Hyaluronic Acid Unique Identification Prints in Perspective of its 3D Microscopical Structure as a Carbohydrate Hydrogel with Various Physiochemical and Biological Functions
Abstract
Purpose: Hyaluronic acid (HA) is used in vast number of medical applications. Yet, its possible microscopical 3D forms compose nearly untouched area of research. This study aims to provide a basis of understanding the HA various microscopical 3D structures reflecting its various physiochemical characteristics and biological effects.
Materials and methods: Using the environmental scanning electron microscopy (ESEM) method, twenty commercial hyaluronic acid products were examined for their 3D microscopic structures.
Main outcome measure(s): Visual comparison of microscopical structure and construction of a comparison table listing the products features.
Main results: The microscopical structure varied widely among the products under investigation. The complexity of the structures of a product increased when hyaluronic acid was blended with other materials. Pure hyaluronic acid products also had varied microscopical structures based on different concentrations.
Conclusion: The microscopical structure seems to be unique for each hyaluronic acid-based product and could be used as a unique identification print. This uniqueness extends to their clinical applications, and thus, it will prove advantageous for future research as well as industrial manufacturers to tag HA-based products with their unique microscopical identification prints (Graphical abstract).
Keywords
Comparison of hyaluronan products, Hyaluronan comparative study, Hyaluronan modification, Hyaluronan morphology, Hyaluronan topographic features, Hyaluronan microscopic structure, Hyaluronic acid
Introduction
Background
Hyaluronan, having the chemical formula (C14H21NO11) η is a natural biopolymer (native HA) in the human body and composes an important component of the extracellular matrix. The extracellular matrix is tissue-specific (including HA-tissue-specificity) corresponding to specific physiological and biochemical functional properties. Native HA molecules (including their receptors, HA synthases, and HA hyaluronidases) are all tissue-specific and vary based on the developmental phase, gender, age, health/disease states, and environmental factors. Native HA is usually obtained from animal tissues, bacterial strains that naturally produce HA, genetically modified bacteria, and marine sources. The purity of the final product can vary depending on the HA source and the methods to isolate it [1,2]. "The HA chain has three chemical groups that can be modified: The carboxylic acid group (COOH), hydroxyl group (OH), and the N-acetyl group (CH3CO), which is located on the acetamido group (C2H4NO)" [2].
Native HA is used for some medical applications without modifying its chemical bonds, which in this case can be called linear or unmodified type, or alternatively, it can be modified by many methods depending on the purpose of its application. There are many factors that play an important role in modification process, such as source and purity of hyaluronan, the conditions during the modification and manufacturing processes, the molecular weight of the used hyaluronan, the chemical agents used during the process, etc. Thus, commercial products based on hyaluronan can be expected to be different from each other in terms of their final physical characteristics such as their solubility, viscosity, elasticity, etc., and are expected to have different 3D dimensional structure, as well as different biological and clinical effects. In addition, HA pores, particle size, and distribution are important on tissue level because the pores can take part in cell migration and proliferation, and the particle size of HA can be a determining factor for the material implantation site. In general, dermal fillers have a particle size ranging from 150 μm up to 300 μm. Other HA products aimed for applications other than fillers have other sizes of particles and pores.
