (P < 0.54).
Comment
The results of this study indicate that the simultaneous administration of low-dose fractionated cisplatin chemotherapy and high-dose radiation therapy (CTRT) is an effective primary treatment for patients with advanced operable Stage III and IV SCCHN of the hypopharynx and base of tongue. Toxicity to treatment was lower with CTRT compared with CONTROL and with other standard regimens. CTRT also resulted in more clinical complete responses. Biopsy revealed more histologic complete responses as well. No differences were found regarding curative surgery. However, significantly fewer patients with a histologic complete response to CTRT required post-treatment curative surgery when compared to those with residual disease. Recurrent cancer occurred less frequently in CTRT than among CONTROL patients, and was only local in CTRT patients. In contrast, distant metastases were increased in CONTROL. Furthermore, while no differences exist between the groups regarding overall survival, complete response patients in the CTRT group had a greater five-year survival achievement than those who only had a partial response. Our review of the literature indicates that these treatment effects of CTRT on Stage III and IV SCCHN of the base of tongue and hypopharynx specifically have not been reported previously and are significant findings of this study.
Compared to other treatment regimens for SCCHN, the CTRT regimen produced little toxicity. Only 14% of CTRT patients suffered grade 3 toxicity, and none had grade 4 or 5 toxicity, significantly less than 40% CONTROL. In addition, 19% of CTRT patients completed treatment with no toxicity at all. Previously published clinical trials of concomitant chemo-radiotherapy almost universally have reported increased toxicities due to the potency of the drug combinations [3]. In their evaluation of high-dose 100 mg/M2 cisplatin on days 2, 16, and 30 of radiotherapy plus 5-FU, Bourhis and colleagues observed grade 3 and higher toxicity in 83% of their patients [12]. Unfortunately, these very high rates of toxicity are common among studies of high-dose cisplatin given every three weeks [4,13]. Alternatively, a study with weekly low-dose cisplatin (30 mg/M2) during radiotherapy still saw grade 3 to 4 mucositosis in 35.2% of patients [14]. In contrast, an early clinical trial of the regimen that became CTRT (20 mg/M2 cisplatin on day 1 to 4 and 22 to 25 of radiotherapy) experienced only 27% grade 3 toxicity and no grade 4 or 5 toxicity, similar to the present results [9,10]. Thus, the direction of chemoradiotherapy study in treating SCCHN needs to move in the direction of low-dose chemotherapy so as to improve patient tolerance of pre-operative treatment without compromising therapeutic effectiveness.
The CTRT chemo-radiotherapy combination analyzed in this study achieved reduced toxicity without compromising treatment effects against the cancers. Our clinical complete response rate (CCR) was 63%, and our negative biopsy, as indicated by a histologically complete response (HCR) was 59%. These outcomes are favorable to those of Paccagnella, et al. who treated SCCHN patients with either two cycles of cisplatin 20 mg/M2, days 1-4, plus 5-FU 800 mg/M2/day during weeks 1 and 6 of radiotherapy or docetaxel 75 mg/M2 plus cisplatin 80 mg/M2, day 1, and 5-FU 800 mg/ M2/day every 3 weeks [15]. The two arms of this study achieved CCR rates of 21.2% and 50%, respectively. Another study tested 100 mg/ M2 cisplatin every 3 weeks plus 5-FU versus the cisplatin regimen plus UFT 200 mg/M2/d and vinorelbine 25 mg/M2 every 21 days [16]. Again, CCR rates were only 36% and 31%, respectively. Conversely, a pilot CTRT study by Goodman, et al. in which patients were treated with cisplatin 20 mg/M2 on days 1 to 4 and 18 to 20 during radiotherapy had an HCR rate of 54% [17]. Consequently, CTRT has comparable and even slightly better rates of complete response and negative biopsy than other studies regarding the treatment of SCCHN.