Published studies should mention those specific characteristics to enable researchers and scientists to compare different forms of HA effectively. Furthermore, future research should establish clear comparison between HA formulations indicated for the same clinical application to determine the optimal form. Such research should include in vitro studies (mainly for their biological properties and effects), animal studies (mainly for their biocompatibilities), their appearance under microscopy, and their UV Spectrum absorption [1,2].
Objective
Despite the vast number of studies on HA, there is still a large information gap with respect to correlating the HA modifications to their respective cellular, biological, and clinical functions. In our study, we aimed to study the microscopic features of different commercially available HA products using a scanning electron microscope (SEM) to investigate if each product has a unique microscopic print and to try to compare them. We aimed to provide a basis of understanding the modified HA products having various physiochemical properties, biological effects, and clinical applications in terms of their various microscopical 3D structures.
Materials and Methods
Twenty commercial HA-Based products were picked to represent different clinical applications, which included multiple products used for the same indication. The selection included some products having the HA in the form of a linear molecule (without modification to its chemical structure), some having it in a modified form (by the cross-linking process), and others having a mix of both. Some samples contained HA in addition to another material (Prilocaine, Mannitol, Dextranomer). The inclusion of these samples aimed to capture the blending of HA with other materials and to include them in the comparison as they are available products in the market used by physicians. Table 1 shows the information and characteristics of the products.
The testing of samples was performed by Vedrana Spada, PhD (Institution for material analysis of the Istarska County, Metris, Pula, Croatia, www.centarmetris.hr). The samples were received on 19th November 2019, and the test results were obtained on 20th December 2019. A Thermo-Fischer Scientific™ Quanta™ FEG 250 SEM was used to perform this analysis. This SEM has a magnification capability of 14-1 million x and includes different imaging modes of low and high vacuum, and Environmental Scanning Electron Microscopy (ESEM), accommodating a wide range of samples from metals to non-conductive soft materials such as gels, polymers, tissues, and cells. It enables dynamic in situ analysis of diverse samples in their natural state above or below ambient temperatures from minus 165 °C to 1400 °C with specialized in situ stages.
The samples were placed in the SEM chamber according to the ESEM method/mode at 100 Pa pressure conditions, a temperature of 5 °C, and humidity of about 20%. A Peltier base was used to cool the samples, and a large field low vacuum (LFD) secondary electron detector (SED) was used to distinguish the differences in surface morphology and record the images. A voltage of 10 kV was used, and the spot size of the electron beam was 5.
Results
After performing ESEM, an official report was generated (No. 2019/98) listing the topographic images for the 20 different HA-based products (Product 5 was omitted). The images held the same numbering as the test samples, and the lab technician was blinded to the samples’ commercial names.
Microfilms of morphology were recorded for each product, at least one for each magnification of (200x × 500 μm), (400x × 200 μm), (800x × 100 μm), and (1600x × 50 μm).
For comparison reasons, the 100x and 200x images are the most representative for the overall structure of each product. However, this paper demonstrates other magnifications for some products which had interesting parts to explore and examine closely. Images of Product 6 were excluded as they were not clear enough.
The results are shown in the following sequence:
• Figure 1: Represents the results for product 1
• Figure 2: Represents the results for product 2
• Figure 3: Represents the results for product 3
• Figure 4: Represents the results for product 4
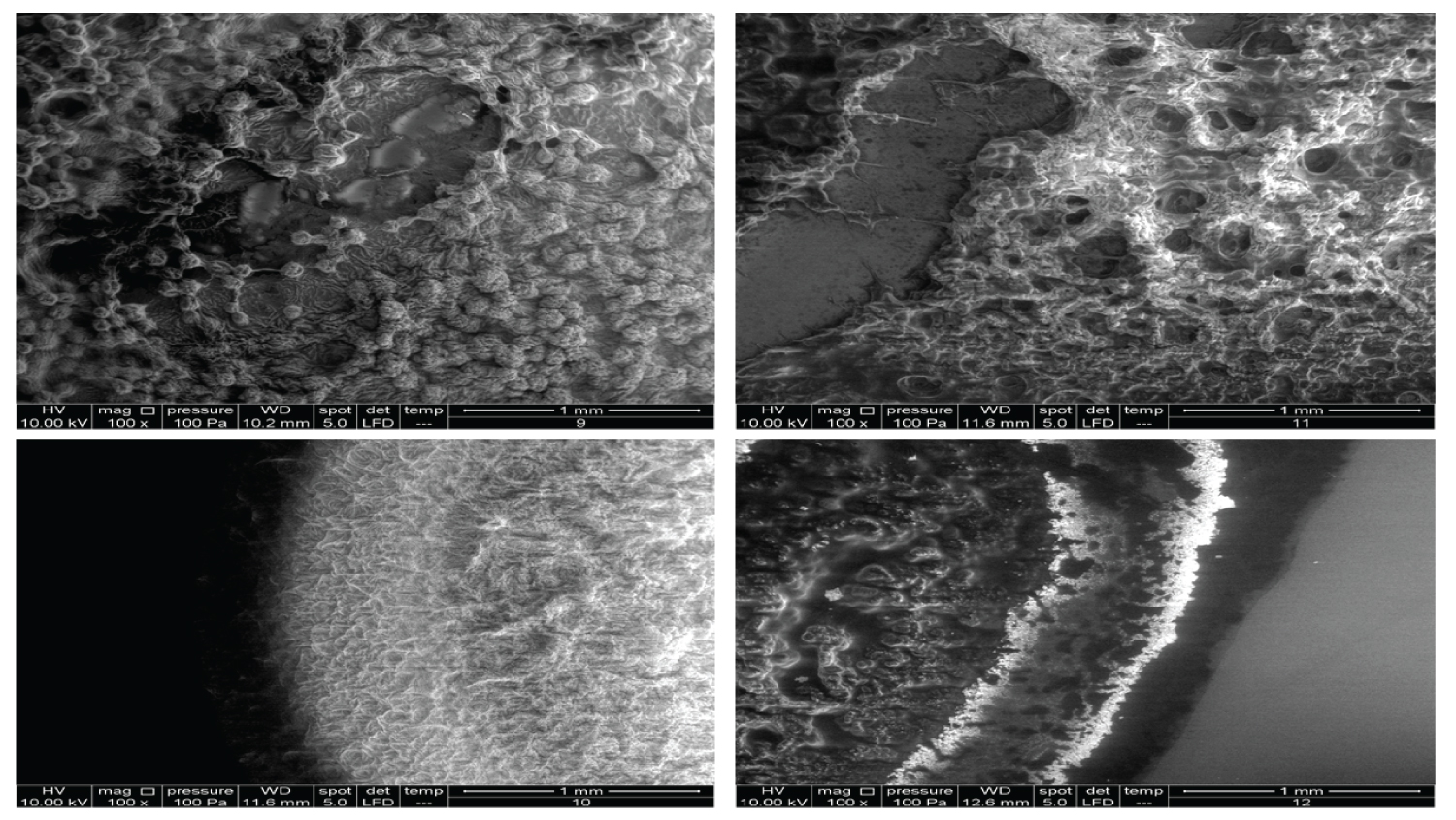
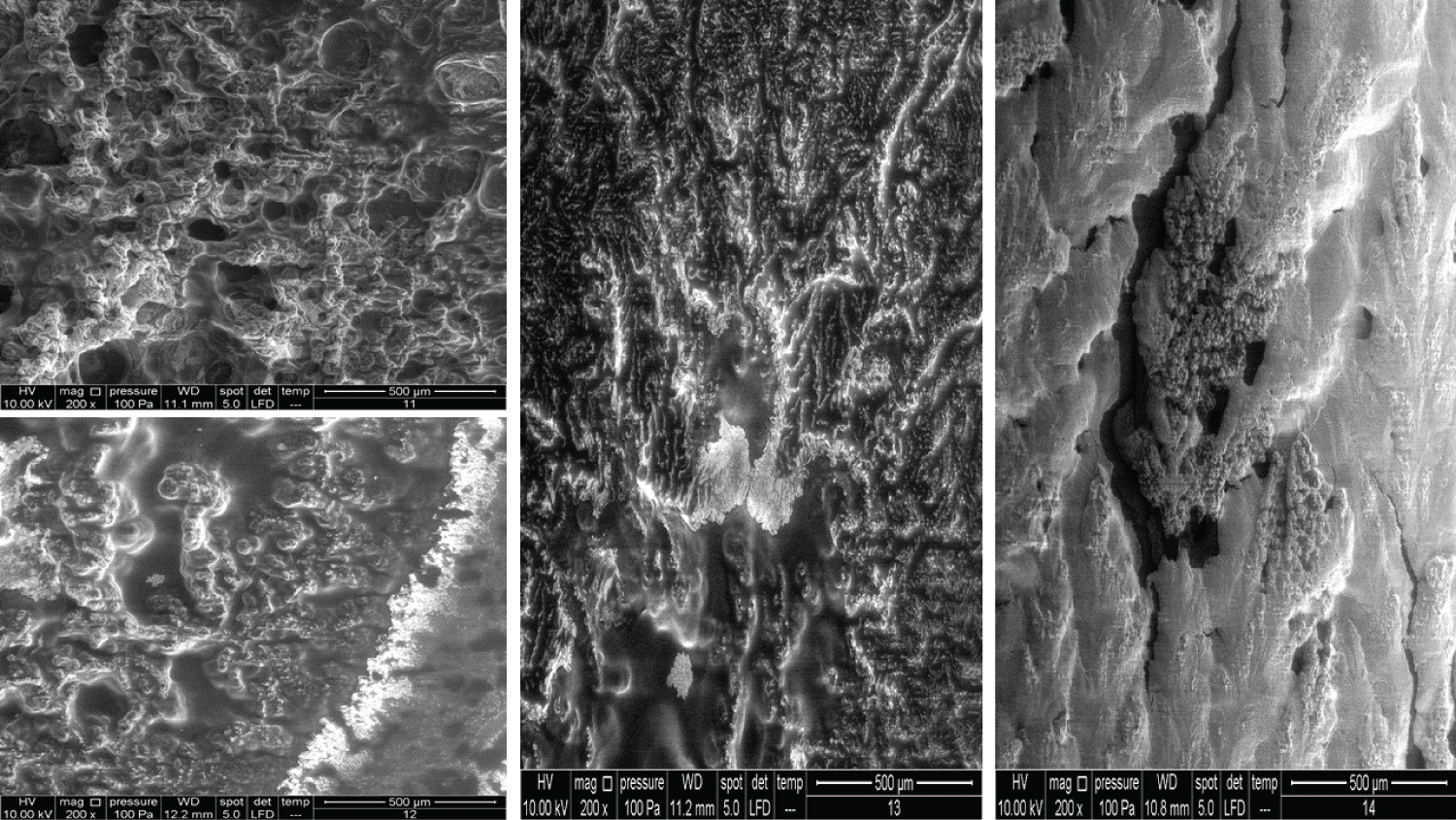
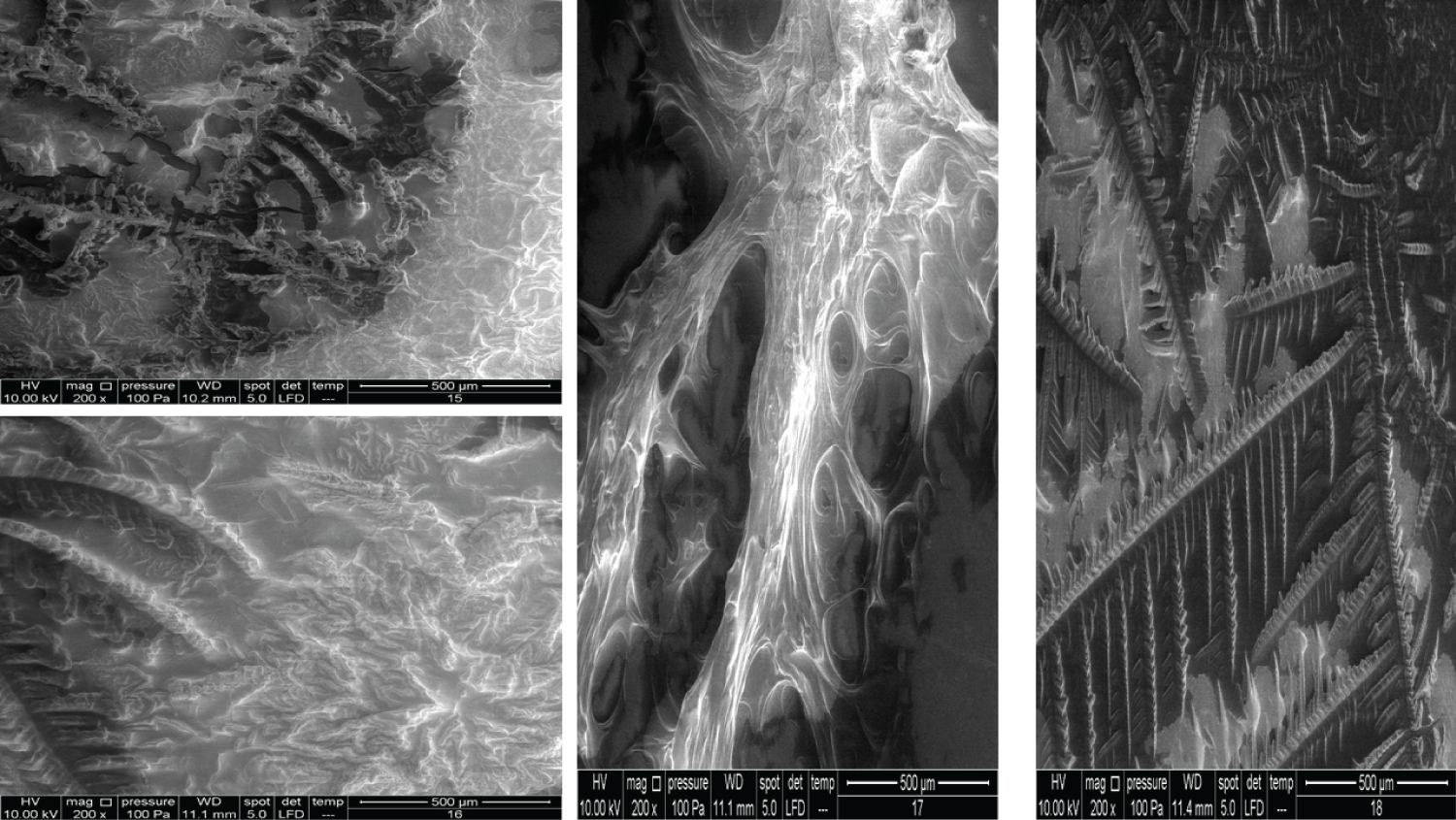
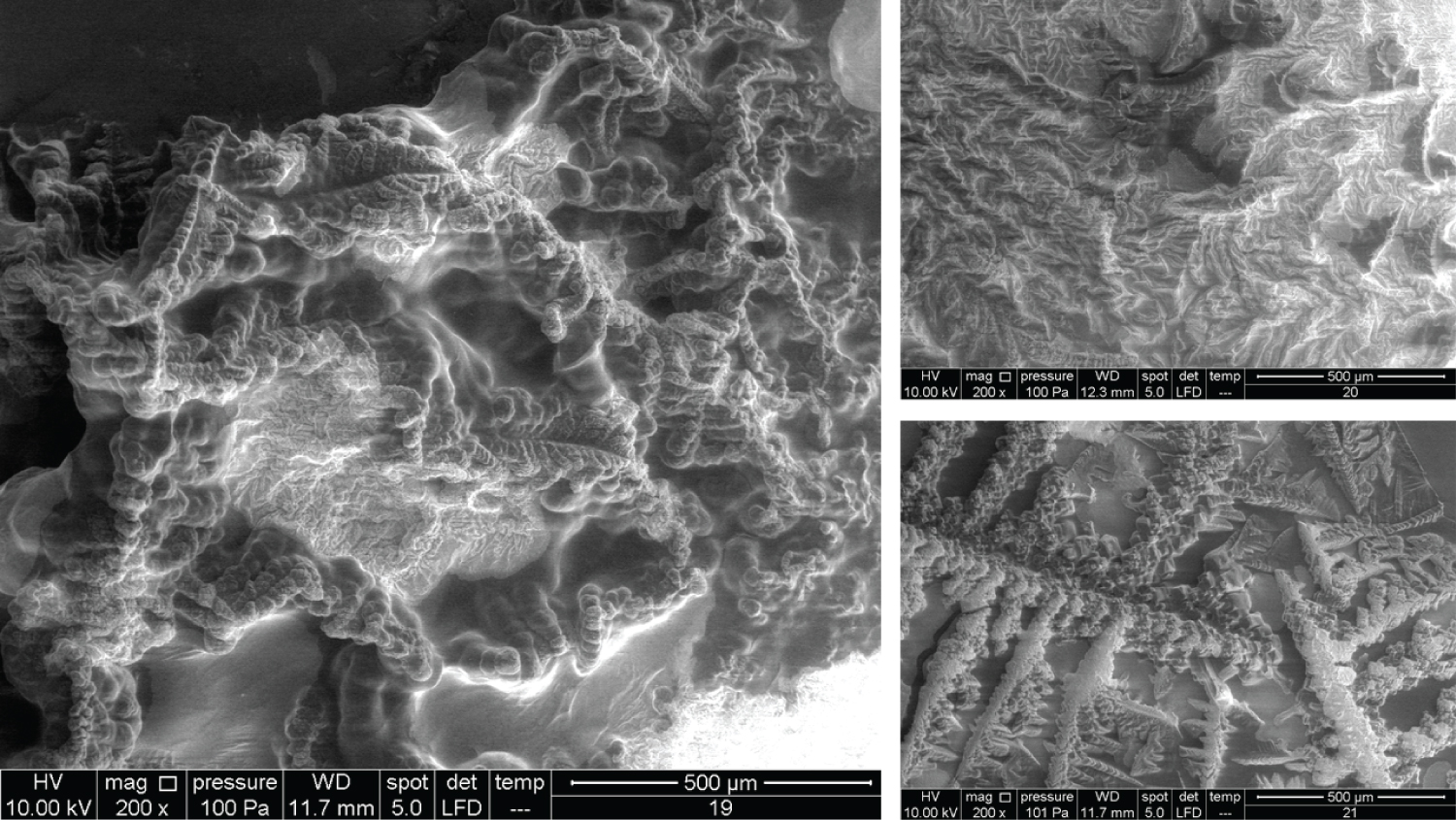
• Figure 5: Represents the results using 100x magnification for products (9, 10, 11, 12).
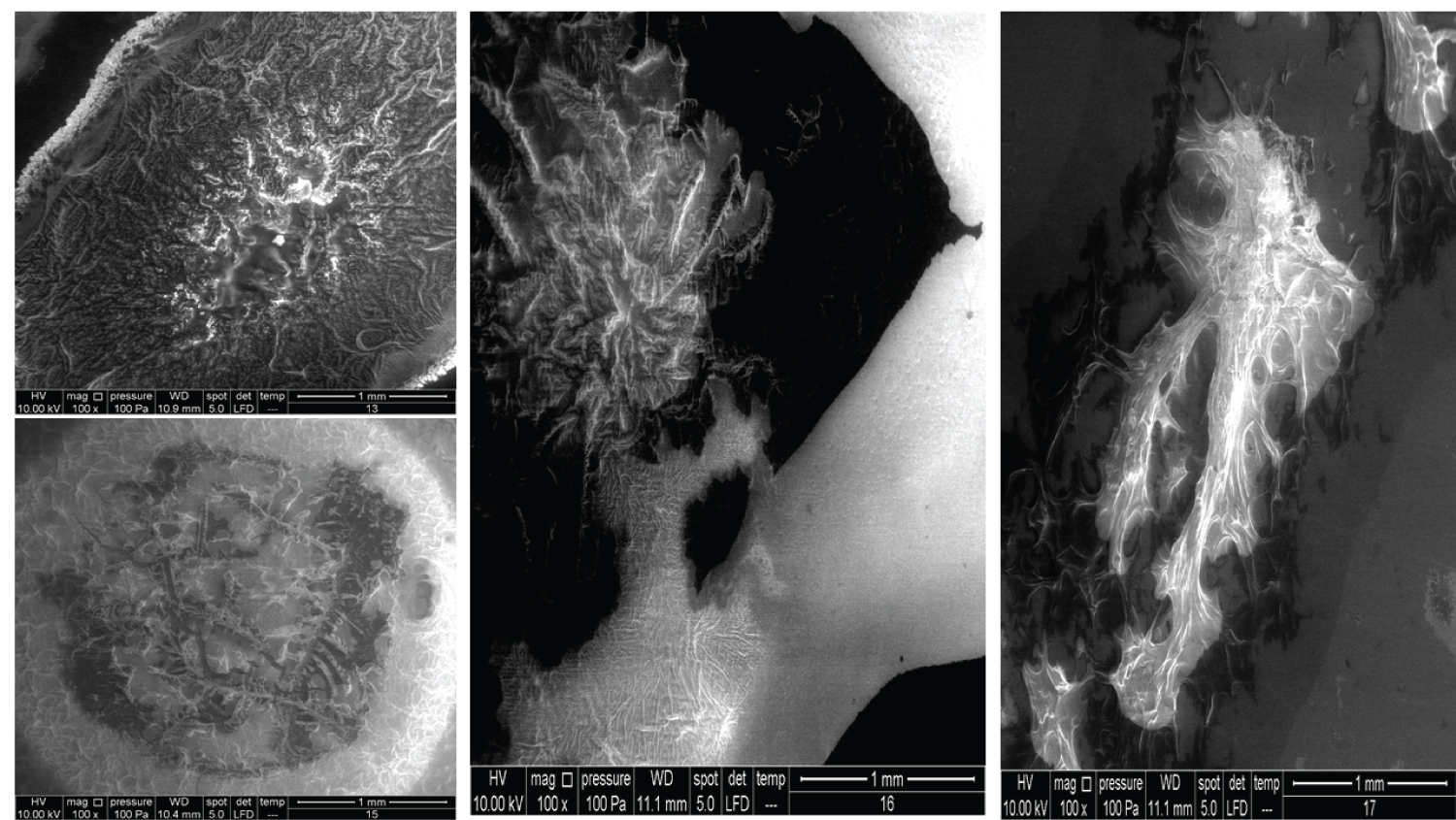
• Figure 6: Represents the results using 100x magnification for products (13, 15, 16, 17).
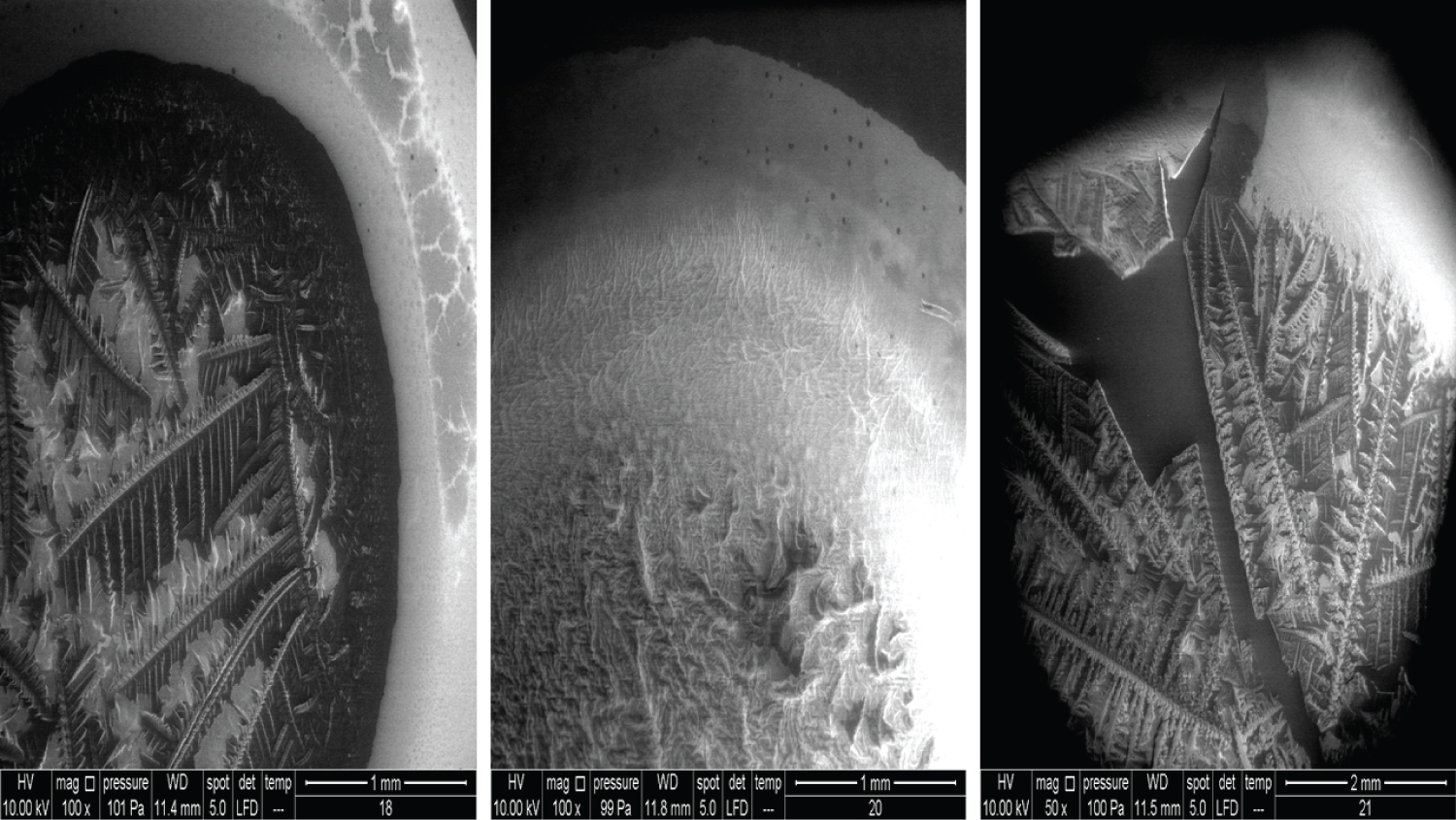
• Figure 7: Represents the results using 100x magnification for products (18, 20) and 50x for (21).