Radical, curative head and neck surgery, with its high complication rates and resulting cosmetic and functional morbidities, has been a major concern in the treatment of SCCHN, particularly in elderly patients [18]. Organ preservation is extremely important to the patient; however, organ function is often compromised when surgery is used to treat SCCHN. Additionally, patients with SCCHN frequently present with unresectable, advanced stage disease at diagnosis [19]. Thus, CTRT was specifically designed to eliminate surgery from the treatment regimen whenever possible. The decrease in post-treatment surgeries among HCR patients in the CTRT treatment group reveals the success of CTRT in preserving organ function. Patients who responded to this treatment not only had a negative biopsy, but also were able to retain full function of their upper aerodigestive tract. Furthermore, only 19% of post-treatment surgeries in CTRT required composite resections with complex reconstruction. A comparison study of two treatments, cisplatin 100 mg/M2 on day 1, 23, and 45 during radiotherapy versus cisplatin 40 mg/M2 weekly for 6 weeks found that 44.6% and 37% of patients, respectively, required post-treatment surgery [20]. Thus, although CTRT did not differ from CONTROL regarding surgery, it was more successful in preventing post-treatment surgery when compared to other regimens.
No metastatic disease was present in the CTRT group after treatment, while the CONTROL group had 25% of patients with distant recurrence. The study by Posner, et al. found distant metastasis in 5% of the TPF regimen group and in 9% of the PF group [2].
Regarding survival, only 50% of patients in CTRT expired with disease, compared to 100% of the CONTROL patients. The median survival for CTRT was 20 months, which is comparable to other treatment regimens, which have ranged from 25 to 85 months [3]. CTRT also achieved increased five-year survival for patients in which an HCR was achieved. Lastly, the trend toward increased long-term survival as evidenced by both of the Kaplan-Meier curves for overall survival and disease-free survival show that CTRT is at least comparable with other treatment regimens in terms of survival.
There are several limitations in the present study. Of course, a retrospective review is lower on the evidence-based medicine scale than would be a prospective investigation. Incomplete information on individual patients and uncontrolled follow-up data restricted analyses. Additionally, the CONTROL group was small in number, was not enrolled by any pre-established criteria, and therefore, varied widely in the treatments that were applied. Consequently, not only was this study not a strict two-armed investigation, but the CONTROL group may be viewed as too heterogeneous for valid comparison. Nevertheless, in the authors' opinion, the CONTROL group may reflect community practice and thus provides a clinical perspective beyond the value of this report as a single arm protocol. Radiation therapy also varied within both patient groups, as state-of-the-art evolved over time. However, the CTRT chemotherapy regimen was administered consistently. The small number of patients in this series was a limitation as well, especially in trying to determine equivalence.
The improved CCR and HCR rates achieved in this study while simultaneously reducing toxicity are major improvements to the multi-modality treatment of squamous cell carcinoma of the head and neck. The lack of distant metastases is another positive outcome of CTRT. Lastly, CTRT is comparable in terms of survival with other published regimens. Based on the results presented in this paper, we believe pre-surgery low-dose cisplatin in combination with high-dose radiotherapy is a feasible and useful first line treatment regimen for SCCHN.
References
- Cancer Facts and Figures 2013. American Cancer Society.
- Posner MR, Hershock DM, Blajman CR, et al. (2007) Cisplatin and fluoracil alone or with docetaxel in head and neck cancer. N Engl J Med 357: 1705-1715.
- Cohen EE, Lingen MW, Vokes EE (2004) The expanding role of systemic therapy in head and neck cancer. J Clin Oncol 22: 1743-1752.
- Adelstein DJ, Li Y, Adams GL, et al. (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21: 92-98.
- Wolff HA, Overbeck T, Roedel RM, et al. (2009) Toxicity of daily low dose cisplatin in radiochemotherapy for locally advanced head and neck cancer. J Cancer Res Clin Oncol 135: 961-967.