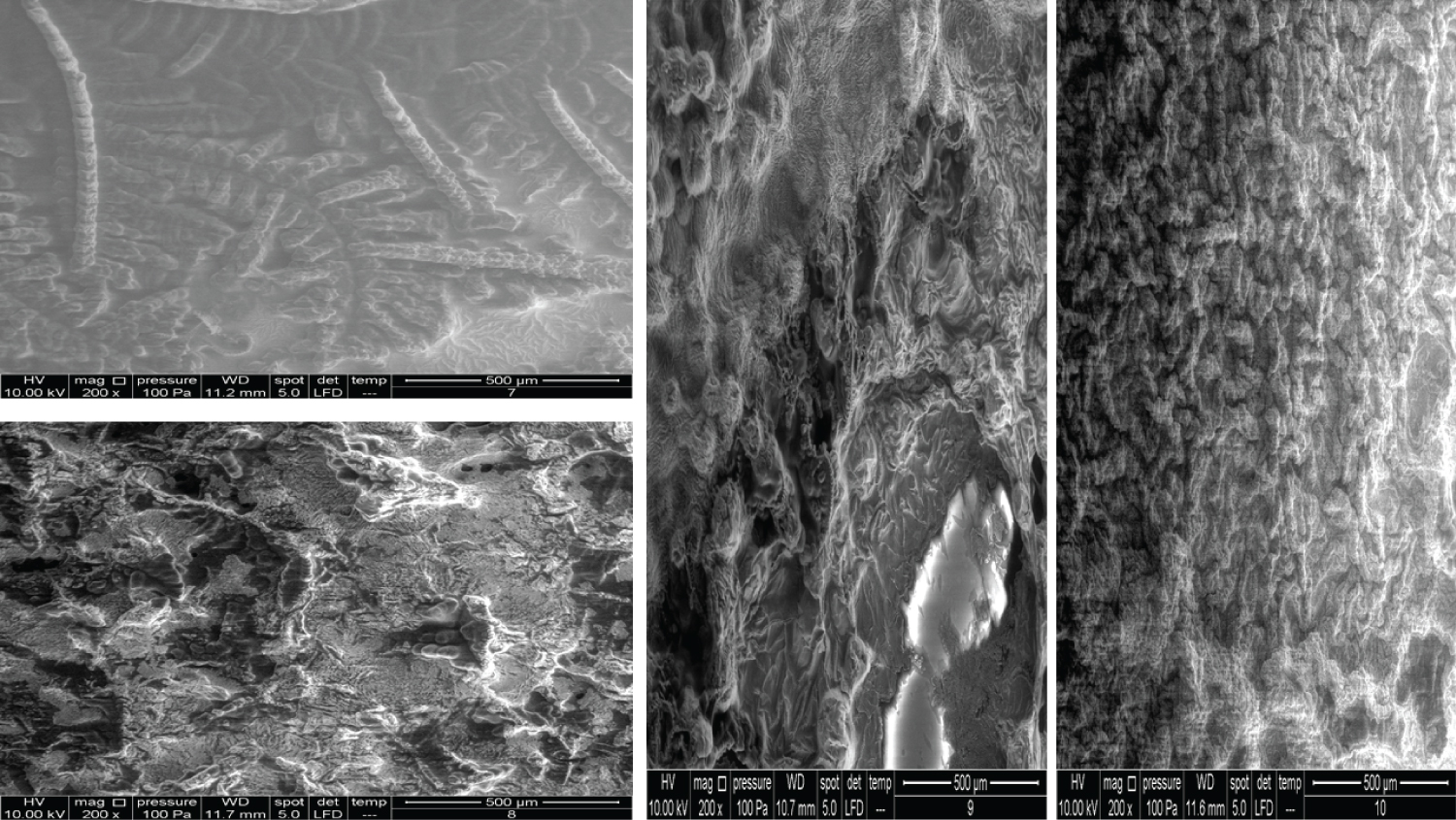
• Figure 8: Represents the results using 200x magnification for products (7, 8, 9, 10).
• Figure 9: Represents the results using 200x magnification for products (11, 12, 13, 14).
• Figure 10: Represents the results using 200x magnification for products (15, 16, 17, 18).
• Figure 11: Represents the results using 200x magnification for products (19, 20, 21).
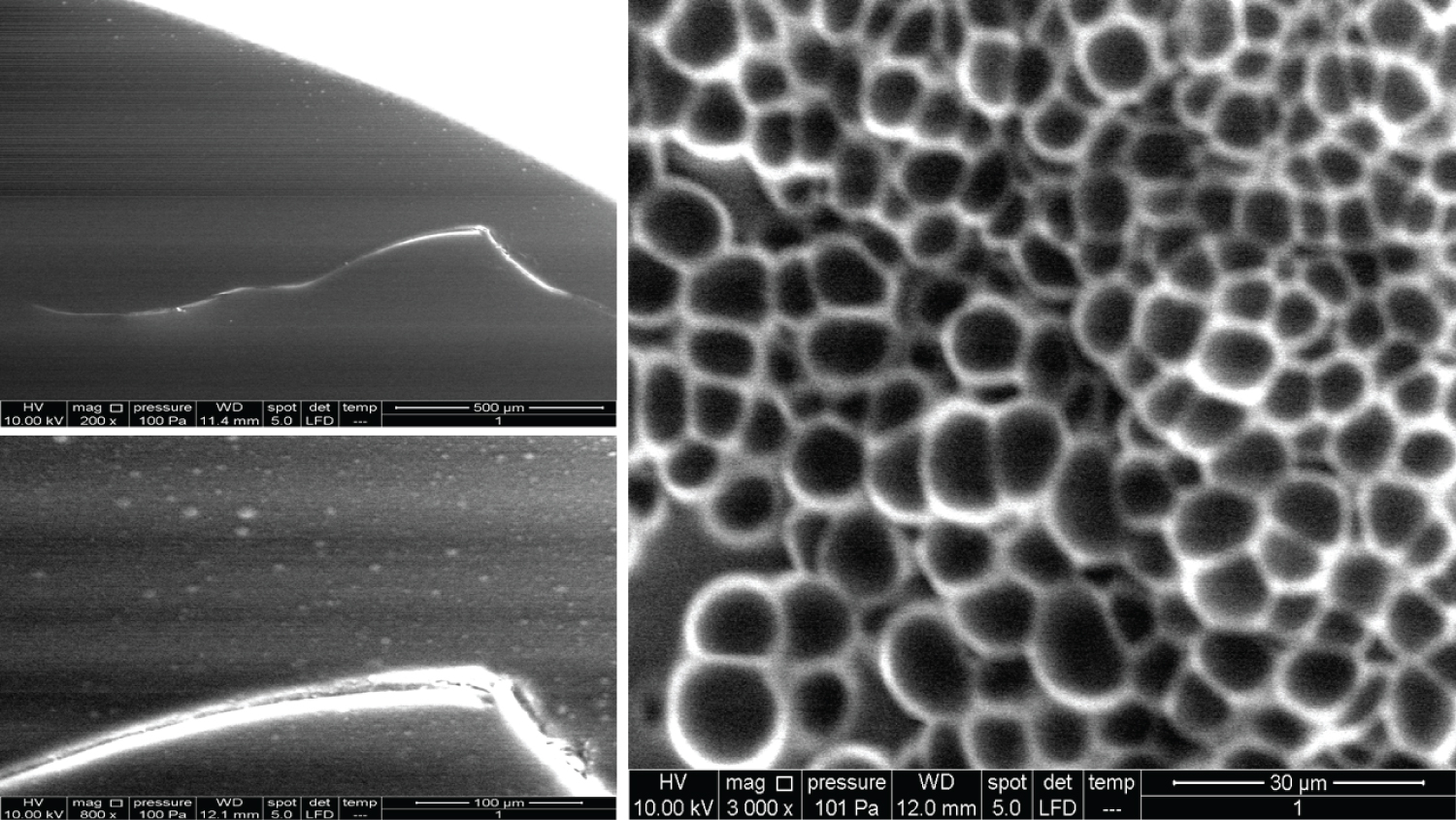
As Figure 1 shows, identifiable morphology was only observed once the magnification reached 3000x. The overall microscopical structure shows compact units that seem similar in shape but with various sizes, much like a honeycomb. The edges of each unit tend to take a spherical form, and there is a possible compacting of units to form clusters.
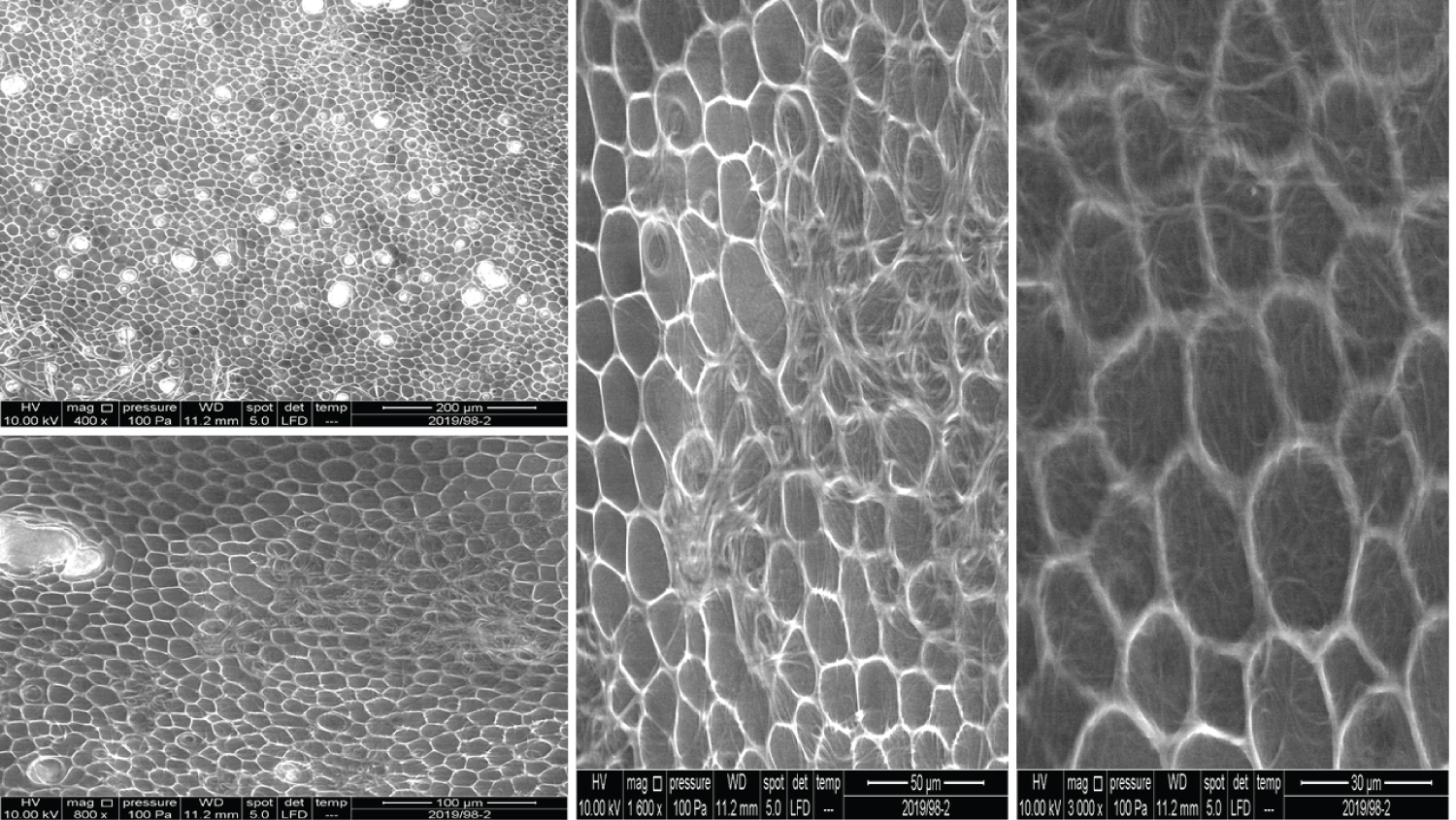
As Figure 2 shows, the units of structure in Product 2 looked quite similar to the units seen in Product 1. Pore-like structures were observed at 400x, which seemed scattered in a random fashion (a lower magnification might give more information in this regard). Higher magnification (800x) revealed another structure lying over the units. Going on to a 1600x, we saw a fibrous structure that accumulated densely at some locations. With 3000x magnification, it seemed there were two distinct layers (the fibrous structure, and the units), which overall looked like fishing nets.
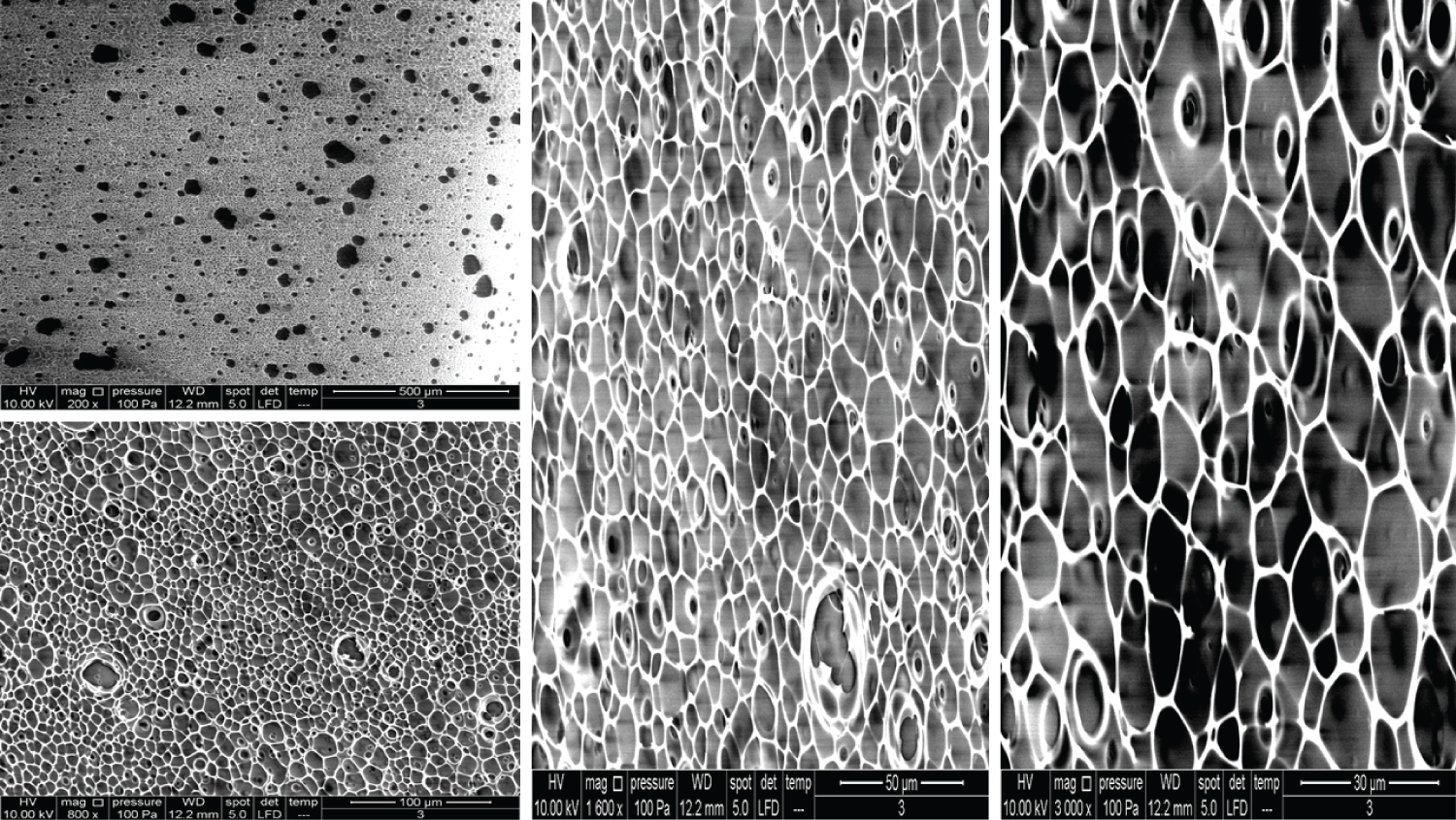
The images for Product 3 appear similar to Products 1 and 2, but 200x magnification showed a more uniform distribution of pore-like structures and their sizes (Figure 3). Higher magnifications showed a 3D nature, which might explain the extraordinary capacity of HA to absorb water.