- Agarwala SS, Cano E, Heron DE, et al. (2007) Long-term outcomes with concurrent carboplatin, paclitaxel and radiation therapy for locally advanced, inoperable head and neck cancer. Ann Oncol 18: 1224-1229.
- Puc MM, Chrzanowski FA Jr, Tran HS, et al. (2000) Preoperative chemotherapy-sensitized radiation therapy for cervical metastases in head and neck cancer. Arch Otolaryngol Head Neck Surg 126: 337-342.
- Koness RJ, Glicksman A, Liu L, et al. (1997) Recurrence patterns with concurrent platinum-based chemotherapy and accelerated hyperfractionated radiotherapy in stage III and IV head and neck cancer patients. Am J Surg 174: 532-535.
- Glicksman AS, Wanebo HJ, Slotman G, et al. (1997) Concurrent platinum-based chemotherapy and hyperfractionated radiotherapy with late intensification in advanced head and neck cancer. Int J Radiat Oncol Biol Phys 39: 721-729.
- Davis MA, Tyrrell J, Slotman GJ, et al. (2015) Preoperative simultaneous fractionated cisplatin and radiation therapy in the treatment of advanced operable stage III and IV squamous cell carcinorma of the head and neck. Am J Surg 209: 575-579.
- (2009) SAS/STAT(R) 9.22 User's Guide. The SAS Institute, Cary, NC, USA.
- Bourhis J, Lapeyre M, Tortochaux J, et al. (2011) Accelerated radiotherapy and concomitant high dose chemotherapy in non resectable stage IV locally advanced HNSCC: results of a GORTEC randomized trial. Radiother Oncol 100: 56-61.
- de Castro G Jr, Snitcovsky IM, Gebrim EM, et al. (2007) High-dose cisplatin concurrent to conventionally delivered radiotherapy is associated with unacceptable toxicity in unresectable, non-metastatic stage IV head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 264: 1475-1482.
- Rampino M, Ricardi U, Munoz F, et al. (2011) Concomitant adjuvant chemoradiotherapy with weekly low-dose cisplatin for high-risk squamous cell carcinoma of the head and neck: a phase II prospective trial. Clin Oncol (R Coll Radiol) 23: 134-140.
- Paccagnella A, Ghi MG, Loreggian L, et al. (2010) Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol 21: 1515-1522.
- Rivera F, Vega-Villegas ME, Lopez-Brea M, et al. (2008) Randomized phase II study of cisplatin and 5-FU continuous infusion (PF) versus cisplatin, UFT and vinorelbine (UFTVP) as induction chemotherapy in locally advanced squamous cell head and neck cancer (LA-SCHNC). Cancer Chemother Pharmacol 62: 253-261.
- Goodman MD, Tarnoff M, Kain M, et al. (1997) Interactions between outcomes and tumor response to preoperative cisplatin-sensitized radiotherapy in advanced head and neck cancer. Southern New Jersey Head and Neck Cancer Treatment Group. Am J Surg 174: 527-531.
- Argiris A (2013) Current status and future directions in induction chemotherapy for head and neck cancer. Crit Rev Oncol Hematol 88: 57-74.
- Fortin A, Caouette R, Wang CS, et al. (2008) A comparison of treatment outcomes by radiochemotherapy and postoperative radiotherapy in locally advanced squamous cell carcinomas of head and neck. Am J Clin Oncol 31: 379-383.
- Jean-Pascal Machiels, Maarten Lambrecht, François-Xavier Hanin, et al. (2014) Advances in the management of squamous cell carcinoma of the head and neck. F1000Prime Reports 6: 44
Corresponding Author
Gus J Slotman, Director of Clinical Research, Inspira Health Network, 1505 West Sherman Avenue, Suite B, Vineland, NJ, 08360, USA, Tel: +856-641-8635, Fax: +856-641-8636.
Copyright
© 2017 Tyrrell J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.