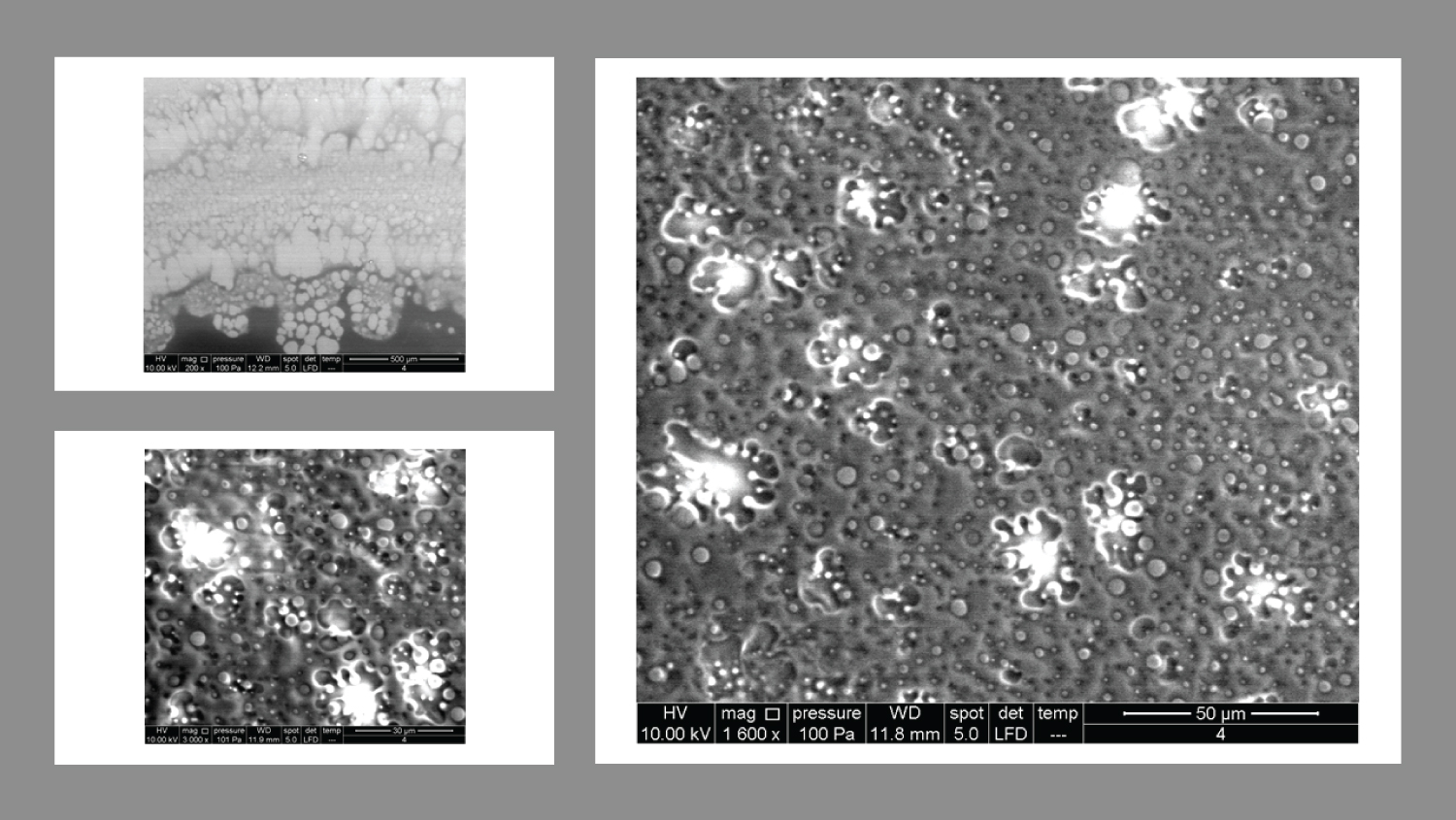
Product 4 is composed of Vitamin C and 20% HA, and the images showed a completely different pattern (Figure 4) At 200x magnification, it looked like a thick layer with finger-like projections, which looked like sacks containing spherical bodies of different sizes. At higher magnifications, there were quite a big number of spherical bodies that seemed scattered in two groups of different sizes.
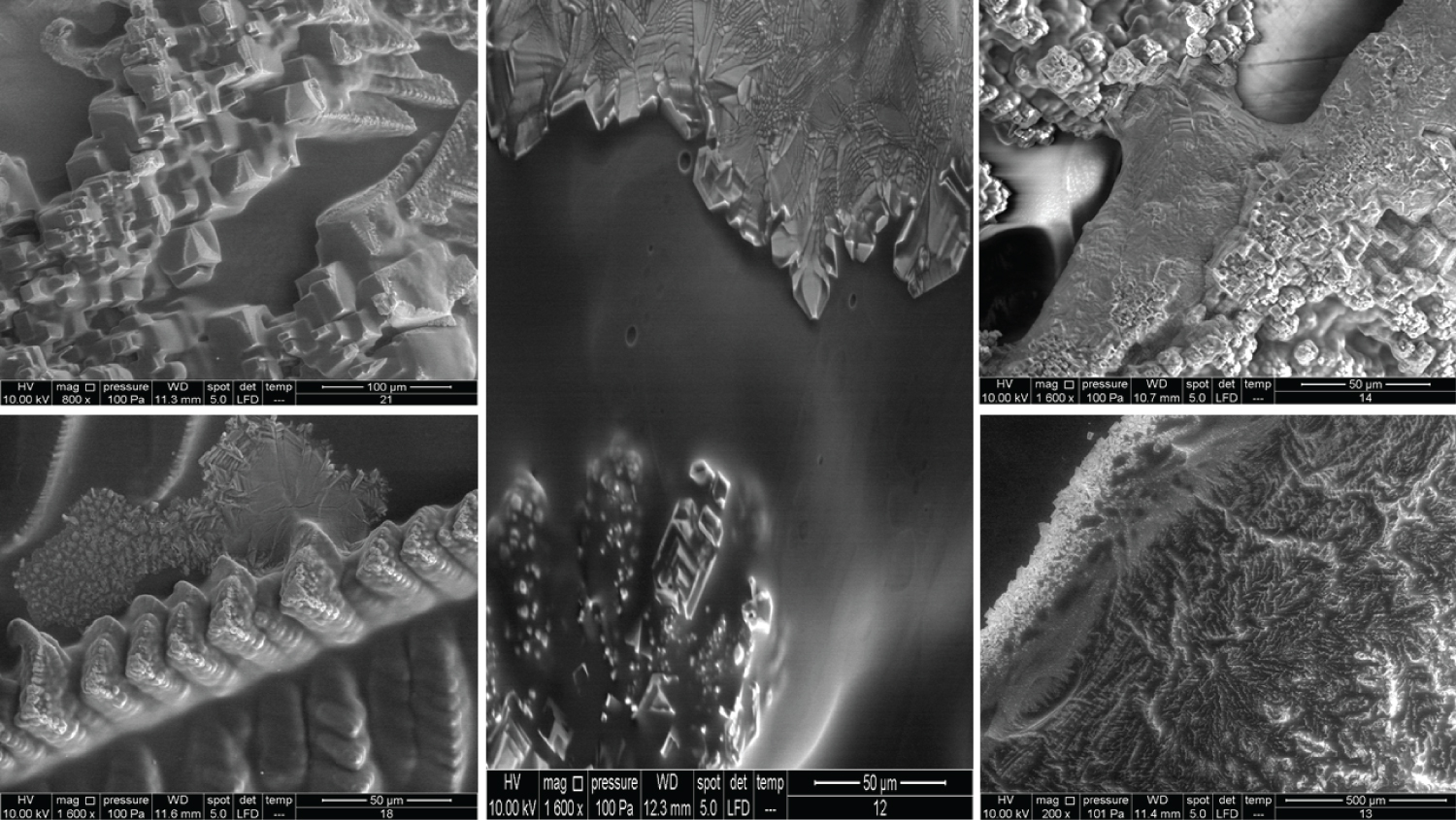
Figure 5 shows very interesting images for four different products. Product 9 contains 10 mg/ml HA and 20 mg/ml dextranomer; it is used for the treatment of vesicoureteral reflux in children. Dextranomer is in the form of a salt and thus cannot be introduced into the tissue directly; mixing it with HA provides a carrier system. As the image shows, the dextranomer particles were coated with HA, forming nodule-like structures. Product 10 contains 1.0 mg/ml of a linear form of HA and 14.0 mg/ml of a cross-linked form of HA. It is used as an intraarticular injection for the reduction of pain and improved functionality of osteoarthritic joints. As seen in the image, there were two different zones, a cortex and a core, which might represent the two different forms of HA (the linear and the cross-linked). Product 11 contains the same composition as Product 10 but is blended with prilocaine (3 mg/ml) as an enhancement. Very obviously, the enhancement resulted in a new structure. Product 12 contains 23 mg/ml of the cross-linked form of HA in addition to lidocaine (3 mg/ml) and is used as a face filler to correct moderate to severe wrinkles. Once again, we noticed the (Cortex-Core) morphology feature, but it had a different structure from the other products. This was probably due to the different concentrations and molecular weights used in addition to the implementation of different manufacturing techniques.
The images in Figure 6 clearly show the variety of structures and how much they can differ from one product to another despite all them being based on HA. Product 13 contains 25 mg/ml of a cross-linked form of HA and lidocaine and is used as a face filler to correct deep wrinkles. Product 15 contains 2.0 mg/ml of the linear form of HA and 16.0 mg/ml of the cross-linked form of HA and is used as a regenerative material and a gel scaffold in dental treatments. Product 16 contains 3 mg/ml of the linear form of HA and 21 mg/ml of the cross-linked form of HA and is used as a face filler to correct light to medium wrinkles. Products 13, 15, and 16 also showed the cortex-core feature. Product 17 contains 24 mg/ml of the cross-linked form of HA and is used as a face filler to correct medium to deep wrinkles. This product demonstrated a unique structure that was totally homogenous through all images obtained. We named this unique morphology as "HA flames."
In Figure 7, all the products showed the cortex-core feature but were nevertheless different from each other. Product 18 contains 22 mg/ml of the crossed linked HA and is used as an intraarticular injection for the reduction of pain and improved functionality of osteoarthritic joints. Product 20 contains 16 mg/ml of the cross-linked HA and is used as a face filler to correct light wrinkles. Product 21 contains 10 mg/ml of HA and is used as an intraarticular injection.
The images for Products 7 and 8 showed a big difference between them (Figure 8). Product 7 contains 20 mg/ml of linear HA and 5 mg/ml of mannitol and is used as an intraarticular injection. Product 8 contains 20 mg/ml of a cross-linked HA and 3 mg of lidocaine and is used as a face filler to correct deep face impressions. Product 9 and Product 10 have been mentioned previously, but the images represented here are of the 200x magnification.
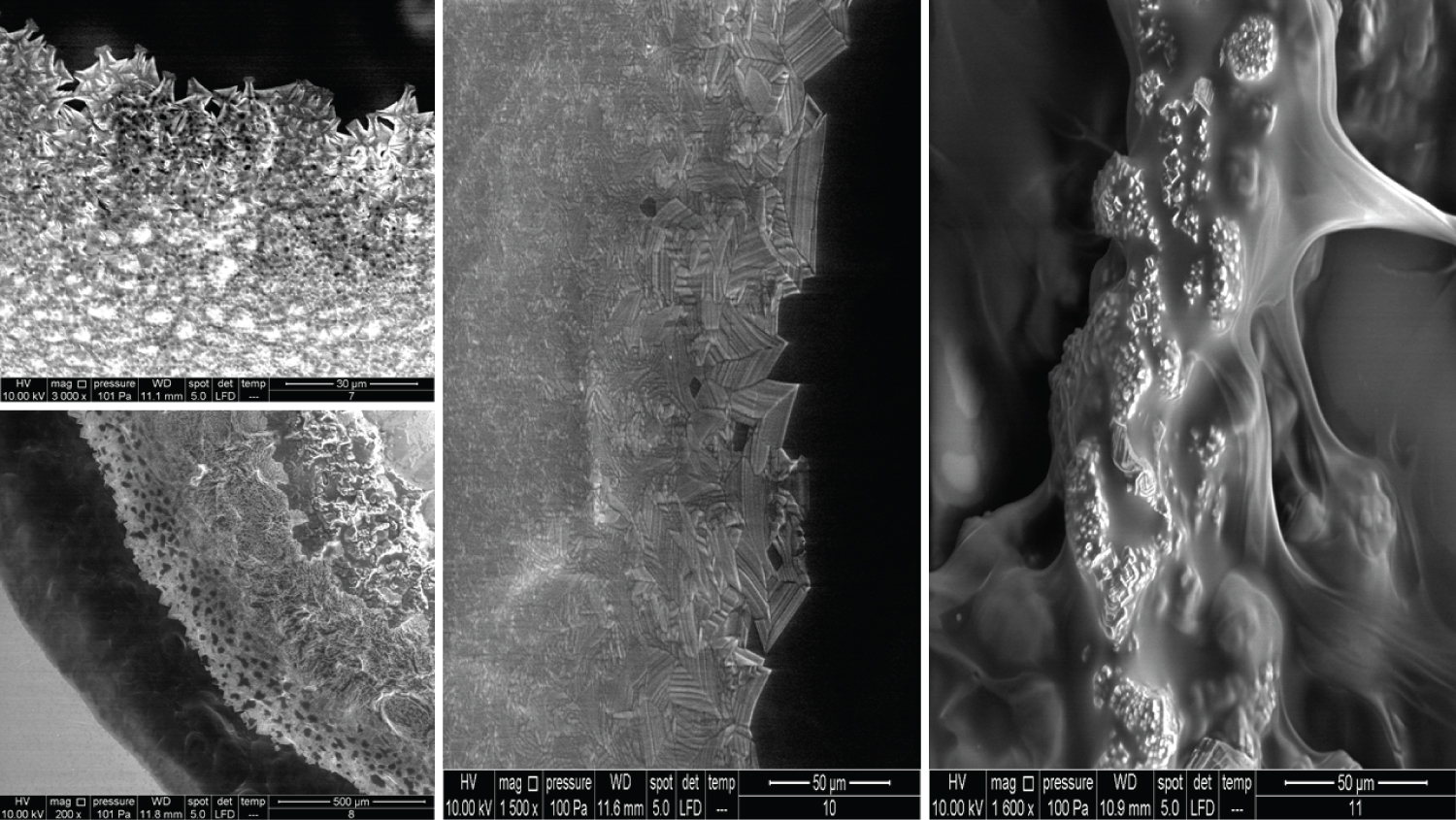
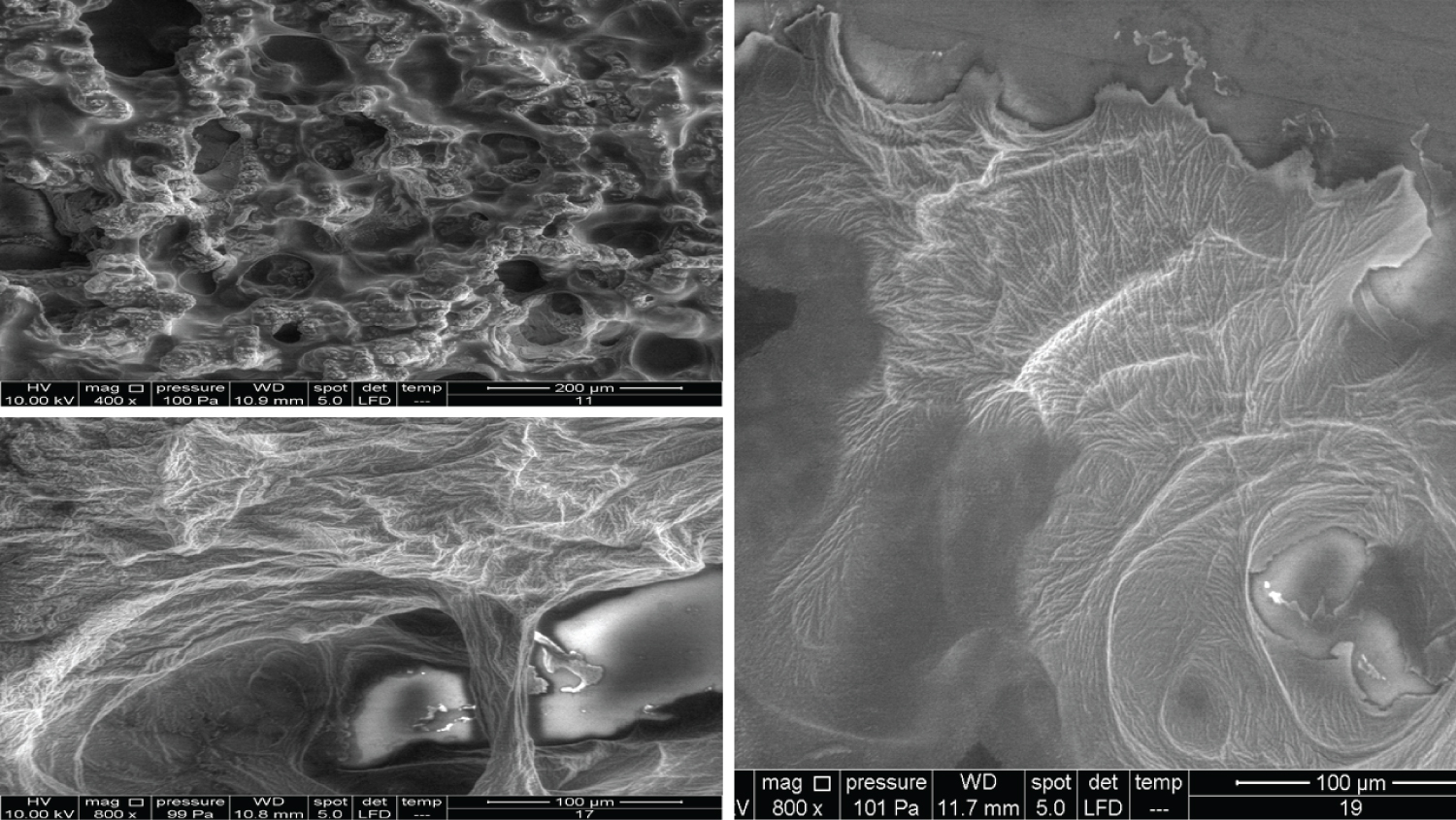
Figure 9 shows Products 11, 12, 13 and 14 imaged using a 200x magnification. Product 11 showed some parts with membrane-like structures, whereas Product 13 amazingly showed an obvious crystalline structure, where two large crystals met each other and were located at the centre of the material. Product 14 contains 10 mg/ml of a linear HA and is used as an intraarticular injection. The images of this product showed a very high compact crystalline formation, which upon investigation (using higher magnifications up to 3000x), showed an enormous amount of crystals as small cubes all compacted together.
Products 15, 16, 17, and 18 were mentioned previously, but using 200x magnification revealed more details (Figure 10). Product 17 contains 24 mg/ml of cross-linked HA and upon examination with 400x, 800x, and 1600x showed a membranous nature and demonstrated a completely different pattern from the other HA products.
Product 19 contains 16 mg/ml of cross-linked HA and is used as a filler to correct light to medium wrinkles (Figure 11). It also showed some regions with a membranous nature. Products 20 and 21 were mentioned previously. It was suspected that product 21 has a very similar nature to Product 18 (using 100x magnification). Using higher magnifications revealed that some parts have a similar structure, while other parts amazingly have similarities with Product 14 and its cubical crystal structure.
Discussion
The obtained results are consistent with the observations by Flynn, et al. who examined three different types of HA dermal fillers and found that differences in morphologic architecture were evident among all of them [3].
Reviewing the scientific literature, there are very few studies that explored the possible correlations between the different types of commercially available HA products and their clinical applications. In fact, this approach will enable the advancement in HA molecule knowledge, which still needs a lot of effort and time to reach a satisfactory point.
The study by Micheels, et al. aimed to examine seven commercial products of different crosslinking technologies [4]. The investigators conducted tests for cohesivity and resistance to stretch, and light microscopy examinations; they concluded that knowledge of HA gels characteristics is essential to select the most appropriate product for a specific treatment indication as HA products are produced with different crosslinking technologies that can influence their behaviour.
The study by Kim, et al. compared different types of fillers (twenty biphasic, and nine monophasic) using MRI scanner; they concluded that the fillers exhibited different MRI properties [5]. We should draw the reader's attention here that the terminology of monophasic and biphasic should not be used in the context of describing the HA products [6].
The study by Fagien, et al. aimed to collect the rheologic and physicochemical measurements for 18 commercial products where relationships were observed between the elastic modulus of HA and the swelling factor, and between the elastic modulus and cohesion, but with limitation to the products manufactured by the same crosslinking technology [7]. They concluded that the elastic modulus for HA represents a useful parameter to differentiate products and understand how to select a product to contribute to an optimal outcome.
Also, the study by Santoro, et al. compared different types of fillers and correlated their rheological and physical properties to their clinical effectiveness, and their results demonstrated that the viscoelastic properties of different dermal fillers vary considerably even within the same family and that the physical properties significantly influence the filler performance [8]. They concluded that the rheological data could be a tool for measuring the efficacy and longevity of a dermal filler, but that consideration should be taken that in vivo conditions, production technology, and injector skills, also have an influence.
Furthermore, the study by Migliore, et al. evaluated the evidence from in vitro and in vivo studies for HA products used as intraarticular injections and marketed in Italy [9]. They concluded that product-specific studies are necessary to provide guidance to clinicians to conclude an appropriate choice regarding HA-intraarticular injections.
The study by Nicholls, et al. investigated the rheological properties for various HA formulations used for the treatment of knee osteoarthritis [10]. They concluded that the standard assessment of the HA formulations as a class might not be appropriate; instead, each product should be investigated individually, and future research should seek to link the differences in the formulations’ rheological properties to their clinical efficacy.
In a study by Mondon and Dadras, the investigators assessed seven different types of HA soft tissue fillers crossed linked by 1,4-Butanediol Diglyceryl Ether under optical microscopy and cryo-scanning electron microscopy [11]. They assessed the influence of the structure for the products under study on drug release time using lidocaine and bovine serum albumin. They indicated that the crosslinked HA hydrogels are manufactured with different technologies which substantially affect the network of the hydrogel, which in turn affects the drug release time. Thus, by varying specific parameters such as the HA concentration, HA molecular weight, and cross-linking ratio/density, scientists can modify the properties of the cross-linked HA hydrogels to achieve certain desired characteristics. In their study, most of the results they obtained were also consistent with ours, and they concluded that all examined samples had a different structure on a macroscopic scale but exhibited the same fibrous structure on a microscopic scale (10 μm and 5 μm), having different homogeneity levels and pore sizes between 0.5 μm and 18 μm.
Such studies furnish the road for future efforts to explore and standardize the methods for HA analysis and investigation. Protocols should be developed for each specific clinical indication using HA as a treatment to capture the differences between available products and to find out the best formulation for use. Such protocols should capture the HA-based product physiochemical characteristics, in vitro biological properties, animal studies for its biocompatibility, microscopical characteristics, and its clinical aspects. Handling any of these characteristics separately cannot provide enough information on the product behaviour in biological and clinical settings. The 3D microscopical structure can serve as a unique identification method for modified HA-based products, and thus providing additional valuable information in profiling of such products. We should draw attention that the 3D microscopical structure can be considered as the scaffold that enables cells migration and proliferation (design dependent), and where the fragments of HA resulting out of its degradation after implantation in certain tissue have certain length and 3D (depending on its design) that might promote different cellular effects.
We should mention that the superiority of an HA product in certain biological or clinical aspects over other products does not mean its superiority in other aspects, and thus, the best formulation can be determined according to the biological and clinical effect of interest. For example, multiple HA products having different characteristics in terms of their average molecular weight, concentration, viscosity, elasticity, etc., might be used for the same clinical application but having different modes of application, duration of effect, residency time, and biological effects. This should be the logic behind investigating each HA product for its exact characteristics to enable the physician to select the most appropriate one according to each specific clinical case.
Interpretations
In an effort to explain the outcome results, and upon examination of all available images, we constructed Table 2 in conjunction with Table 1 to group the products according to their morphological characteristics. Then, we searched for correlations between the product's chemical composition and the differences in morphological features.
The images for the honeycomb-like units and the Cortex-Core feature have been illustrated previously. Figure 12 and Figure 13 show the crystal formation feature, whereas Figure 14 shows the membranous feature for the products mentioned in Table 2.
While an HA molecule is composed of basic repeating units like any other natural molecule (protein, DNA, etc.), the many forms or isoforms of this molecule perform totally different biological tasks. What contributes to such differences are the types of chemical or physical bonds that exist between the basic units of a molecule and give it a unique geometric form. Furthermore, different manufacturing techniques results in alteration of HA geometric form and thus its physiochemical characteristics. Due to vast number of variables, explaining the HA physiochemical characteristics in terms of its 3D microscopical structure seems not possible. Yet, the 3D microscopical structure can serve as a unique identification method for modified HA-based products.
Limitations
The present study included 20 different available commercial products representing multiple clinical application categories (Orthopaedic, Aesthetic, Paediatric, Dentistry), where some of them are used for the same clinical application. The study should only be considered as a blueprint for further detailed research. Exact investigations that compare all the available forms of HA for a specific clinical indication will be of high interest and value. Furthermore, we did not perform any exact statistical comparative analysis for the images as the results were visually clear, but statistics could reveal other interesting information in this regard.
In addition, we took a live streaming video for the non-modified type of hyaluronan under the scanning electron microscopy, and amazingly it showed that the HA network moves in a dynamic steady way with preserving its overall structure. We concluded that, by noticing the movement of the pores (shifting movement). Presumably, this is due to breaking some bonds between HA molecules and initiating new ones in milliseconds, which eventually composes a very interesting untouched area of study.
Conclusion
According to all the information mentioned in this paper and considering that the difference in physical and rheological properties for a material leads to altering or modifying its biological and clinical effects, it seems that each HA-based product has its unique microscopical structural print. The factors affecting the final HA product characteristics are numerous, and the comparisons between different HA products based on their molecular weight, concentration, etc. take tremendous effort and rarely (if ever) can be linked to their final form and biological function. Instead, examining the final HA product’s physical, chemical, and biological characteristics provides a detailed overview regardless of the many interfering factors during its manufacturing process, and where the microscopical structure characteristics serve as a unique identifier.
Every single HA-based product should be considered unique. Each HA-based product should be profiled in detail (including its physiochemical properties, in vitro studies, animal and human studies) to establish its exact indications and expected outcome results. A newly published study represents a nice model to examine in detail each HA formulation and identify the most appropriate one to use for a specific indication [12]. Furthermore, another study provides the possible cellular pathways and modes of action of HA in biological processes, which could be experimentally tested [13].
Finally, our recommendation in conducting morphological studies investigating the differences between different products is to use the magnification ranges starting from (50x × 2 mm) up to (200x × 500 μm). Further magnifications up to 3000x × 30 μm can be very useful to capture very specific details, but it does not provide an image for the overall morphological form of the products under investigation.
The recent study is a small contribution to the cumulative knowledge about the HA molecule and to the huge efforts of scientists and researchers over nearly 80 years since its discovery.
Author Contributions
Professor Jelena Prpic supervised the marking of the samples and their analysis.
Disclosure
The corresponding author declares that Elaf Medical Supplies has a business relationship with BioScience GmbH, Biopolymer GmbH, and Naturelize GmbH, but that he performed this research independently with his personal funds and acted as scientist and author without any orientation toward a specific product.
Data Availability
The complete set of Scanning Electron Microscopy images with their original resolution can be provided for any researcher if required and can be obtained by emailing the corresponding author at alkhateeb@elaf-me.com.
References
- Al-Khateeb R, Prpic J (2019) Hyaluronic Acid: The reason for its variety of physiological and biochemical functional properties, Appl Clin Res Clin Trials and Regul Aff 6: 112-159.
- Al-Khateeb R, Olszewska-Czyz I (2020) Biological molecules in dental applications: Hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon 6: e03722.
- Flynn T, Thompson D, Hyun S, et al. (2015) Ultrastructural analysis of 3 hyaluronic acid soft-tissue fillers using scanning electron microscopy. Dermatol Surg 41: S143-S152.
- Micheels P, Sarazin D, Tran C, et al. (2016) Effect of different crosslinking technologies on hyaluronic acid behaviour: A visual and microscopic study of seven hyaluronic acid gels. J Drugs Dermatol 15: 600-606.
- Kim M, Moon W, Hur M, et al. (2017) Ex vivo magnetic resonance imaging using hyaluronic acid fillers: Differences between monophasic and biphasic fillers. Skin Res Technol 24: 16-19.
- Öhrlund J, Edsman K (2015) The Myth of the “Biphasic” Hyaluronic Acid Filler. Dermatol Surg 41: S358-S364.
- Fagien S, Bertucci V, von Grote E, et al. (2019) Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plast Reconstr Surg 143: 707e-720e.
- Santoro S, Russo L, Argenzio V, et al. (2011) Rheological properties of cross-linked hyaluronic acid dermal fillers. J Appl Biomater Biomech 9: 127-136.
- Migliore A, Bizzi E, De Lucia O, et al. (2016) Differences among branded hyaluronic acids in Italy, Part 1: Data from in vitro and animal studies and instructions for use. Clin Med Insights Arthritis Musculoskeletal Disord 25: 89-101.
- Nicholls M, Manjoo A, Shaw P, et al. (2018) Rheological properties of commercially available hyaluronic acid products in the United States for the treatment of osteoarthritis knee pain. Clin Med Insights Arthritis and Musculoskeletal Disord 11: 117954411775162.
- Mondon K, Dadras M, Tillier J, et al. (2016) Influence of the Macro- and/or Microstructure of cross-linked hyaluronic acid hydrogels on the release of two model drugs. J Glycobiol 5: 1.
- Baraldi E, Veronica M, Fabio G, et al. (2019) Differences among Three Branded Formulations of Hyaluronic Acid: Data from environmental scanning electron microscope profile, rheology behaviour and biological activity. Biomed J Sci Tech Res 17: 12468-12478.
- Ji X, Yu T, Li X, et al. (2019) Luo, Effect of hyaluronic acid: Mechanistic investigations via topological and functional analysis of its protein interaction network. Trop J Pharm Res 18: 1919-1925.
Corresponding Author
Rami Al-Khateeb, Elaf Medical Supplies®, Al-Madena Al Monawara Street, Rana Centre, 5th floor, P.O. Box 1348, Zip 11941, Amman, Jordan, Tel: +00962-795516617
Copyright
© 2021 Al-Khateeb R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.